Abstract
The burrowing activity of fiddler crabs inhabiting intertidal flats creates visually distinct patches within these habitats. However, differences in the composition and abundance of shorebirds and their macroinvertebrate prey between areas inhabited or not by crabs are yet to be studied. Here, we compare the macroinvertebrate and shorebird assemblages in low and high crab density areas in the intertidal flats of the Bijagos archipelago, Guinea-Bissau. High crab density areas are associated with lower richness and densities of macroinvertebrates. Shorebird assemblages were also less rich at high crab density areas and the differences in species composition occurred according to prey type preferences. Fiddler crab density was the most important variable explaining macroinvertebrate abundance, after accounting for the effects of fine fraction of sediment and distance to coast. Nonetheless, a controlled experimental setup would be required to attribute differences found to the engineering activity of fiddler crabs rather than other unaccounted habitat features. Our findings suggest that crab patches should be taken into account when assessing the distribution and abundance of macroinvertebrates and shorebirds in intertidal areas. Since low and high crab density areas differ markedly in terms of shorebird carrying capacity, monitoring variations in their extent will be important to interpret past and present population trends.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal areas are very important ecosystems for many vertebrate species including birds and fishes (Wallace et al., 1984; Burger et al., 1997). Namely, intertidal flats are especially important for shorebird populations during migratory and non-breeding periods, when many species feed extensively on benthic macroinvertebrates at low tide (Evans et al., 1999). While the value of these habitats for shorebirds is undisputed, the mechanisms determining the distribution of birds within these areas are not completely understood (e.g., Burger et al., 1997; Ribeiro et al., 2004; Martins et al., 2013; Yu et al., 2020). Prey availability, although not exclusively, has been widely considered a key factor determining the spatial distribution of foraging shorebirds at several spatial scales (Goss-Custard et al., 1977; Piersma et al., 1994; Kelly, 2001; Harrington et al., 2010). Thus, variables influencing benthic macroinvertebrate distribution on the tidal flats will indirectly impact the whereabouts of shorebirds.
Intertidal flats may often seem a simple, two-dimensional and homogenous habitat, but their structure and composition can vary greatly in space in terms of abiotic factors, such as sediment grain size, topography, and water content (Ribeiro et al., 2004; Wooldridge et al., 2018) and biotic factors, such as organic matter content (Galois et al., 2000) and bacterial and microalgal density (Cammen & Walker, 1986; Weerman et al., 2010). This heterogeneity is associated with other compartments of the system, including infaunal communities. Indeed, the composition of benthic macroinvertebrates assemblages and the densities of each species are known to be strongly related with variables such as sediment grain size and organic matter content (Pearson & Rosenberg, 1978; Raffaelli & Hawkins, 1999; Ysebaert & Herman, 2002). Therefore, the heterogeneity of intertidal flats will be associated with different macroinvertebrate communities across space (Ysebaert & Herman, 2002; Rodrigues et al., 2006).
The spatial variation in the structure of intertidal flats is often rather subtle and, therefore, usually difficult to detect without close inspection, especially regarding sediment and microbiotic features. However, some organisms can create quite dramatic discontinuities in these otherwise smooth gradients of conditions. This is the case of intertidal flats inhabited by ecosystem engineers whose presence and action leads to the formation of sharply marked patterns in the sediment surface. Among animals, crabs are renowned to have a major role as habitat modifiers, most especially in tropical environments (Mouton & Felder, 1996; Botto & Iribarne, 2000; Kristensen, 2008). The burrowing activity of fiddler crabs (Subfamily: Ucinae Dana, 1851) is known to dramatically change the topography and biogeochemistry of the sediment (Mouton & Felder, 1996; Botto et al., 2000; Kristensen, 2008), turning their patches visually very distinct from the surrounding “crab-free” flats. These effects are driven by sediment reworking that causes bioturbation, i.e., the forced ascension of deep organic matter and sediment to the surface, promoting changes in sediment characteristics and in the growth and activity of bacteria, and increasing carbon flow and decomposition efficiency (Katz, 1980; Kristensen, 2008). The areas inhabited by fiddler crabs, thus, seem to constitute a distinctive intertidal habitat, visually different from the adjacent areas where crab borrows are absent or occur in very low densities. Strikingly, differences in benthic macroinvertebrate and shorebird communities between such contrasting areas have seldom been investigated (but see Iribarne et al., 2005). Studies conducted on sand prawn and ghost shrimps have shown that the sediment reworking promoted by these crustaceans has a negative impact on the richness and abundance of benthic macroinvertebrate communities (Tamaki, 1988; Pillay et al., 2007a, b). Whether fiddler crabs can generate the same effects is yet to be determined.
While potential disparities in the composition, density, or availability of benthic macroinvertebrates associated with crab presence may naturally influence shorebird assemblages, fiddler crabs themselves are also a common food source for several shorebird species in many tropical intertidal flats (Zwarts, 1985; Iribarne & Martinez, 1999; Lourenço et al., 2017). Therefore, regardless of the differences in the remaining macroinvertebrate community, the presence of crabs may also be associated with the spatial segregation among shorebirds according to their feeding preferences.
The Bijagós archipelago, Guinea-Bissau, is an internationally important area for shorebirds during the non-breeding period, holding approximately 10% (ca. 700,000) of all birds that migrate along the East Atlantic Flyway (EAF; Delany et al., 2009). West African fiddler crabs Afruca tangeri are an abundant and widespread element in most of the extensive intertidal flats of this archipelago, forming distinct patches, and are known to be an important prey item for shorebirds there (Zwarts, 1985; Lourenço et al., 2017). Although the importance of this site is globally acknowledged, many aspects of the foraging ecology of shorebirds are still poorly understood. In particular, the distribution of shorebirds and their prey in relation to the presence of fiddler crabs is yet to be studied.
In this study, we investigated the differences between intertidal areas inhabited and free of fiddler crabs in the Bijagós archipelago concerning the structure of the benthic macroinvertebrate and shorebird communities. To achieve this, we compared (1) species composition, abundance, total biomass, and harvestable biomass of the benthic macroinvertebrate community and (2) species composition and abundance of the shorebird community, in areas with low and high density of fiddler crabs. We also account for any differences in habitat features known to influence macroinvertebrate communities, namely sediment type (granulometry) and distance to coast (as a proxy for sediment exposure time).
Methods
Study area
The Bijagós archipelago (11° 12′ N, 15° 53′ W) lies off the coast of Guinea-Bissau, in West Africa, and comprises 88 islands and islets. The intertidal flat area covers c.a. 1000 km2, often bordered by mangrove forests, and it is mostly dominated by large areas of soft sediment beds interspersed with smaller areas of sandy sediments (Campredon & Catry, 2016). The archipelago supports a remarkable biodiversity, which has led to its classification as a Biosphere Reserve (1996) by UNESCO and as a Ramsar Site (2014) and has justified the establishment of three marine protected areas.
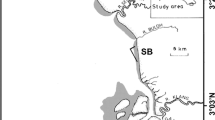
This study was conducted between February and April 2019 in the intertidal flats of Adonga islet, in the south of Orango National Park (Fig. 1). This area consists of a sand cord approximately 9 km long by 0.5 km wide which, at low tide, reveals a large extension of intertidal flats including sandy and muddy patches, permeated by a channel network (Fig. 1). The study area is characterized by a semi-diurnal tidal regime, where the tidal range varies from approximately 3 m during neap tides to approximately 4.5 m during spring tides. Fiddler crabs occur in dense patches in ca. 50% of the study area (authors’ unpublished data), and their presence creates a noticeable heterogeneity in the intertidal flat landscape, as a result of sediment reworking (Online Resource 1). The limits between crab areas and their surroundings are, thus, very noticeable, and the latter show only low densities of very small crabs (see “Results”), with no signs of reworked sediment.
Study area showing the intertidal flats of Adonga islet in the Bijagós archipelago, Guinea-Bissau. The intertidal flats are shown in dark gray. White squares represent the plots used for shorebird counts (each square 250 × 250 m). Circles represent the sites selected for macroinvertebrate sampling, sediment sampling, and crab video recordings: white circles—low crab density areas; black circles—high crab density areas. Blue ellipses represent the blocks of low and high crab density areas sampled
Macroinvertebrate collection and processing
Three paired areas of low and high crab density were selected for macroinvertebrate sampling, based on a visual assessment of the presence of crabs (Fig. 1, Online Resource 2). Paired areas were adjacent (separated by less than 50 m) in order to minimize any heterogeneity in abiotic variables between low and high crab density related to exposure time, sediment type, or proximity to shore and channels. Fifteen sediment cores (86.6 cm2, ca. 20 cm deep) were collected at random in each of the six areas of about 10,000 m2, totaling 45 cores per type of area. Since most small macroinvertebrates inhabit the upper layers of the sediment (Cardoso et al., 2010), the top 5 cm of collected sediment was sieved through a 0.5 mm mesh size and the remaining 15 cm was sieved using a 1 mm mesh size, following established protocols (e.g., Schneider & Harrington, 1981; Mercier & McNeil, 1994; França et al., 2009; Lourenço et al., 2017; Herbert et al., 2018). All invertebrates were collected from the sieves and immediately stored in ethanol 96% until further analysis.
In the laboratory, all invertebrates were identified to the lowest possible taxonomic level using guides for east Atlantic polychaetes (Day, 1967; Fauchald, 1977), bivalves (Carpenter & De Angelis, 2016; Cosel & Gofas, 2019), gastropods (Carpenter & De Angelis, 2016), and crustaceans (Carpenter & De Angelis, 2014). To determine their biomass (measured as mg of ash-free dry weight, AFDW), each individual was dried to constant weight (at 60°C, during 48 h) and then incinerated in a muffle furnace (580°C, for 2 h). The samples were weighed after drying and again after incineration, and the biomass was calculated as the difference between dry and ash-free weight. We further determined the biomass of all prey that is accessible and ingestible for shorebirds (hereafter harvestable biomass), i.e., the macroinvertebrate biomass in the top 5 cm of sediment excluding invertebrates outside the size range consumed by any shorebird species (following Lourenço et al., 2017).
Fiddler crabs are not adequately sampled using cores because they are highly mobile and can either escape from the areas targeted for sampling or hide deep in their burrows far from the core sampler reach. Therefore, we estimated their density using video cameras in 60 quadrats (70 × 70 cm) selected at random within the 6 selected areas (10 quadrats filmed per area). Quadrats were never placed less than 30 m apart from each other. Films were 4–4.5 min long and were shot using a Canon PowerShot SX60 HS, with 1980 × 640 pixel resolution at 25 frames per second. The camera was set on a tripod on the side of the quadrat and immediately after starting the filming session the observer moved at least 50 m away from the camera until the end of the footage, to avoid any disturbance. Videos were analyzed using the software VLC media player 3.0.6, excluding the initial and final parts of the film (30–60 s), when the presence of the observer disturbed the crabs. The total number of individuals was recorded as soon as crabs resumed their normal activity. The carapace width of all crabs filmed was estimated using a ruler (nearest mm) previously placed within the quadrat. The biomass of fiddler crabs was estimated from the carapace width, using the regression equations determined by Lourenço et al. (2017) and then averaged by crab density area.
Shorebird counts
Shorebird density in low and high crab density areas was estimated from counts performed during low tide. Sixty-seven plots of 250 × 250 m were defined with a set of wooden poles positioned with a GPS, in order to cover a representative part of the study area (Fig. 1). During a single day, five successive counts were performed sequentially on a set of two to four plots by one observer positioned at the middle intersection point. This point was reached by boat during ebbing tide to avoid disturbing foraging birds. Counts were carried out at 1-h intervals in the period between 2 h before until 2 h after low tide peak. This period was selected since most of the intertidal flat is completely available for foraging birds (i.e., without water cover) 2 to 3 h before and after low tide peak. The counts were repeated in two additional days in 55 plots and in three other days in 12 plots between February and April. Counts were carried out simultaneously by three observers using telescopes (20–60 times of magnification). All bird species actively feeding on the intertidal flat, including all Charadriiformes species, egrets and the Sacred ibis Threskiornis aethiopicus were counted. Within each plot, birds could easily be assigned to be feeding in low and high crab density areas and were counted separately. We visually estimated the high crab density area coverage in each counting plot. Overall, eight plots had 100% of high crab density coverage, 12 plots had 0% of high crab density coverage, and 47 plots included both low and high crab density areas.
Sediment sample collection and processing
Sediment sample collection took place in the same areas selected for macroinvertebrate sampling. In total, 30 samples (ca. 40 ml each) of sediment were collected from the top 5 cm of sediment, 15 in low, and 15 in high crab density areas. Samples were air dried after removing all visible particles of detritus. In the laboratory, approximately 5 g of each sediment sample was used to determine organic content (AFDW) following the method previously described for determining the biomass of macroinvertebrates (mass loss after ignition at 580°C). To determine the fine fraction of sediment (i.e., particles < 63 µm), we first determined the dry weight of each sample. Then, samples were immersed in a sodium pyrophosphate solution for 6 h to disperse the sediment prior to wet sieving through a 63 µm mesh. The dry weight of the remaining material on the mesh was measured. The fine fraction of sediment was calculated as the difference between the two weights divided by the dry weight of the initial sample.
Data analysis
The study design used to compare the macroinvertebrate community in terms of density and biomass (both total and harvestable) between areas with different crab densities consisted of three locations of two paired “treatments” (i.e., low and high crab density). Hence, we used a random block analysis with three blocks (each block corresponding to one location; Fig. 1) to test all the differences between low and high crab density areas. We tested all pairwise correlations in the density and biomass of different invertebrate species, to conclude that 80% of the inter-taxa correlations are within r = [− 0.06, 0.180]; therefore, we chose not to use a multivariate approach, opting instead for using univariate models. The tests were performed using generalized linear mixed models assuming a negative binomial error distribution. Since the objective was to compare areas with low and high crab densities within blocks and not among blocks, blocks were used as random factors. We included the fine fraction of sediment (sediment type) and distance to coast (as a proxy for exposure period) as covariates in all models, to account for any effect of these factors on the dependent variables under analysis. Organic matter of the sediment (measured as AFDW) was strongly correlated with its fine fraction (Pearson correlation, r = 0.86, n = 15, P < 0.001), and therefore, the former was not included in any models. One model was run for each macroinvertebrate class (Bivalvia, Polychaeta, Gastropoda, and Malacostraca) and for fiddler crabs. Given the low number of observations regarding Gastropoda and Malacostraca, the models concerning these 2 macroinvertebrate classes were run using transformed response variables. The transformation was achieved by ranking the value of each observation using the average method for ties. The ranking was performed within each block independently of other blocks as suggested by Conover & Iman (1981) for random block designs. The final models were obtained by comparing the AIC of all candidate models for each response variable and selecting the one with the lowest AIC value.
Bird densities (expressed as number of birds per hectare) within each counting plot were calculated by averaging repeated counts within the same day (± 2 h from low tide), among days and plots. In plots where both low and high crab density areas were present, estimates were carried out separately for each class of crab density. Following the approach used for invertebrates, a correlation analysis of the densities of birds of different species in the plots, revealed that 75% of the interspecies correlations were low, within r = [− 0.4 < r < 0.4]. Therefore, comparisons of the number of birds of each species between areas of low and high crab densities were tested using generalized linear models, assuming a negative binomial error distribution. The same approach was used to compare species richness of macroinvertebrate and bird communities (in cores and plots, respectively) between crab density areas.
The overall variabilities in the invertebrate and shorebird communities between low and high crab density areas were also explored with a multivariate analysis. Principal component analysis (PCA) was carried on (centered and reduced (log + 1) − transformed) values of biomass of the four macroinvertebrate classes (Bivalvia, Polychaeta, Gastropoda and Malacostraca), using data from 90 cores (45 in each type of habitat). The same was done with (center and reduced (log + 1) − transformed) data of shorebird densities in the 67 areas where birds were quantified (59 and 55 in low and high crab density areas, respectively). All PCA were computed with prcomp function in R and no rotation was applied.
Fiddler crab size-class differences in density between low and high crab density areas were tested using a generalized linear model assuming a Poisson error distribution using crab density area as independent variable.
We also determined the accumulation curves for the macroinvertebrate communities in low and high crab density areas to assess if the sampling effort was adequate to accurately describe the macroinvertebrate community and to investigate differences between sampling areas (Online Resource 2).
All analysis were performed using R software (R Core Team, 2020).
Results
Macroinvertebrate communities in low and high fiddler crab density areas
A total of 37 macroinvertebrate taxa were found, 13 of which were exclusive to low crab density areas. Macroinvertebrate communities of low crab density areas showed a significantly higher taxonomic richness (Mixed-effects model with negative binomial distribution: effect of crab density P < 0.001) when compared with high crab density areas (8.3 ± 0.4 (SE) species per core versus 2.6 ± 0.3 (SE) species per core). These differences are evident even when considering a small sampling effort (Online Resource 2).
Overall, all four macroinvertebrate classes showed almost fivefold higher densities in areas with low crab density (Fig. 2, Online Resource 3), and we found no significant effects of the fine fraction of sediment or distance to the coast in explaining this difference (Table 1). Similar results were recorded for total and harvestable invertebrate biomass (Fig. 3, Tables 2 and 3, see Online Resource 3). Unsurprisingly, the sole taxa significantly more abundant in high crab density areas were the fiddler crab, both in terms of density and biomass (Figs. 2 and 3, Tables 1 and 2).
Density (individuals/m2 ± SE) of the main macroinvertebrate classes sampled in low and high crab density areas (n = 45 sediment cores in each density area). Estimates of fiddler crab Afruca tangeri densities were obtained from video recordings (n = 30 in each area, see “Methods” for details). Note the log scale on y-axis
Total and harvestable biomass (mg AFDW/m2 ± SE) of the main macroinvertebrate classes sampled in both low and high crab density areas (n = 45 sediment cores in each density area). Fiddler crab estimates were obtained by measuring all crabs in the video recordings (low crab density: n = 138; high crab density: n = 919). A Total biomass estimates using all samples collected up to a depth of 20 cm. B Estimates of harvestable biomass for shorebirds using samples collected only in the top 5 cm of sediment, thus excluding invertebrates outside the size range consumed by shorebirds (based in (Lourenço et al., 2017)). Note the log scale on y-axis
The PCA analysis on the (log + 1) biomass of each core in both types of habitats is represented in Fig. 4. The first axis (ca. 45% of the variance) represents a general gradient of biomass of all taxa (larger values of biomass on the left-hand side), while the axis 2 (with ca. 26% of the variance) represents a distinction between cores with higher Bivalvia and Polychaeta biomasses versus those with higher biomass of Gastropoda and Malacostraca. Overall, cores in low crab density areas showed more dispersion among themselves and higher biomass of all invertebrate groups when compared to the high crab density areas.
The size-class structure of fiddler crabs differed significantly between areas of low and high crab density (GLM Poisson: effect of fiddler crab density class P < 0.001; interaction P < 0.001, Online Resource 4). Crabs measuring < 0.5 cm comprised less than 20% of the individuals in areas with high crab density, while they represented ca. 42% in low crab density areas (Online Resource 4). In the later areas, individuals with carapace length larger than 1.5 cm were virtually absent.
Shorebird communities in low and high fiddler crab density areas
The shorebird community of low crab density areas showed higher species richness when compared to high crab density areas (GLM.nb: effect of fiddler crab presence P < 0.001; Theta = 0.421). Nine out of the 18 most common shorebird species studied showed significant differences in density between the two different crab density areas (Fig. 5, see Online Resource 5). Among these, four species showed significantly higher densities in low crab density areas (Red knot Calidris canutus, Curlew sandpiper Calidris ferruginea, Ringed plover Charadrius hiaticula and Sanderling Calidris alba), while five species showed the opposite pattern (Eurasian curlew Numenius arquata, Sacred ibis Threskiornis aethiopicus, Common redshank Tringa totanus, Common sandpiper Actitis hypoleucus, and Whimbrel Numenius phaeopus). There were no significant differences for the remaining species. The largest differences (more than tenfold) were found for Common sandpipers, Red knots, and Ringed plovers (Fig. 5).
Density (individuals/ha ± SE) of the most abundant shorebird species recorded in low and high crab density areas. The vertical line separates species that have higher densities in low crab density areas from species with higher densities in high crab density areas. Differences between low and high crab density areas were assessed with GLM.nb’s relating bird density with crab density class. Significant effects (α ≤ 0.05) are highlighted with an asterisk
The PCA on the values of density of the 18 most common shorebird species show that counting areas with and without fiddler crabs are aligned according to two perpendicular axes (Fig. 6). A good part of the variation in areas with fiddler crabs is captured by axis 1 (ca. 27% of the variance), where species like Common redshank, Sacred ibis, Curlew, and Whimbrel are abundant. Axis 2 (with 20%) represents variation associated with species typically occurring in areas without fiddler crabs (Fig. 6), such as Ringed plover, Sanderling, Curlew sandpiper, and Red knot.
Principal component analysis of (log + 1 transformed) densities of the 18 most common species of shorebirds in areas with and without fiddler crabs. Ninety-five percent confidence regions are represented for counting areas in both types of habitats. Species represented in bold show significant differences in their densities between low and high crab density areas (while species in gray do not, see Fig. 5)
Discussion
Areas inhabited by fiddler crabs have been almost exclusively studied at the microhabitat level, focusing on the impacts of bioturbation on sediment topography and biogeochemistry, and on primary production (e.g., Kristensen, 2008; El-Hacen et al., 2019). Therefore, whether habitats modified by this ecosystem-engineer support distinct faunal assemblages, such as macroinvertebrates and shorebirds, remains mostly elusive. Our results show a clear difference in the composition and abundance of benthic macroinvertebrate and shorebird communities between low and high crab density areas. Areas with low crab densities showed a significantly higher abundance and biomass of invertebrates (except for the fiddler crab itself) and shorebird assemblages occurred in each of the two habitats mostly according to their main prey (Zwarts, 1985; Lourenço et al., 2017).
Ecosystem engineers have been more often described as species that facilitate the colonization by other species by promoting habitat changes (Bouma et al., 2005; van der Zee et al., 2016) or indirectly increasing the complexity of food webs by raising primary productivity (Smith et al., 2009). Nevertheless, negative effects had already been observed with burrowing ecosystem engineers, namely sand prawn and ghost shrimps (Tamaki, 1988; Pillay et al., 2007a, b).
We found that areas with high crab density were significantly less rich and supported lower densities of benthic macroinvertebrates (with several species being two to six times less abundant in these areas) while also being less variable among samples in terms of its macroinvertebrate community. In all our comparisons between low and high crab density areas, we accounted for variations in the fine fraction of sediment and exposure period, two habitat features that are reported to influence the densities or the biomass of invertebrate taxa (Pearson & Rosenberg, 1978; Ysebaert & Herman, 2002; Lourenço et al., 2018), and still, the density of fiddler crabs appeared as the most important variable explaining macroinvertebrate abundance. Although preferences of foraging shorebirds for one habitat type were shown to be species-specific, shorebird species richness was also higher in low crab density areas. While our study focused only on a limited number of taxonomic groups (shorebirds and benthic macroinvertebrates), the results suggest that habitats occupied and modified by fiddler crabs may have inhibiting rather than facilitating effects on at least some biodiversity compartments of intertidal ecosystems.
Several previous studies demonstrated that the activity of fiddler crabs increases carbon flow and decomposition efficiency and changes the topography and biogeochemistry of the sediment (Mouton & Felder, 1996; Botto & Iribarne, 2000; Kristensen, 2008). These changes in the sediment, in particular the direct disturbance effects caused by the burrowing behavior of crabs, as well as trophic effects such as predation and competition, have been proved to impact other sediment-dwelling organisms (Hoffman et al., 1984; Reinsel, 2004; Weis & Weis, 2004; Iribarne et al., 2005). As fiddler crabs are reported to feed mainly on vegetable detritus, microalgae, nematodes and bacteria (Kristensen & Alongi, 2006; Kristensen, 2008), competition may reduce the abundance of other macroinvertebrates in high crab density areas. Also, by reducing habitat suitability or even survival of mobile species such as errant polychaetes (Tamaki, 1988) and gastropods (Pillay et al., 2007a, b), and sessile species such as sedentary polychaetes (Pillay et al., 2007b) and bivalves (Pillay et al., 2007a, b), sediment reworking may also play a role in justifying the differences observed in macroinvertebrate communities between low and high crab density areas. Nevertheless, since our study focused on a limited number of habitat variables, the causes for observed differences are difficult to assess, and both abiotic variables such as salinity and currents (e.g., van der Meer, 1991; Ysebaert et al., 2002; Compton et al., 2013), and biotic variables such as the interaction with other species (e.g., Piersma, 1987; Piersma et al., 1993; van der Zee et al., 2012) may also be affecting benthic macroinvertebrate communities. Thus, a controlled experiment based upon inclusion/exclusion enclosures (e.g., Smith et al., 2009) and/or laboratory manipulation in terrariums (e.g., Natálio et al., 2017) would be further necessary to answer these questions and unequivocally attribute the differences found in shorebird and benthic invertebrate communities to the activity of fiddler crabs rather that to other potentially unaccounted habitat features or mechanisms.
Our results also explicitly showed that the composition of shorebird assemblages foraging in these intertidal areas differs in relation to fiddler crab densities. While the density of some shorebird species was drastically different between low and high crab density areas (up to 20-fold in some cases), preferences for one or another varied among species. Given that areas with high densities of fiddler crabs presented lower specific richness and biomass of harvestable macroinvertebrates (other than fiddler crabs) while also having a less varied macroinvertebrate community, these areas are likely less attractive for most foraging shorebirds. However, trophic interactions between shorebirds and fiddler crabs also play a role in explaining shorebird distribution, since these crabs are important prey items for several shorebird species in the Bijagós archipelago (Zwarts, 1985; Lourenço et al., 2017). Species such as the Whimbrel Numenius phaeopus, Common sandpiper Actitis hypoleucos and Common redshank Tringa totanus, show high proportions of fiddler crabs in their diet (Zwarts, 1985; Lourenço et al., 2017). Unsurprisingly, these species, together with the Sacred ibis Threskiornis aethiopicus and the Curlew Numenius arquata, showed significantly higher densities in high crab density areas. Despite foraging extensively upon crabs (Zwarts, 1985; Lourenço et al., 2017), Grey plovers Pluvialis squatarola and Bar-tailed godwits Limosa lapponica also showed no preference for any of the two studied habitats, suggesting that these birds can switch between areas according to their prey selection. Shorebird species that feed to a lesser extent on fiddler crabs, such as the Ringed plover Charadrius hiaticula and the Sanderling Calidris alba, or do not consume fiddler crabs and rely mainly upon bivalves (such as the Red knot Calidris canutus), or polychaetes (such as the Curlew sandpiper Calidris ferruginea; Lourenço et al., 2017), preferred low crab density areas. This may be mainly due to the fact that other macroinvertebrate prey are much scarcer in areas with high crab density. Strikingly, the fiddler crab size classes mainly preyed upon by these shorebird species (0–1 cm) have higher densities in high crab density areas and one might expect that such areas could attract more shorebird species that feed on these small crabs. The foraging strategies of each species may explain these results. Birds that detect their prey by sight and capture them with swift pecks, like the Ringed plovers, can have their ability to detect and capture small crabs hampered by the distracting effect of the presence and activity of large crabs, which are not suitable prey items. This is also true for birds that are mainly tactile foragers and use their beaks to probe for prey, like the Sanderlings (Zwarts, 1985; Lourenço et al., 2017, 2018). The lack of differences in habitat use for species that are known to prey either upon bivalves or polychaetes, such as the Eurasian Oystercatcher Haematopus ostralegus, the Dunlin Calidris alpina (Lourenço et al., 2016), and the Little stint Calidris minuta (Bengtson & Svensson, 1968), may be due to their low abundance in the study area. The same probably occurs with species that have a high proportion of crabs in their diet (author’s unpublished information), such as the Kentish plover Charadrius alexandrinus, the White-fronted plover Charadrius marginatus and the Greenshank Tringa nebularia.
Previous studies conducted in two SW Atlantic estuaries showed that the presence of the burrowing crab Chasmagnathus granulatus affects the small- and large-scale habitat use of shorebirds, while also describing species-specific habitat selection of foraging birds (Botto et al., 2000; Iribarne et al., 2005). These studies suggest that crabs are responsible for decreasing the habitat quality for shorebirds through a physical decrease in habitat area and, to a lesser extent, a decrease in prey availability (as crabs change the vertical distribution of polychaete and thus their availability to shorebirds), which may also be happening in our study area.
Conclusion
Our study represents an important contribution for the overall understanding of the spatial patterns of foraging shorebirds and of their prey, since fiddler crab patches are common and widespread in many tropical intertidal habitats. These patches show very clear differences in terms of the shorebird community structure when compared with low crab density areas, which suggest that predicting the density of shorebirds and the composition of the assemblages in foraging areas requires an adequate sampling stratification that takes into consideration the presence of fiddler crabs (see also Botto et al., 2000; Iribarne et al., 2005). Therefore, the presence and the spatial extent of these areas should be included in the set of predictors to model shorebird distribution. These findings are also important from a conservation perspective, as ultimately, fiddler crab areas seem to have a noticeably different carrying capacity for shorebirds, and therefore monitoring variations in their extent of occurrence is important to interpret their population trends. Preliminary results (authors’ unpublished data and Iribarne et al., 2005) suggest that fiddler crab patches can be efficiently mapped using satellite imagery or even drones. These can be essential tools to use in the future to continuously monitor future changes in the spatial spread distribution of fiddler crabs over time, in order to assess whether their location and extent varies significantly over time.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available since data collection was carried out under the funding of an ongoing project for which final outputs were not yet released. However, data supporting the findings of this study are available from the corresponding author [J.P.] on (reasonable) request.
References
Bengtson, S. A. & B. Svensson, 1968. Feeding habits of Calidris alpina L. and C. minuta Leisl. (aves) in relation to the distribution of marine shore invertebrates. Oikos 19: 152–157.
Botto, F. & O. Iribarne, 2000. Contrasting effects of two burrowing crabs (Chasmagnathus granulata and Uca uruguayensis) on sediment composition and transport in estuarine environments. Estuarine, Coastal and Shelf Science 51: 141–151.
Botto, F., G. Palomo, O. Iribarne & M. M. Martinez, 2000. The effect of southwestern Atlantic burrowing crabs on habitat use and foraging activity of migratory shorebirds. Estuaries 23: 208–215.
Bouma, T. J., M. B. De Vries, E. Low, G. Peralta, I. C. Tánczos, J. Van De Koppel & P. M. J. Herman, 2005. Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86: 2187–2199.
Burger, J., L. Niles & K. E. Clark, 1997. Importance of beach, mudflat and marsh habitats to migrant shorebirds on Delaware Bay. Biological Conservation 79: 283–292.
Cammen, L. M. & J. A. Walker, 1986. The relationship between bacteria and micro-algae in the sediment of a Bay of Fundy mudflat. Estuarine, Coastal and Shelf Science 22: 91–99.
Campredon, P. & P. Catry, 2016. Bijagos Archipelago (Guinea-Bissau). In Finlayson, C. M., G. R. Milton, C. Prentice & N. C. Davidson (eds.), The Wetland Book. Springer, Amsterdam.
Cardoso, I., J. P. Granadeiro & H. Cabral, 2010. Benthic macroinvertebrates’ vertical distribution in the Tagus estuary (Portugal): the influence of tidal cycle. Estuarine, Coastal and Shelf Science 86: 580–586.
Carpenter, K. E. & N. De Angelis, 2014a. The Living Marine Resources of the Eastern Central Atlantic Volume 1 Introduction, Crustaceans, Chitons, and Cephalopods. Food and Agriculture Organization of the United Nations, Rome.
Carpenter, K. E. & N. De Angelis, 2014b. The Living Marine Resources of the Eastern Central Atlantic Volume 2 Bivalves, Gastropods, Hagfishes, Sharks, Batoid Fishes and Chimaeras. Food and Agriculture Organization of the United Nations, Rome.
Compton, T. J., S. Holthuijsen, A. Koolhaas, A. Dekinga, J. ten Horn, J. Smith, Y. Galama, M. Brugge, D. van der Wal, J. van der Meer, H. W. van der Veer & T. Piersma, 2013. Distinctly variable mudscapes: distribution gradients of intertidal macrofauna across the Dutch Wadden Sea. Journal of Sea Research 82: 103–116.
Conover, W. J. & R. L. Iman, 1981. Rank transformations as a bridge between parametric and nonparametric statistics. American Statistician 35: 124–128.
Cosel, R. & S. Gofas, 2019. Marine bivalves of tropical West Africa. Muséum national d’Histoire naturelle de Paris, Marseille.
Day, J. H., 1967. A Monograph on the Polychaeta of Southern Africa. London Museum of Natural History, London.
Delany, S., D. Scott, T. Dodman & D. Stroud, 2009. An Atlas of Wader Populations in Africa and Western Eurasia. Wetlands International and International Wader Study Group, Wageningen.
El-Hacen, E. H. M., T. J. Bouma, P. Oomen, T. Piersma & H. Olff, 2019. Large-scale ecosystem engineering by flamingos and fiddler crabs on West-African intertidal flats promote joint food availability. Oikos 128: 753–764.
Evans, P. R., R. M. Ward, M. Bone & M. Leakey, 1999. Creation of temperate-climate intertidal mudflats: factors affecting colonization and use by benthic invertebrates and their bird predators. Marine Pollution Bulletin 37: 535–545.
Fauchald, K., 1977. The polychaete worms. Definitions and keys to the orders, families and genera. Science Series 28: 188.
França, S., C. Vinagre, M. A. Pardal & H. N. Cabral, 2009. Spatial and temporal patterns of benthic invertebrates in the Tagus estuary, Portugal: comparison between subtidal and an intertidal mudflat. Scientia Marina 73: 307–318.
Galois, R., G. Blanchard, M. Seguignes, V. Huet & L. Joassard, 2000. Spatial distribution of sediment particulate organic matter on two estuarine intertidal mudflats: a comparison between Marennes-Oleron Bay (France) and the Humber Estuary (UK). Continental Shelf Research 20: 1199–1217.
Goss-Custard, J. D., R. E. Jones & P. E. Newbery, 1977. The ecology of the wash. I. Distribution and diet of wading birds (Charadrii). The Journal of Applied Ecology 14: 681–770.
Harrington, B. A., S. Koch, L. K. Niles & K. Kalasz, 2010. Red knots with different winter destinations: differential use of an autumn stopover area. Waterbirds 33: 357–363.
Herbert, R. J. H., C. J. Davies, K. M. Bowgen, J. Hatton & R. A. Stillman, 2018. The importance of nonnative Pacific oyster reefs as supplementary feeding areas for coastal birds on estuary mudflats. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 1294–1307.
Hoffman, J. A., J. Katz & M. D. Bertness, 1984. Fiddler crab deposit-feeding and meiofaunal abundance in salt marsh habitats. Journal of Experimental Marine Biology and Ecology 82: 161–174.
Iribarne, O. & M. Martinez, 1999. Predation on the southwestern Atlantic Fiddler Crab (Uca uruguayensis) by migratory shorebirds (Pluvialis dominica, P. squatarola, Arenaria interpres, and Numenius phaeopus). Estuaries 22: 47–54.
Iribarne, O., M. Bruschetti, M. Escapa, J. Bava, F. Botto, J. Gutierrez, G. Palomo, K. Delhey, P. Petracci & A. Gagliardini, 2005. Small- and large-scale effect of the SW Atlantic burrowing crab Chasmagnathus granulatus on habitat use by migratory shorebirds. Journal of Experimental Marine Biology and Ecology 315: 87–101.
Katz, L. C., 1980. Effects of burrowing by the fiddler crab, Uca pugnax (Smith). Estuarine and Coastal Marine Science 11: 233–237.
Kelly, J. P., 2001. Hydrographic correlates of winter Dunlin abundance and distribution in a temperate estuary. Journal of the Waterbird Society 24: 309–475.
Kristensen, E., 2008. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. Journal of Sea Research 59: 30–43.
Kristensen, E. & D. M. Alongi, 2006. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnology and Oceanography 51: 1557–1571.
Lourenço, P. M., T. Catry, T. Piersma & J. P. Granadeiro, 2016. Comparative feeding ecology of shorebirds wintering at Banc d’Arguin, Mauritania. Estuaries and Coasts Estuaries and Coasts 39: 855–865.
Lourenço, P. M., T. Catry & J. P. Granadeiro, 2017. Diet and feeding ecology of the wintering shorebird assemblage in the Bijagós archipelago, Guinea-Bissau. Journal of Sea Research Elsevier 128: 52–60.
Lourenço, P. M., J. P. Granadeiro & T. Catry, 2018. Low macroinvertebrate biomass suggests limited food availability for shorebird communities in intertidal areas of the Bijagós archipelago (Guinea-Bissau). Hydrobiologia 816: 197–212.
Martins, R., L. Sampaio, A. M. Rodrigues & V. Quintino, 2013. Soft-bottom Portuguese continental shelf polychaetes: diversity and distribution. Journal of Marine Systems 123–124: 41–54.
Mercier, F. & R. McNeil, 1994. Seasonal variations in intertidal density of invertebrate prey in a tropical lagoon and effects of shorebird predation. Canadian Journal of Zoology 72: 1755–1763.
Mouton, E. C. & D. L. Felder, 1996. Burrow distributions and population estimates for the fiddler crabs Uca spinicarpa and Uca longisignalis in a gulf of Mexico salt marsh. Estuaries 19: 51–61.
Natálio, L. F., J. C. F. Pardo, G. B. O. Machado, M. D. Fortuna, D. G. Gallo & T. M. Costa, 2017. Potential effect of fiddler crabs on organic matter distribution: a combined laboratory and field experimental approach. Estuarine, Coastal and Shelf Science 184: 158–165.
Pearson, T. H. & R. Rosenberg, 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: An Annual Review 16: 229–311.
Piersma, T., 1987. Production by intertidal benthic animals and limits to their predation by shorebirds: a heuristic model. Marine Ecology Progress Series 38: 187–196.
Piersma, T., R. Hoekstra, A. Dekinga, A. Koolhaas, P. Wolf, P. Battley & P. Wiersma, 1993. Scale and intensity of intertidal habitat use by knots Calidris canutus in the Western Wadden Sea in relation to food, friends and foes. Netherlands Journal of Sea Research 31: 331–357.
Piersma, T., Y. Verkuil & I. Tulp, 1994. Resources for long-distance migration of knots Calidris canutus islandica and C. c. canutus: how broad is the temporal exploitation window of benthic prey in the western and eastern Wadden Sea? Oikos 71: 393–407.
Pillay, D., G. M. Branch & A. T. Forbes, 2007a. The influence of bioturbation by the sandprawn Callianassa kraussi on feeding and survival of the bivalve Eumarcia paupercula and the gastropod Nassarius kraussianus. Journal of Experimental Marine Biology and Ecology 344: 1–9.
Pillay, D., G. M. Branch & A. T. Forbes, 2007b. Experimental evidence for the effects of the thalassinidean sandprawn Callianassa kraussi on macrobenthic communities. Marine Biology 152: 611–618.
Raffaelli, D. & S. J. Hawkins, 1999. Intertidal Ecology. Chapman and Hall, London.
Reinsel, K. A., 2004. Impact of fiddler crab foraging and tidal inundation on an intertidal sandflat: season-dependent effects in one tidal cycle. Journal of Experimental Marine Biology and Ecology 313: 1–17.
Ribeiro, P. D., O. O. Iribarne, D. Navarro & L. Jaureguy, 2004. Environmental heterogeneity, spatial segregation of prey, and the utilization of southwest Atlantic mudflats by migratory shorebirds. Ibis 146: 672–682.
Rodrigues, A. M., S. Meireles, T. Pereira, A. Gama & V. Quintino, 2006. Spatial patterns of benthic macroinvertebrates in intertidal areas of a Southern European estuary: the Tagus, Portugal. Hydrobiologia 555: 99–113.
Schneider, D. C. & B. A. Harrington, 1981. Timing of shorebird migration in relation to prey depletion. The Auk 98: 801–811.
Smith, N. F., C. Wilcox & J. M. Lessmann, 2009. Fiddler crab burrowing affects growth and production of the white mangrove (Laguncularia racemosa) in a restored Florida coastal marsh. Marine Biology 156: 2255–2266.
Tamaki, A., 1988. Effects of the bioturbating activity of the ghost shrimp Callianassa japonica Ortmann on migration of a mobile polychaete. Journal of Experimental Marine Biology and Ecology 120: 81–95.
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena, Austria, https://www.r-project.org/.
van der Meer, J., 1991. Exploring macrobenthos-environment relationship by canonical correlation analysis. Journal of Experimental Marine Biology and Ecology 148: 105–120.
van der Zee, E. M., T. van der Heide, S. Donadi, J. S. Eklöf, B. K. Eriksson, H. Olff, H. W. van der Veer & T. Piersma, 2012. Spatially extended habitat modification by intertidal reef-building bivalves has implications for consumer–resource interactions. Ecosystems 15: 664–673.
van der Zee, E. M., C. Angelini, L. L. Govers, M. J. A. Christianen, A. H. Altieri, K. J. van der Reijden, B. R. Silliman, J. van de Koppel, M. van der Geest, J. A. van Gils, H. W. van der Veer, T. Piersma, P. C. de Ruiter, H. Olff & T. van der Heide, 2016. How habitat-modifying organisms structure the food web of two coastal ecosystems. Proceedings of the Royal Society B: Biological Sciences 283: 20152326.
Wallace, J. H., H. M. Kok, L. E. Beckley, B. Bennett, S. J. M. Blaber & A. K. Whitfield, 1984. South African estuaries and their importance to fishes. South African Journal of Science 80: 203–207.
Weerman, E. J., J. De Van Koppel, M. B. Eppinga, F. Montserrat, Q. X. Liu & P. M. J. Herman, 2010. Spatial self-organization on intertidal mudflats through biophysical stress divergence. American Naturalist 176: E15–E32.
Weis, J. S. & P. Weis, 2004. Behavior of four species of fiddler crabs, genus Uca, in southeast Sulawesi, Indonesia. Hydrobiologia 523: 47–58.
Wooldridge, L. J., R. H. Worden, J. Griffiths, J. E. P. Utley & A. Thompson, 2018. The origin of clay-coated sand grains and sediment heterogeneity in tidal flats. Sedimentary Geology 373: 191–209.
Ysebaert, T. & P. M. J. Herman, 2002. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Marine Ecology Progress Series 244: 105–124.
Ysebaert, T., P. Meire, P. M. J. Herman & H. Verbeek, 2002. Macrobenthic species response surfaces along estuarine gradients: prediction by logistic regression. Marine Ecology Progress Series 225: 79–95.
Yu, C., D. Ngoprasert, T. Savini, P. D. Round & G. A. Gale, 2020. Distribution modelling of the endangered spotted Greenshank (Tringa guttifer) in a key area within its winter range. Global Ecology and Conservation 22: e00975.
Zwarts, L., 1985. The winter exploitation of Fiddler Crabs Uca tangeri by waders in Guinea-Bissau. Ardea 73: 3–12.
Acknowledgements
We thank Instituto da Biodiversidade e Áreas Protegidas Dr. Alfredo Simão da Silva (Guinea-Bissau), namely Justino Biai, Aissa Regalla, Samuel Pontes, and IBAP staff, for the permissions and critical logistic support to work on the field. Jorge Gutiérrez, Filipe Moniz, and Edna Correia provided help in different phases of the fieldwork. Two anonymous referees provided valuable comments to an earlier version of this manuscript. This study was funded by MAVA Foundation through project “Waders of the Bijagós: Securing the ecological integrity of the Bijagós archipelago as a key site for waders along the East Atlantic Flyway.” Thanks are due to Fundação para a Ciência e Tecnologia (FCT) and Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) for the financial support to CESAM (UIDP/50017/2020 and UIDB/50017/2020), through national funds, and to project “MigraWebs: Migrants as a seasonal ecological force shaping communities and ecosystem functions in temperate and tropical coastal wetlands” (ref. PTDC/BIA-ECO/28205/2017). TC and MH were also supported by FCT through contract IF/00694/2015 and a doctoral grant (SFRH/BD/131148/2017), respectively.
Author information
Authors and Affiliations
Contributions
JP, TC, and JPG conceived the study and designed methodological protocols. JP, MH, and JB collected the data. JP, TC, and JPG analyzed the data and wrote the manuscript. All authors reviewed and approved the final manuscript. TC and JPG provided funding for the project.
Corresponding author
Additional information
Handling editor: Daniele Nizzoli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paulino, J., Granadeiro, J.P., Henriques, M. et al. Composition and abundance of shorebird and macroinvertebrate communities differ according to densities of burrowing fiddler crabs in tropical intertidal flats. Hydrobiologia 848, 3905–3919 (2021). https://doi.org/10.1007/s10750-021-04601-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04601-1