Abstract
Fishers’ ecological knowledge (FEK) has contributed to a better understanding about the reproduction of fishery resources, especially where biological data are scarce or unavailable. We analyzed FEK on the reproduction of five fishery resources in the Paranaguá estuarine complex and adjacent coastal area, Brazil, including fishes (Genidens barbus, Centropomus parallelus, Chaetodipterus faber, Pseudobatos sp.) and shrimp (Litopenaeus schmitti). We also sought to compare FEK with biological data and investigate which characteristics (age, fish evisceration and fishers’ location) influence fishers' knowledge. We interviewed 132 artisanal fishers. FEK indicated reproductive peaks in the spring and summer for the fish species and in late winter and early spring for the shrimp. The maturation sizes according to FEK did not differ from sizes at first maturity (L50) as reported by biological studies for G. barbus and L. schmitti. Fishers’ residence sites and fish evisceration influenced more their knowledge related to species reproduction than age. Fishers showed a detailed ecological knowledge about some reproductive aspects, including size at first maturity of females, site of occurrence and reproductive peaks. Furthermore, fishers were able to identify sexual dimorphism, parental care and ovoviviparity. These results can be applied to improve fisheries management in the context of data poor fisheries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The local ecological knowledge (LEK) is a form of traditional knowledge that has been studied in recent decades by a multidisciplinary science known as ethnobiology, the study of knowledge developed by human societies regarding biology (Berkes, 1999; Hanazaki, 2015). In turn, studies addressing fishers’ LEK (or FEK) can provide an alternative source of information to fill knowledge gaps on reproductive behavior, abundance, size and seasonal occurrence of fishery resources (Johannes et al., 2000; Batista & Lima, 2010; Zukowski et al., 2011). In recent decades, studies on FEK have contributed to the understanding of several aspects of fish ecology, such as reproduction, migration, feeding interactions, habitat preferences and the formulation of new biological hypotheses that could be investigated with scientific biological data (Aswani & Lauer, 2006; Silvano & Valbo-Jorgensen, 2008; Hamilton et al., 2012; Herbst & Hanazaki, 2014; Nunes et al., 2019). Furthermore, considering the lack of data on broad temporal scales, the knowledge held by older fishers is often the only source of information for understanding environmental changes and temporal trends on abundance, size and catch composition of fishery resources (Saenz-Arroyo et al., 2005; Azzurro et al., 2011; Bender et al., 2013; Tesfamichael et al., 2014; Hallwass et al., 2013, 2020). Worldwide, fishers’ knowledge has provided useful data on spawning aggregations of reef fish in the Pacific (Hamilton et al., 2012), relevant nursey sites for sharks in Mexico (Cuevas-Gómez et al. 2020), use of rivers and estuaries by sharks in Fiji (Rasalato et al., 2010), essential habitats for reproduction (nursery and spawning grounds) of rays in the Portuguese coast (Serra-Pereira et al., 2014), besides use of habitat and reproductive migrations of the sea lamprey in a Portuguese estuary (Braga et al., 2019). Nevertheless, there are fewer studies addressing FEK on the reproduction of estuarine fishes (Le Fur et al., 2011; De Souza Jr. et al., 2020), especially studies that include both fish and invertebrates (Leite & Gasalla, 2013) in the subtropical region of the Southern Brazilian coast.
Artisanal small-scale fishers and their FEK have been neglected by the top-down centralized approach to fisheries management adopted by Brazilian authorities, especially for coastal fisheries, upon which management rules are often imposed to fishers without prior consultation (Begossi, 1998, 2006; Diegues, 1998, 2008). For example, a restrictive marine protected area has been enforced in the southeastern Brazilian coast without consultation nor consideration of fishers’ knowledge, which caused conflicts with nearby coastal communities (Lopes et al., 2013a, b). Similarly, a red list of threatened aquatic species, including fish regularly exploited by fishers, was issued by Brazilian authorities and contested by fishers, also generating conflicts (Di Dario et al., 2015). The fishers in a tropical Brazilian estuary mention the need for a closed fishing season for an important fish species, as there are currently no closed seasons and the abundance of this fish has declined according to fishers’ perceptions (De Souza Jr. et al., 2020). In this sense, the study and applications of FEK to improve management could help to empower fishers and increase their participation in fisheries management and conservation polices (Ruddle & Hickey, 2008; Le Fur et al., 2011; Hamilton et al., 2012; Silvano & Begossi, 2012).
When conducting FEK studies, most researchers interview "experts" or key informants and specifically adopt age or fishing experience as criteria to define and select participants (Davis & Wagner, 2003). Fishers’ age seems plausible due to the accumulation of fishing experience and direct observations of exploited fishery resources and their environment (Saen’z-Arroyo et al., 2005; Silvano et al., 2006; Begossi et al., 2008). However, the age criterion may limit the number of "expert" (older fishers) available to be interviewed in each community, which usually leads to more qualitative based research. Furthermore, sometimes FEK may not differ between younger and older fishers (De Souza Jr. et al., 2020) or younger fishers engaged in more recent fishing methods, such as spear fishing, can also have detailed FEK on exploited fishes (Gerhardinger et al., 2006). Therefore, it would be useful to investigate other factors or fishers’ characteristics, besides fishers’ age, that may affect or influence FEK, for example fishers’ residence sites or the practice of fish evisceration.
Overall, we investigate three key research objectives. First, we analyze and describe FEK about the reproductive aspects of five fishery resources in the Paranaguá estuarine complex and adjacent coastal zone, Southern Brazil, including four fish species [the white catfish—Genidens barbus (Lacépède, 1803); the fat snook—Centropomus parallelus Poey, 1860; the Atlantic spadefish—Chaetodipterus faber Broussonet, 1782 and the guitarfish—Pseudobatos sp.] and the white shrimp [Litopenaeus schmitti (Burkenroad, 1936)]. Through this objective we address the following research question: can FEK provide new information about reproductive aspects, such size at first maturity, seasonality, habitat, dimorphism, and parental care? Second, we compare the information provided by FEK with biological data available in the literature. This comparison aims to highlight complementarities between these two knowledge systems (Johannes et al., 2000; Silvano & Valbo-Jorgensen, 2008) and address this question: what are the differences and similarities between FEK and biological literature regarding reproductive aspects of the studied species? Third, we seek to evaluate which characteristics of fishers are influencing their FEK, by addressing the question: which attributes of fishers, such as location of residence, fish evisceration activity and age, influence FEK about the reproduction of fishery resources?
Materials and methods
Study area and data sampling
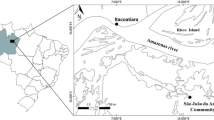
Located in Southern Brazil, the coast of the state of Paraná is 98 km long and has two important estuarine systems: the Paranaguá estuarine complex, located in the north, and Guaratuba Bay, located in the south. This study focuses on fishing communities distributed in the Paranaguá estuarine complex and the adjacent coastal zone (Fig. 1), where the ecosystem includes an extensive area with mangroves and dynamic environments (Lana et al., 2001). The Paranaguá estuarine complex is located under the coordinates 25° 15 'S/48° 45′ W and 25° 35 'S/48° 10 'W, and consists of the Antonina, Paranaguá, Laranjeiras, Guaraqueçaba and Pinheiros bays (SPVS, 1992). This estuarine complex is divided into two main orientation axes: the East–West axis being approximately 56 km long and the North–South axis being about 30 km long (Andriguetto Filho et al., 2006). The estuarine complex is inhabited by approximately 3075 artisanal fishers living in fishing communities, which depend on fishing as the basis of their economies (Mendonça et al., 2017). Most of these communities reside in rural areas and many of them are situated on islands. Local fishers who live in communities in the estuarine complex fish in the estuary and those who inhabit the coastal area fish in the coastal zone. These fishers utilize small boats, which limit fishing to some hours a day, especially in estuarine communities. In the coastal area, fishers may have larger vessels, able to spend a few days at sea, mainly for shrimp fishing (M.U.S.N. direct observation).
We interviewed 132 fishers, who were interviewed along six zones at the estuary and adjacent coastal area, according to the location of the communities to which they belong (participants’ residence sites, Fig. 1). At least three interviewees would be needed to perform quantitative analyses or statistical tests (Davis & Wagner, 2003), so we aimed to obtain a minimum of 15 participants (fishers) from each studied community. We obtained more interviews than this minimum sample size but the number of interviews varied among areas according to population density and the availability of fishers to be interviewed in each zone (Antonina Bay, n = 17, Guaraqueçaba Bay, n = 24, Pinheiros Bay, n = 19, Paranaguá and Laranjeiras Bays, n = 27, Southern Coastal Zone, n = 24 and Northern Coastal Zone, n = 21). Previous visits and consultations with leadership of the associations and fishing communities aided in identifying fishers to be interviewed. During these visits and meetings, we asked for communities’ consent to participate in our study. In these previous visits the researcher conducted his direct observation on fishing and approached the fishers to create a more favorable environment for the interviews and pre-test the questionnaires (Online Resource 1).
We conducted the interviews between January 2017 and February 2018. We utilized the snowball sampling technique in each fishing community, by forming chains of references on which the community leaders and the initial participants indicate potential fishers to be interviewed (Bernard, 1995). To avoid selecting occasional or recreational fishers, we included only artisanal fishers who cited fishing as their main activity for subsistence. We also obtained free and informed consent from each individual fisher we interviewed, respecting the rights of prior consultation of these populations established by the International Labor Organization (ILO) Convention 169 on Indigenous and Tribal Peoples and in accordance with the Law nº 13.123/15 (Brazil, 2015), which establish rights and obligations to access the traditional knowledge on biodiversity. Our research was authorized by the Biodiversity Authorization and Information System (SISBIO), authorization number 56890-1, which includes an assessment on ethics and merit of the project, since there are fishing communities in the studied protected area managed by Chico Mendes of Biodiversity Conservation Institute (ICMBIO), from the Ministry of the Environment, Brazil.
Interviews were conducted with each participant individually and were undertaken by the same researcher (the first author). Interviews were supported by a semi-structured questionnaire with closed and open-ended questions (Silvano et al., 2006) about the socio-economic profile (age, income and local of residence), the fishing activity (species caught and participation in fish evisceration) and FEK about species reproduction. The duration of each interview varied between 20 and 60 min.
The five fishery resources included in this study were observed in the fishing markets of Paranaguá, Antonina and Pontal do Paraná, and are thus considered important resources for the subsistence of these fishing communities. The fishery resources elected are the siluriform Genidens barbus, the perciforms Centropomus parallelus and Chaetodipterus faber, the rajiform Pseudobatos sp. and the crustacean decapoda Litopenaeus schmitti. The taxonomic validation of the information obtained from fishers for each of these species occurred during the interviews through photos of the studied species (Nunes, 2020). The set of photos was shown to each fisher as they were asked to indicate the corresponding local names (Silvano et al., 2006). However, it was not possible to validate the guitar fish Pseudobatos sp. at the species level because most fishers recognized only one species, while two biological species for this genus exist in the studied region (Pseudobatos percellens, Walbaum, 1792 and Pseudobatos horkelii, Müller & Henle, 1841).
The first maturity lengths of females were obtained by using a tape measure, by which fishers indicated the corresponding sizes of the analyzed species, as done in previous studies (Nunes et al., 2019; De Souza Jr. et al., 2020). In this survey, we asked fishers about the body size (cm) at which most individuals were reproductively mature. We focused only on the reproduction of females because the ovaries are larger than the testicles which facilitate the macroscopic visualization and identification made by fishers when eviscerating the fish. Seasonality and habitat of observation of reproductive activity was obtained during the interviews. We also asked about sexual dimorphism, what characteristics fishers use to identify and parental care, which may eventually occur in the analyzed species.
Consulting the already published biological data on species
In the search for biological data on reproduction of the studied species, we used the keywords "fish reproduction", “reproductive biology”, "sexual maturity", and "spawning season" combined with the species names, in the platforms "Web of Science", "Scielo” and “Google Scholar ". The journal articles were selected according to geographic proximity to our study area, to allow the comparison of biological data and our results based on FEK. In the scientific literature, we searched for information on at size of first maturity of the females (L50) to be compared with FEK. According to biological studies, L50 corresponds to the length at which 50% of the females of the population are mature. The same procedure was performed for the analysis of the reproductive period of each studied species.
Data analysis
The sites of observation and/or capture of mature females were categorized in relation to the environments of rivers, bays and coastal zone, and its absolute frequencies of fishers’ responses were converted to percentage frequencies (%) values for graphic representation per fishing communities’ zones. For this graphic representation, we did not consider zones with less than five fishers who responded about reproduction. We defined the reproductive period of the fishery resources according to FEK based on the absolute frequency of fishers' responses (n). Each interviewed indicated a value (cm) corresponding to the L50 of females, generating an average frequency of observations per species. Fishers’ responses about sexual dimorphism and the occurrence of parental care in the fishery resources were analyzed by percentage and absolute frequency.
Considering our second goal (compare FEK and biological data), we used a t-test to compare the average lengths of first maturity of female according to FEK and the L50 obtained from biological studies, for each species analyzed in this study. We also made an overall qualitative comparison between FEK and biological data regarding information on reproductive periods and sites.
To address our third goal (determine the influence of fishers’ characteristics on FEK). We sought to verify if FEK about the reproduction of the fishery resources depends on the zones established in the study area (participants' residence sites). We chose to use the chi-square test of independence (X2) among all studied zones for each species, considering two possibilities of answers (able or not able to give information about reproduction). We have excluded results from areas with less than three participants per species. To test whether the participants' age influenced FEK about reproduction we used a logistic regression analysis, again considering two possible answers (able or not able to give the information about each species). To evaluate whether FEK on the reproductive aspects of each species analyzed depends on participation in the evisceration of fish and shrimp (see Online Resource 2), we used the chi-square test of independence (X2) comparing participants’ responses (able or not able to give the information about reproduction) between fishers that eviscerate and fishers that do not eviscerate fish and shrimp.
The statistical analyses were performed using R software version 3.5.3 (R Development Core Team, 2019), with the vegan packpage. We adopted the significance level of P < 0.05.
Results
FEK on reproduction of fishery resources
Fishers indicated the sites where they observe or capture mature females and reported seasonality the reproduction for all the fishery resources studied. Among those fishers who were able to provide information about reproduction, some were also able to identify sexual dimorphism when present in the species, recognizing external morphological characteristics of male and female (Fig. 2).
The white catfish G. barbus was caught by 95 participants (72%); only six fishers never observed mature females and/or were unable to provide information on reproduction of this species. The fishers indicated the rivers and bays as places of greater occurrence of mature females (Fig. 3a). October, November, and December were cited as having the highest reproductive activity (Fig. 4a). Although most participants (70%; n = 62) did not consider that white catfish G. barbus present sexual dimorphism (or they could not answer), 30% of the fishers (n = 27) indicated the existence of dimorphism in this species (Fig. 2) with the main characteristics being the larger head of the male (29%; n = 26), the larger abdomen of females (9%; n = 8), and the skinny body of the male (7%; n = 6) after the reproductive period. Participants reported the occurrence of parental care, which according to the majority (78%; n = 69), occurs as oral incubation of eggs; some participants (26%; n = 23) indicated that males are responsible for “hatching eggs with its mouth”.
Percentage frequency (%) of responses (y-axis) regarding the observation of mature females per species: a G. barbus, b C. parallelus, c C. faber, d Pseudobatos sp. and e L. Schmitti. The environments indicated by fishers: rivers of the estuarine complex (Rivers), Paranaguá and Laranjeiras bays (PGUA Bay), Guaraqueçaba Bay (GQBA Bay), Antonina Bay (ANT Bay), Pinheiros Bay (PIN Bay) and coastal zone (Coast). The x-axis corresponds to the fishing communities’ zones: Antonina Bay (ANT), Guaraqueçaba Bay (GQBA), Pinheiros Bay (PIN), Paranaguá and Laranjeiras bays (PGUA), Southern coastal zone (SCZ) and Northern coastal zone (NCZ). The total absolute frequency (n) varied for each species: G. barbus (n = 89); C. parallelus (n = 64); C. faber (n = 39); Pseudobatos sp. (n = 34) and L. schmitti (n = 36)
Absolute frequency responses (y-axis) on the reproductive seasonality of the evaluated fishery resources (x-axis): a G. barbus, b C. parallelus, c C. faber, d Pseudobatos sp. e L. schmitti. Gray and black horizontal bars represent the reproductive period and peak, respectively indicated in the literature
The fat snook C. parallelus was caught by 79 fishers (60%) and 64 of these participants (81%) provided information about the reproduction of this species. December was the most cited month for observation of mature females (Fig. 4b), both in the coastal zone as well as in the estuarine complex (Fig. 3b). The occurrence of parental care and sexual dimorphism was not reported for this species.
The Atlantic spadefish C. faber was caught by 59 participants (45%) and 39 of these fishers (66%) were able to indicate information about the reproduction of this species. The Paranaguá and Pinheiros bays were the sites that fishers attributed a higher occurrence of mature females (Fig. 3c). Additionally, higher frequencies of reproductive activity were reported in November, December and January (Fig. 4c). No parental care and sexual dimorphism were reported. Some fishers (26%; n = 10) reported spontaneously that spawning occurs in aggregations close to the surface, when they are easily captured.
The guitarfish Pseudobatos sp. was the least cited fishery resource (32%; n = 42) among the studied species, however, most of these participants (81%; n = 34) were able to indicate information about its reproduction. The coastal zone was the most cited locality where mature females are observed (Fig. 3d), and January and February were reported as the reproductive peak (Fig. 4d). Most fishers (76%; n = 26) recognized differences between males and females. The main characteristics observed by fishers were the male clasper (65%; n = 22), which fishers call pinguela or pistolinhas, and female genitalia (21%; n = 7). Most fishers (79%; n = 27)) associated the species' ovoviviparity with differentiated parental care, stating that "females become pregnant".
The most commonly cited among the studied species was the white shrimp L. schmitti, which was caught by 104 (79%) out of 132 participants. However, only 36 participants (27%) were able to indicate information about the reproduction of this species; the coastal zone being the area with the highest reported frequency of females with mature gonads (Fig. 3e). Regarding the reproductive peak of this species, the highest cited months were September and October (Fig. 4e). Fishers also noted sexual dimorphism in the white shrimp, with the male petasma being the main characteristic referenced (75%; n = 27). The male petasma, which fishers call perninhas or patinhas, refers to the genital structure in male penaeidean shrimps. Most fishers (56%; n = 22) who were able to discuss reproductive aspects did not report parental care for this species. However, 14 participants (38%) indicated the storage of eggs in cephalothoraxes and dorsal parts of females, as a differentiated reproductive aspect of this species.
Comparison between FEK and biological data
While the average lengths of the first maturity of females according to FEK did not differ significantly from the L50 indicated by the biological studies for G. barbus and L. schmitti, differences are apparent for C. faber, C. paralellus and Pseudobatos sp. (Table 1). Overall, the highest cited months for reproduction as indicated by FEK agreed with reproductive seasonality reported by the biological studies for four of the studied species (Fig. 4a, b, c, d), with the exception of L. schmitti (Fig. 4e). The reproductive peak of L. schmitti according to biological knowledge occurs in October and November, while according to FEK it occurs in September and October (Fig. 4e).
Fishers’ characteristics and FEK
FEK about the reproduction varied according to fishing communities’ locations. Fishers’ knowledge about G. barbus and C. parellelus presented detailed reproductive aspects in all locations. However, fishers from communities located in the upper estuary (Antonina and Guaraqueçaba bays) reported less information about the reproduction of C. faber, L. schmitti, and Pseudobatos sp. Therefore, FEK regarding the studied species depended on the location of fishing communities (Table 2).
The average age of participants in this study was 53.3 years old (σ = 15.3 years) and fishers ranged between 20 and 84 years old. The average fishing experience of participants was 41.88 years (σ = 15.18), and the mean age when fishers started fishing was 11.29 years old (σ = 4.14). According to the logistic regression analysis, age was not a predictor of FEK about the reproduction of the studied species (plots in Online Resource 3): G. barbus (β1 = 0.006; SE = 0.029; Z = 0.235; P = 0.815), C. faber (β1 = − 0.001; SE = 0.020; Z = − 0.057; P = 0.955), Pseudobatos sp. (β1 = 0.019; SE = 0.027; Z = 0.707; P = 0.479), L. schmitti (β1 = 0.019; SE = 0.013; Z = 1.407; P = 0.159). The exception was C. parallelus, as results demonstrated a positive relationship between fishers age and FEK on reproduction (β1 = 0.062; SE = 0.022; Z = 2.739; P = 0.006).
Our results also revealed that the practice of fish evisceration, performed by most of the participants (75%), was positively correlated with FEK on the reproduction of G. barbus (X2 = 21.975; df = 1, P < 0.001), Pseudobatos sp. (X2 = 14.947, df = 1, P < 0.001), and L. schmitti (X2 = 9.75, df = 1, P = 0.001). However, no relationship was found for C. paralellus (X2 = 3.119; df = 1, P = 0.077) and C. faber (X2 = 3.006, df = 1, P = 0.082). It was observed that many women participated in the fish evisceration activity, but few women were indicated as fishers by communities, which is reflected in the small number of women interviewed (6%; n = 8).
Discussion
This study on FEK generated new information about the reproductive biology of the studied species in the Paranaguá estuarine complex and adjacent coastal zone, especially for G. barbus and C. parallelus. We could not find other studies in the scientific literature about the reproduction of G. barbus for our study area. The interviewed fishers indicated that the estuarine zone, including rivers and bays, were the sites with the greatest occurrence of reproductive activity of this species. Similar findings were observed in biological studies in the Patos Lagoon estuary, which is located about 880 km southward from the study area (Reis, 1986; Araujo, 1988). The rivers and bays of the Paranaguá estuarine complex were mentioned by fishers as sites with a higher frequency of mature females of C. parallelus. Fishers reported a higher frequency of mature females mainly in late spring and early summer. A similar pattern was observed by Nogueira (2009) in the Guaratuba Bay, 50 km southward from our study area.
Most of the information from FEK agreed with biological data. The main period of reproductive activity for C. faber according to FEK occurs between November and January, which is corroborated by biological data (Soeth et al., 2018). However, FEK and biological data indicated that C. faber aggregate seasonally to facilitate spawning, which could increase the susceptibility of this species to overexploitation (Sadovy de Mitcheson et al., 2008; Soeth et al., 2018). FEK indicated that Pseudobatos sp. reproduces in the coastal areas, especially during the summer, which agrees with results from biological sampling (Rocha, 2010; Martins et al., 2018). Furthermore, fishers were able to identify sexual dimorphism in this ray, which could be useful to develop management initiatives aiming to prevent the capture of females during the summer. FEK on reproduction of L. schmitt generated similar findings as biological data for estuaries in other regions of the southeastern Brazilian coast, such as São Paulo (Santos et al., 2008) and Santa Catarina (Machado et al., 2009). Biological studies about the reproduction of L. schmitt demonstrate that it occurs mainly in the coastal zone, while estuaries are used as nursery grounds (Santos et al., 2008). The fact that this species grows in estuarine zones and reproduces in the coastal zone influenced the observed FEK about the reproduction of this species, as reproduction may be more often observed by those participants who target shrimp in coastal areas.
In this study, FEK on length of fist maturity of females agreed with the length at first maturity (L50) from biological studies for two species (G. barbus, L. schmitti), while average sizes based on FEK were larger than L50 for two species (C. parallelus, C. faber) and smaller for one species (Pseudobatos sp.). In another study exploring FEK on fish reproduction in a floodplain lake in the Brazilian Amazon, Serrão et al. (2019) observed similarities between FEK and biological studies regarding fish reproductive sizes for 8 of the 10 studied fish species (or groups of species). However, while Serrão et al. (2019) asked fishers about the average reproductive size, in this study we asked about the size of first maturity (cm) for most specimens. Previous research conducted in the estuary and coast of the Brazilian Amazon (De Souza Jr. et al., 2020) also concluded that minimum reproductive sizes according to FEK differ and are larger than the L50 from biological studies for the commercial fish pescada-amarela (Cynoscion acoupa). A possible reason for this difference is that fishers may target larger fish, so even minimum sizes tend to be larger, besides the fact that the empirical basis to establish L50 by biological studies (half of the spawning population) differ from FEK, which is based on accumulated experiences of individual fishers (De Souza Jr. et al., 2020). Compared to biological data (Nogueira, 2009) in our study, fishers observed a larger size of the first maturity of females of C. parallelus. Nevertheless, Rodrigues (2005) found a value of L50 (28 cm) for this species located at the mouth of the Doce river in Southeastern Brazil, which is close to the value indicated by the participants of this study (28.9 cm). The lack of long term data on reproductive biology of Brazilian estuarine fishes makes it difficult to conduct more realistic comparisons, as a time lag of 10 years between our study on FEK and biological sampling (Nogueira, 2009) could be sufficient to allow for changes in females' maturation sizes (Chuwen et al., 2011). We also noted differences for C. faber. However, Soeth et al. (2018) determined L50 values during the development of ovaries (in the earlier phase of gonadal development) through microscopic analysis, while fishers observed C. faber females at an advanced maturation stage, near the point of spawning when gonads are more visible. Therefore, these different empirical approaches to assess spawning individuals could explain the observed differences between FEK and biological data. The observed difference for females of Pseudobatos sp. could be related to the fact that FEK was based on two biological species combined, while biological studies are always species specific. The size of L50 adopted in this study is for P. percellens (Rocha, 2010), the most abundant species in the study area (Possatto et al., 2017) and is probably the most frequently caught species by fishers.
The determination of the first maturity lengths according to FEK is an estimate, obtained through the daily observations of fishers based on their acquired knowledge, which may be connected to their cultural heritage. Therefore, fishers’ ability to indicate fish sizes, such as reproductive sizes, or maximum and minimum catch sizes (Nunes et al., 2019, De Souza Jr. et al., 2020) may constitute important source of knowledge that can help to fill information gaps, generate hypotheses and support the understanding of the ecology of fishery resources (Johannes et al., 2000; Silvano & Valbo-Jorgensen, 2008). For example, the observed FEK on size of mature females of fish and shrimp indicated that fishers are able to understand, discuss or negotiate management initiatives aimed to protect juveniles (minimum size) or prevent the capture of larger and older females, which have higher reproductive potential (Birkeland & Dayton, 2005; Hixon et al., 2013). Furthermore, this method can be improved through participatory monitoring, with fishers recording measurements when they eviscerate fish, which could improve estimates of L50 (Schemmel et al. 2016; Silvano & Hallwass 2020).
We showed that some characteristics of the participants, such as residence sites and the practice of fish evisceration, were correlated to FEK for most of the studied species. The residence sites of the artisanal fishers influenced their FEK about the reproduction of most fishery resources. FEK could be correlated to the different reproductive strategies and the life cycle of each species, which may occur in specific areas. For some species, such as the guitarfish, few fishers captured and mentioned this resource in the upper reaches of the estuary, which may be due to the low abundance of this species in this zone, where the influence of freshwater is greater, thus reflecting the preference of this species for marine environments (Rocha, 2010; Martins et al., 2018). However, for the white catfish, the observation of females with mature gonads was reported by many participants in all analyzed zones, which may indicate that this species is spread over the region or travels throughout all zones during its reproduction. Our results showed that age was less important than other attributes for most of the analyzed species. This result differs from other studies where fishers’ age was deemed important and adopted as a criterion for the selection of participants (Silvano et al., 2006; Hallwass et al., 2020). Moreover, in the Amazonian coast some studies have shown that older and younger fishers can provide similar information about the biology of the fish Cynoscion acoupa (De Souza Jr. et al., 2020), or the migration and sizes of fish caught in the Tapajos River, Brazilian Amazon (Nunes et al., 2019). However, when considering other aspects of knowledge, such as changes in species composition, variation in abundances of fishery resources, or environmental changes over time, participants’ age is an important factor, as older fishers could be the only source of temporal information (Saenz Arroyo et al., 2005; Azzurro et al., 2011; Tesfamichael et al., 2014; Hallwass et al., 2013, 2020). We should also consider that the snowball sampling method used for selecting participants in this study tends to favor more experienced fishers, as experienced fishers are more likely to be recognized and identified by the community. Perhaps if we sampled a larger number of fishers less than 20 years old or novice fishers, age and fishing experience could be a more relevant factor in our analysis. However, in our sampling, younger fishers were also able to provide relevant information related to fish reproduction. The inclusion of younger fishers increases the number of participants, strengthening quantitative approaches. Thus, younger fishers should also be included in similar studies on FEK, including those that adopt a snowball sampling method. FEK on the reproduction of the analyzed species was positively correlated to the practice of evisceration of fish and shrimp, except for C. faber. Fish evisceration allows for the observation of the gonads, especially for the females, which usually reach higher gonadal volumes in comparison with males (Fávaro et al., 2005; Bryan et al., 2007). We thus recommend that future studies on reproduction consider fish and shrimp evisceration as a possible criterion for participants’ selection. Women participation in the fish and shrimp evisceration is common (M.U.S.N. personal observation), although we experienced a greater difficulty in obtaining interviews with women. Although women participated in fishing activities repairing fish gears and evisceration, they are not always recognized as fishers by the communities and fisher's entities. Thus, future studies may intensify the search for fisherwomen and see if there is a gender difference in FEK on reproduction of fishery resources.
Conclusion
We demonstrated that the fishers have detailed ecological knowledge about the reproductive aspects of fishery resources of different taxonomic groups, including first maturity lengths of females, site of occurrence and reproductive peaks. Furthermore, fishers were able to identify sexual dimorphism, parental care and ovoviviparity. These results were presented in the fishing communities in the form of meetings, generating debate on the topic. Furthermore, fisheries managers were also contacted about this study and its results. Overall, for most of the analyzed species, FEK was positively correlated to fish evisceration and was influenced by participants’ residence site, but not by fishers’ age. A limitation to be considered in this approach is the identification of species by fishers, which does not always correspond to taxonomic classifications adopted in biological studies. In conclusion, FEK, when analyzed following a qualitative and quantitative approach, can provide relevant information to conservation and management initiatives.
FEK can contribute to achieve sustainable fisheries on two main ways. First, by providing scarce or unavailable data on biology and ecology of fishing resources, which have the potential to improve the management of fishery resources by supporting strategies to protect the species reproduction. Second, by informing managers, researchers, and decision makers about the aquatic environment and exploited organisms, which can help to minimize conflicts and build consensus towards sound fisheries management. We thus recommend that FEK must be considered in the formulation and implementation of fisheries management policies, such as establishing closed fishing periods, minimum or maximum catch sizes and marine protected areas.
References
Andriguetto Filho, J. M., P. T. C. Chaves, C. Santos & S. A. Liberati, 2006. Diagnóstico da Pesca no estado do Paraná. In Pesca marinha e estuarina do Brasil no início do século XXI: Recursos, tecnologias, aspectos socioeconômicos e institucionais. Editora Universitária UFPA, Belém do Pará.
Araújo, F. G., 1988. Distribuição, abundância relativa e movimentos sazonais de bagres marinhos (Siluriformes, Ariidae) no estuário da Lagoa dos Patos (RS), Brasil. Revista Brasileira de Zoologia 5: 509-543.
Aswani, S. & M. Lauer, 2006. Benthic mapping using local aerial photo interpretation and resident taxa inventories for designing marine protected areas. Environmental Conservation 33: 263–273.
Azzurro, E., P. Moschella & F. Maynou, 2011. Tracking signals of change in Mediterranean fish diversity based on local ecological knowledge. PLoS One 6: e24885.
Batista, V. S. & L. G. Lima, 2010. In search of traditional bio-ecological knowledge useful for fisheries co-management: the case of jaraquis Semaprochilodus spp.(Characiformes, Prochilodontidae) in Central Amazon, Brazil. Journal of Ethnobiology and Ethnomedicine 6(1): 15.
Begossi, A., 1998. Cultural and ecological resilience among Caiçaras of the Atlantic Forest and caboclos of the Amazon, Brazil. In: Folke, C. & F. Berkes (Eds). Linking social and cultural systems for resilience. Cambridge, Cambridge University Press, pp. 129-157.
Begossi, A., 2006. Temporal stability in fishing spots: conservation and co-management in Brazilian artisanal coastal fisheries. Ecology & Society 11: 5.
Begossi, A. & R. A. M. Silvano, 2008. Ecology and ethnoecology of dusky grouper garoupa, Epinephelus marginatus (Lowe, 1834) along the coast of Brazil. Journal of Ethnobiology and Ethnomedicine 4: 20.
Bender, M. G., S. R. Floeter & N. Hanazaki, 2013. Do traditional fishers recognise reef fish species declines? Shifting environmental baselines in Eastern Brazil. Fisheries Management and Ecology 20(1): 58-67.
Berkes, F., 1999. Sacred ecology: traditional ecological knowledge and resource management. Taylor & Francis, Philadelphia 209 p.
Bernard, H. R., 1995. Research Methods in Anthropology—Qualitative and Quantitative Approaches. 2ªed. United States of America, Altamira Press.
Birkeland, C. & P. K. Dayton, 2005. The importance in fishery management of leaving the big ones. Trends in Ecology & Evolution 20(7): 356-358.
Braga, H.O., M.J. Pereira, F. Morgado, A.M.V.M. Soares, and U.M. Azeiteiro (2019) Ethnozoological Knowledge of Traditional Fishing Villages about the Anadromous Sea Lamprey (Petromyzon Marinus) in the Minho River, Portugal. Journal of Ethnobiology and Ethnomedicine. https://doi.org/10.1186/s13002-019-0345-9.
Bryan, B. J., M. L. Wildhaber, D. M. Papoulias, A. J. DeLonay, D. E. Tillitt & M. L. Annis, 2007. Estimation of gonad volume, fecundity, and reproductive stage of shovelnose sturgeon using sonography and endoscopy with application to the endangered pallid sturgeon. Journal of Applied Ichthyology 23(4): 411-419.
Chuwen, B. M., I. C. Potter, N. G. Hall, S. D. Hoeksema & L. J. B. Laurenson, 2011. Changes in catch rates and length and age at maturity, but not growth, of an estuarine plotosid (Cnidoglanis macrocephalus) after heavy fishing. Fishery Bulletin 109: 247–260.
Cuevas-Gómez, G. A., J. C., Pérez-Jiménez, I. Méndez-Loeza, M. Carrera-Fernández,J. L. Castillo-Géniz, 2020. Identification of a nursery area for the critically endangered hammerhead shark (Sphyrna lewini) amid intense fisheries in the southern Gulf of Mexico. Journal of Fish Biology. https://doi.org/10.1111/jfb.14471
Davis, A., & J. R. Wagner, 2003. Who knows? On the importance of identifying “experts” when researching local ecological knowledge. Human ecology 31(3): 463-489.
Di Dario, F., C. Alves, H. Boos, F. L. Frédou, R. Lessa, M. M. Mincarone, M. A. A. Pinheiro, C. N. M. Polaz, R. E. Reis, L. A. Rocha, 2015. A Better Way Forward for Brazil’s Fisheries. Science 1079
Diegues, A. C., 1998. Environmental impact assessment: the point of view of artisanal fishermen communities in Brazil. Ocean & Coastal Management 39: 119-133.
Diegues A. C., 2008. Marine protected areas and artisanal fisheries in Brazil. In Samudra monograph. International Collective in Support of Fishworkers.
Fávaro, L. F., F. D. A. Frehse, R. N. D. Oliveira & R. Schwarz Júnior, 2005. Reproduction of the Madamango sea catfish, Cathorops spixii (Agassiz) (Siluriformes, Ariidae), of the Pinheiros Bay, estuarine coastal area of Paraná, Brazil. Revista Brasileira de Zoologia 22: 1022-1029.
Gerhardinger, L. C., R. C. Marenzi, A. A. Bertoncini, R. P. Medeiros & M. Hostim-Silva, 2006. Local ecological knowledge on the goliath grouper Epinephelus itajara (Teleostei: Serranidae) in southern Brazil. Neotropical Ichthyology 4(4): 441-450.
Hallwass, G., P. F. Lopes, A. A. Juras & R. A. M. Silvano, 2013. Fishers' knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecological Applications 23(2): 392-407.
Hallwass, G., A. Schiavetti & R. A. M. Silvano, 2020. Fishers’ knowledge indicates temporal changes in composition and abundance of fishing resources in Amazon protected areas. Animal Conservation 23: 36–47.
Hamilton, R. J., M. Giningele, S. Aswani & J. L. Ecochard, 2012. Fishing in the dark-local knowledge, night spearfishing and spawning aggregations in the Western Solomon Islands. Biological Conservation 145: 246–257.
Hanazaki, N., 2015. Why are we so attached to the “ethno” prefix in Brazil? Scientometrics 103(2), 545-554.
Herbst, D. F & N. Hanazaki, 2014. Local ecological knowledge of fishers about the life cycle and temporal patterns in the migration of mullet (Mugil liza) in Southern Brazil. Neotropical Ichthyology 12: 879-890.
Hixon, M. A., D. W. Johnson & S. M. Sogard, 2013. BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES Journal of Marine Science 71(8): 2171-2185.
Johannes, R. E., M. M. Freeman & R. J. Hamilton, 2000. Ignore fishers’ knowledge and miss the boat. Fish and Fisheries 1: 257-271.
De Souza Jr., O. G., J. L. G. Nunes, & R. A. M. Silvano, 2020. Biology, ecology and behavior of the acoupa weakfish Cynoscion acoupa (Lacepède, 1801) according to the local knowledge of fishermen in the northern coast of Brazil. Marine Policy 115: 103870.
Lana, P. C., E. Marone, R. M. Lopes & E. D. C. Machado, 2001. The subtropical estuarine complex of Paranaguá Bay, Brazil. In Coastal marine ecosystems of Latin America. Springer, Berlin, Heidelberg: 131-145.
Le fur, J., A. Guilavogui & A. Teitelbaum, 2011. Contribution of local fishermen to improving knowledge of the marine ecosystem and resources in the Republic of Guinea, West Africa. Canadian Journal of Fisheries and Aquatic Sciences 68:1454-1469.
Leite, M. C. F., M. A., Gasalla, 2013. A method for assessing fishers’ ecological knowledge as a practical tool for ecosystem-based fisheries management: seeking consensus in Southeastern Brazil. Fisheries Research 145: 43–53.
Lopes, P. F. M., E. M. Rosa, S. Salyvonchyk, V. Nora, A. Begossi, 2013a. Suggestions for Fixing Top-down Coastal Fisheries Management through Participatory Approaches. Marine Policy 40 (1): 100–110.
Lopes, P. F. M., R. A. M. Silvano, V. A. Nora, A. Begossi, 2013b. Transboundary Socio-Ecological Effects of a Marine Protected Area in the Southwest Atlantic. AMBIO 42(8): 963–74.
Machado, I. F., L. F. C. Dumont & F. D'Incao, 2009. Stages of gonadal development and mean length at first maturity of wild females of white shrimp (Litopenaeus schmitti - Decapoda, Penaeidae) in southern Brazil. Atlântica 31: 169-175.
Martins, M. F., A. F. Pasquino, & O. B. F. Gadig, 2018. Reproductive biology of the Brazilian guitarfish, Pseudobatos horkelii (Müller & Henle, 1841) from southeastern Brazil, western South Atlantic. Journal of Applied Ichthyology 34: 646-652.
Mendonça, J. T., A. C. M. Lucena, L. D. Muehlmann & R. P. Medeiros, 2017. Fisheries Socioeconomics on the Coast of the State of Paraná (Brazil) for the Period from 2005 to 2015. Desenvolvimento e Meio Ambiente 41: 140-157.
Nogueira, A. B., 2009. Biologia de Centropomus parallelus Poey, 1860 no sistema Baía de Guaratuba, Paraná, Brasil. Dissertação de Mestrado, Universidade Federal do Paraná.
Nunes, M. U. S., 2020. The fishers’ ecological knowledge on the reproduction and migration of fishing resources in a subtropical coastal ecosystem of the Southern Atlantic. PhD thesis, Universidade Federal do Paraná.
Nunes, M. U. S., G. Hallwass, & R. A. M. Silvano, 2019. Fishers’ local ecological knowledge indicate migration patterns of tropical freshwater fish in an Amazonian river. Hydrobiologia 833: 197-215.
Possatto, F. E., M. K. Broadhurst, C. A. Gray, H. L. Spach & M. R. Lamour, 2017. Spatiotemporal variation among demersal ichthyofauna in a subtropical estuary bordering World Heritage-listed and marine protected areas: implications for resource management. Marine and Freshwater Research 68: 703-717.
Rasalato, E., V. Maginnity, J. M. Brunnschweiler, 2010. Using Local Ecological Knowledge to Identify Shark River Habitats in Fiji (South Pacific). Environmental Conservation 37 (1): 90–97.
R Development Core Team, 2019. R: A language and environment for statistical computing. R Foundation for statistical computing. Viena, Austria. http://www.R-project.org. Accessed 01 Feb 2019.
Reis, E. G., 1986. Reproduction and feeding habits of the marine catfish Netuma barba (Siluriformes, Ariidae) in the estuary of Lagoa dos Patos, Brazil. Atlântica 8: 35-55.
Rocha, F., 2010. Biologia reprodutiva da raia-viola Rhinobatos percellens Walbaum, 1792 (Chondrichthyes, Rhinobatidae), da plataforma continental de São Paulo. Dissertação de Mestrado, Universidade Estadual Paulista.
Rodrigues P. P., 2005. Aspectos reprodutivos do robalo peba, Centropomus parallelus, na foz do rio doce, Linhares/ES. Monografia, Universidade Federal do Espírito Santo.
Ruddle, K. & F. R. Hickey, 2008. Accounting for the mismanagement of tropical nearshore fisheries. Environment Development and Sustainability 10: 565-589.
Sadovy de Mitcheson, Y., A. Cornish, M. Domeier, P. L. Colin, M. Russell & K. C. Lindeman, 2008 A global baseline for spawning aggregations of reef fishes. Conservation Biology 22: 1233–1244.
Saenz-Arroyo, A., C. Roberts, J. Torre, M. Cariño-Olvera & R. Enríquez-Andrade, 2005. Rapidly shifting environmental baselines among fishers of the Gulf of California. Proceedings of the Royal Society B: Biological Sciences 272(1575): 1957-1962.
Santos, J. L., E. Severino-Rodrigues, & M. André, 2008. Estrutura populacional do camarão-branco Litopenaeus schmitti nas regiões estuarina e marinha da Baixada Santista, São Paulo, Brasil. Boletim do Instituto de Pesca 34: 375-389.
Schemmel, E., A. M. Friedlander, P. Andrade, K. Keakealani, L. M. Castro, C. Wiggins, B. A. Wilcox, Y. Yasutake, J. N. Kittinger (2016). The codevelopment of coastal fisheries monitoring methods to support local management. Ecology and Society 21(4).
Serrão, E. M., T. M. P. Braga, Y. K. S. Côelho, D. P. F. Campos, A. A. Santos, L. C. Imbiriba & D. M. Zacardi, 2019. Traditional knowledge of fishermen of the reproductive behavior of fish in a flood lake in western Pará, Brazil. Sociedade e Natureza 31: 1-21.
Serra-Pereira, B. K. Erzini, C. Maia, I. Figueiredo, 2014. Identification of potential essential fish habitats for skates based on fishers’ knowledge. Environmental Management 53(5): 985–98.
Silvano, R. A. M. & A. Begossi, 2012. Fishermen’s local ecological knowledge on Southeastern Brazilian coastal fishes: contributions to research, conservation, and management. Neotropical Ichthyology 10: 133–147.
Silvano, R. A. M., & G. Hallwass, 2020. Participatory research with fishers to improve knowledge on small-scale fisheries in tropical rivers. Sustainability 12(11): 4487.
Silvano, R. A. M., P. F. L. MacCord & A. Begossi, 2006. When does this fish spawn? Fishermen’s local knowledge of migration and reproduction of Brazilian coastal fishes. Environmental Biology of Fishes 76: 371-381.
Silvano, R. A. M. & J. Valbo-Jorgensen, 2008. Beyond fishermen’s tales: contributions of fishers’ local ecological knowledge to fish ecology and fisheries management. Environment, Development and Sustainability 10: 657–675.
Soeth, M., L. F. Fávaro, H. L. Spach, F. A. Daros, A. E. Woltrich & A. T. Correia, 2018. Age, growth, and reproductive biology of the Atlantic spadefish Chaetodipterus faber in southern Brazil. Ichthyological Research 66: 140-154.
SPVS (Sociedade de Pesquisa em Vida Selvagem), 1992. Plano Integrado de Conservação para a região de Guaraqueçaba, Paraná, Brasil. Curitiba, Ícone computação gráfica Ltda
Tesfamichael, D., T. J. Pitcher, & D. Pauly, 2014. Assessing changes in fisheries using fishers’ knowledge to generate long time series of catch rates: a case study from the Red Sea. Ecology and Society 19: 18.
Zukowski, S., A. Curtis, & R. J. Watts, 2011. Using fisher local ecological knowledge to improve management: the Murray crayfish in Australia. Fisheries Research 110(1): 120–127.
Acknowledgements
First, we must thank all fishers who contributed to this study! We are thankful for the financial support and grants for M.U.S.N., O.R.C., and M.S., by the Higher Education Personnel Training Coordination (CAPES), for the Post-graduate degree at Federal University of Paraná. One author (R.A.M.S.) also received research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, grant 303393/2019-0) and from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES-PRINT, grant 88887.467553/2019-00). And finally, thank you to colleagues and professors from the Post-graduate Program in Ecology and Conservation at the Federal University of Paraná and Sydney Stenekes, a graduate student from the University of Alberta for reviewing and providing feedback on the previous manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nunes, M.U.S., Cardoso, O.R., Soeth, M. et al. Fishers’ ecological knowledge on the reproduction of fish and shrimp in a subtropical coastal ecosystem. Hydrobiologia 848, 929–942 (2021). https://doi.org/10.1007/s10750-020-04503-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04503-8