Abstract
The diversity of aquatic macrophytes can offer different local conditions required to support an increased number of microhabitats, therefore resulting in diverse biotic communities. This study assessed the influence of macrophyte diversity on the ecological attributes of the associated invertebrates (diversity, richness and abundance), as well as the diet composition of a small fish, Moenkhausia forestii, that inhabits the Upper Paraná River floodplain in abundance, and present great contributions of invertebrates in its diet. The richness and diversity of invertebrates increased with increasing macrophyte diversity, while the abundance of invertebrates didn’t show a significant relationship. The diet of M. forestii differed among stands and the consumption of invertebrates increased with increasing macrophyte diversity, while the consumption of aquatic plants decreased. Moreover, the trophic niche breadth of M. forestii expanded, a probable result of the increase in the ecological opportunity promoted by higher macrophyte diversity. Our study emphasizes the importance of the diversity of these plants as they structure the environment and the associated communities, and changes in the attributes of these plants could be reflected on other trophic levels and even on a local scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrophytes comprise a diverse mosaic of organisms that colonize aquatic and transitional habitats (Thomaz & Cunha, 2010). These plants can influence the structure and functioning of systems through their role in biomass production (Schneider et al., 2018), ecological processes, and nutrient storage (Rejmankova, 2011; Tang et al., 2017). In fact, macrophytes are considered to be key components in aquatic environments, especially due to their positive effects on abundance and richness of taxa, such as fish and invertebrates (Petry et al., 2003; Rennie & Jackson, 2005; Cremona et al., 2008; Gallardo et al., 2017). They are also recognized for their production of substrate for foraging (Jeffries, 1993; Taniguchi et al., 2003; Warfe & Barmuta, 2004) and for decreasing predation pressures by minimizing the encounters between predator and prey (Fisher et al., 2012; Farina et al., 2014). In addition, they can enhance reproductive dynamics of organisms due to the availability of food and shelter resources (Sánchez-Botero & Araújo-lima, 2001; Sánchez-Botero et al., 2008).
The diversity of these plants translates into different architecture, forms of growth and physiology, increasing the number of microhabitats. With a variety of local conditions, including microclimate, stream flow, food source and shelter against predators, there is increased support for diverse biotic communities to thrive (Petry et al., 2003; Habib & Yousuf, 2015). Different morphologies, such as those found in submerged forms, have complex architectures that offer a wide availability of resources. They have a large surface area for colonization of periphyton, affecting the diversity, biomass, richness, and abundance of other associated communities, such as invertebrates (Strayer & Malcom, 2007; Hansen et al., 2011). Likewise, submerged portions of floating macrophytes present complex systems of roots and rhizomes that provide habitat for so many other organisms (Ohtaka et al., 2011; Tóth et al., 2012).
Thus, a change in the diversity of these plants can modify the composition and biomass of invertebrates and indirectly modify biological relationships between species (Warfe & Barmuta, 2004; Cremona et al., 2008; Thomaz & Cunha, 2010; Ávila et al., 2011). Invertebrate communities associated with macrophytes have different life forms with different requirements (McAbendroth et al., 2005) and can perceive and use their habitat in unique ways (Stahr & Kaemingk, 2017). Factors such as the release of biochemicals by macrophytes can attract, repel or be lethal to invertebrates (Habib & Yousuf, 2015). Invertebrates can also be attracted to the palatability and nutritional content of plants, which are determined according to their chemical and physical characteristics (Jiménez-Ramos et al., 2018).
Macrophytes and fish are primarily linked by trophic interactions that involve invertebrate communities (Schultz & Dibble, 2012). Small fish whose life cycle depends on these plants exhibit specific foraging behaviors, such as high exploitation of interstices among the roots of these plants. The success of foraging depends on the availability of the prey and the predator–prey interaction (Priyadarshana et al., 2001). Due to this relationship, changes in invertebrate communities can lead to changes in fish diet, leading to the expansion or contraction of the populations' diet (Svanbäck & Bolnick, 2007; Moreno-Rueda et al., 2018; Zhang et al., 2019), since low abundance and diversity of food can modify foraging patterns and a narrowing of diet breadth (Prejs & Prejs, 1987; Layman et al., 2007). For example, monospecific macrophyte stands may have a reduced environmental heterogeneity, and they may alter the food resources for fish, both qualitatively and quantitatively (Theel et al., 2008).
Considering that the diversity of macrophytes can affect associated communities, this study hypothesized that the diversity of macrophytes influences the attributes of the invertebrate community and the diet of Moenkhausia forestii Benine, Mariguela & Oliveira, 2009, a small characid fish that inhabits the Upper Paraná River floodplain in abundance, with invertebrates constituting a part of its diet. The following predictions were tested: (i) the highest values of diversity, richness and abundance of invertebrates will be found in higher macrophyte diversity; (ii) the fish diet differs between stands of macrophytes with different levels of diversity and (iii) the trophic niche breadth will be greater in stands with a greater diversity of macrophytes. These predictions were based on the fact that macrophyte stands with greater diversity will support more diverse communities of invertebrates, therefore offering a variety of resources, changing diets, and expanding niche breadth for fish (Pelicice & Agostinho, 2006; Kliemann et al., 2019). In order to test these predictions, we assessed the attributes of the invertebrate community and the Moenkhausia forestii diet in stands with different macrophyte diversity.
Materials and methods
Study area
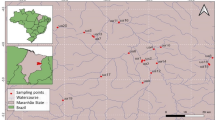
Our study was carried out in the Upper Paraná River floodplain, a region located in the upper region of the Environmental Protection Area of the islands and várzeas of the Paraná River, which is the last dam-free stretch of the Paraná River in Brazil. We conducted the sampling in the Baía River (22° 43′23″ S, 53°17′25″ W—Fig. 1), which presents a variable width and a margin covered mainly by herbaceous grazing vegetation (Lopes et al., 2006). The sampled region presents an expressive littoral zone with nearly an entire perimeter of aquatic macrophytes stands, and is home to a great richness of macrophyte species (e.g. Paspalum repens Berg., Polygonum stelligerum Cham., Polygonum ferrugineum Wedd., Hydrocotyle ranunculoides L.f., Eichhornia azurea (Swartz) Kunth, Eichhornia crassipes (Mart.) Solms, Pistia stratiotes L., (Thomaz & Cunha, 2010).
Sampling sites in the Baía River (river-floodplain system of the Upper Paraná River, Brazil) EPSG: 4618. White circles represent low diversity sampling sites (L1, L2, L3), gray circles represent medium diversity sampling sites (L4, L5, L6) and black circles represent high diversity sampling sites (L7, L8, L9)
Sampling
Our sampling was performed in August 2018 (drought period), and included fish, invertebrates and macrophytes. We sampled nine macrophyte stands after prospecting 30 locations distributed over 13.7 km in length of the littoral region of the Baía River, ensuring a minimum distance of approximately 350 m between each macrophyte stand. Each stand sampled was continuous along the river, but not forming a single stand. We applied the following criteria to select the stands: (i) having Eichhornia, one of the most abundant aquatic macrophytes in the upper Paraná River plain (Padial et al., 2009), and (ii) presenting different richness and diversity, which was visually assessed in the field and later confirmed through statistical analyses. To identify possible spatial autocorrelation between macrophyte stands, we used the macrophyte diversity of each stand and geographical distance (latitude and longitude), calculated as dissimilarity matrices using the Euclidean distance, followed by a Mantel test for both matrices. The Mantel test showed a low and non-significant correlation between stand diversity and geographical distance (r = 0.015; P = 0.41), indicating no spatial autocorrelation between any sampled stand.
We collected the fish by using floating Plexiglas traps (minnow trap type; for details see: Dibble & Pelicice, 2010). Three traps were installed per macrophyte stand with the aim of increasing the sampling effort. The traps were positioned inside the stands in order to minimize the effects on the fish species that transit between the pelagic zone and the area structured by the macrophytes. We installed the traps at 7 am and inspected them every eight hours for a total of 24 h. We anesthetized the captured fish with clove oil, according to the current ethical practice, and fixed in formalin (10%). Subsequently, we identified them at the species level according to Ota et al. (2018). The fish species used in this study was Moenkhausia forestii.
Once a final round of minnow trap inspection and fish collection from each stand was concluded, we sampled the macrophytes using a 0.5 m × 0.5 m square and removed all plant material contained to a depth of 0.5 m, totaling 0.125 m3 of plant material. We transferred the plants to a plastic bucket and washed them in order to collect all associated invertebrates (Campos et al., 2017). The material retained in the plastic bucket was thoroughly filtered through a hand net with 160 µm mesh size and preserved in 70% ethanol buffered with sodium tetraborate (Campos et al., 2017). After filtering, we led the macrophytes to the laboratory, where we identified the species in order to determine the diversity and richness in each stand. Finally, we separated and dried them out in an oven at 60°C to constant weight (DW) and weighed them to obtain biomass.
Data analysis
All statistical analyses were performed in R 4.0.2 software (R Core Team, 2020) using the packages “vegan” (Oksanen et al., 2017), “robustbase” (Maechler et al., 2020) and for the graphs the package “ggplot” (Wickham, 2016). Prior to performing the ANOVA, all assumptions (normality and homogeneity of variances) were checked and met. For linear regressions, all assumptions were checked and met (linearity, normality of residuals and homogeneity of variances). For the glm with Poisson distribution, beyond the announced assumptions we also checked for overdispersion. A significance level of P < 0.05 was used for all analyses. The following subsections show the methodological sequence in our analyses.
Normality checking and data transformation for linear models
Prior to modelling, data normality was tested. There were indications of non-normality in some of the variables (Shapiro–Wilk test) and the abundance of invertebrates, fish abundance fish standard length and food items (diet analysis) were log-transformed before the analysis (Zuur et al., 2009).
Macrophytes diversity
The diversity of macrophytes used as the explanatory variable was calculated from the Shannon–Wiener index (H′) according to the following formula: \(H'=- {\sum }_{i=1}^{n}pi \mathrm{ln}pi\), where pi is the proportion of macrophyte biomass found in species i and n is the number of species in the sampled macrophyte stands (Shannon & Weaver, 1949). For evenness, we used the following formula: \({J=H}^{^{\prime}}\mathrm{ln}(S)\) where H' is the Shannon–Weiner index and S is the total number of species in a sampled stand (Pielou, 1966). For the Shannon–Wiener index we used the function “diversity” in the package “vegan” and for evenness we used the function “evenness” in the package “vegan”. Considering that richness and evenness are components of this index, they were used as response variables in a simple linear regression using the function “lm” in the package “vegan” to assess if both of these components have an influence on the diversity of macrophytes.
In order to control the influence of macrophyte biomass along the gradient of macrophyte diversity, we selected stands with similar biomasses so that differences could be mainly attributed to the diversity. For this purpose, we firstly discretize macrophyte diversity into three levels of diversity (Fig. S1). We classified the stands into low, medium and high diversity of macrophytes, after calculating the Shannon–Wiener index (H’) and identifying stands with the lowest values of H’ to constitute the “low diversity level’’, intermediate levels of H’ for the “medium diversity level’’ and the highest values of H’ for the “high diversity level’’. Secondly, macrophytes biomass was tested using an one-way ANOVA (after checking for normality and homogeneity of variance, using the package “vegan”) with macrophytes diversity as the explanatory variable to assess that the selected stands had similar biomasses.
Invertebrates community
Concerning the invertebrates, we identified and counted them at the lowest possible taxonomic level by using identification keys (McCafferty, 1983; Pérez, 1998) and the help of specialists. The abundance of invertebrates was expressed as the number of individuals per stand and the diversity through the Shannon–Wiener index. For taxonomic richness, we identified the taxa at different taxonomic levels, which for this work referred to the sum of taxa identified at different levels of taxonomic resolution. Several studies that estimate taxonomic richness of freshwater invertebrates reveal the occurrence of only a small bias when richness is estimated from different taxonomic units (Marshall et al., 2006; Heino & Soininen, 2007; Vilmi et al., 2016; Oliveira et al., 2020). We used linear models to explore the associations between the response variables (diversity, richness and abundance of invertebrates) and the diversity of macrophytes, our explanatory variable (prediction i). Diversity of invertebrates was modelled using a Gamma distribution using the function “glmrob'' in the package “robustbase” in order to weight extreme values that have an influence in the explanatory variable after checking for outliers (Cantoni & Ronchetti, 2001). Species richness was modelled using a Poisson error distribution using the function “glm” in the package “vegan”. The abundance of invertebrates was log + 1 transformed and modelled by a linear regression using the function “lm” in the package “vegan”.
Fish diet composition
Because fish populations are commonly regulated through density-dependent mechanisms (Henderson & Magurran, 2014) and they are also size-structured, prior to testing our second prediction (that fish diet differs between stands of macrophytes with different levels of diversity), all fish were counted and measured. We assessed the abundance and standard length of fish (mm) in order to make sure these variables had no effects on fish diet, using the macrophyte diversity as the explanatory variable. Fish abundance was expressed as the total number of individuals per stand (3 traps for 24 h in each stand) and modelled using a Gamma distribution on a log + 1 transformed abundance data. The Gamma model was performed using the function “glmrob” in the package “robustbase” in order to weight extreme values that have an influence in the explanatory variable after checking for outliers (Cantoni & Ronchetti, 2001). As for fish length, we modelled using a Gamma distribution on a log + 1 transformed data using the function “glm” in the package “vegan”.
For diet analysis, we examined the fish stomachs (201 individuals, Table S1) under a stereoscopic and optical microscope and identified the items by using the same taxonomic resolution as applied for the invertebrates. Food items were quantified by the frequency of occurrence and by the volumetric method (Hellawel & Abel, 1971; Hyslop, 1980). In order to test differences in diet composition (prediction ii) among macrophyte diversity levels (low, medium, and high; see section “Macrophytes diversity”) it was tested through a Multivariate Permutation Analysis of Variance (PERMANOVA; Anderson, 2005) using the “adonis” function in the package “vegan”. Stands identity was included as a constraint for permutations (i.e. “strata”) in “adonis” model to account for data non independence of the stands. Also, we checked for homogeneity of multivariate dispersions with PERMIDISP and used a balanced design (67 fish gut contents per level of the factor) to account for heterogeneity of variances since PERMANOVA is very robust when design is balanced (Anderson, 2017). To perform the analysis, we used a matrix of food items per individual fish, with volume values transformed into log + 1 in order to reduce the influence of rare items, and created dissimilarity matrices with the Bray–Curtis distance. We generated 9,999 permutations to assess the significance of the pseudo-F derived from PERMANOVA. In order to identify which prey contributed the most to dissimilarities in the diet, we also performed an Analysis of Similarity Percentages (SIMPER; Clarke 1993) between levels of diversity using the function “simper” in the package “vegan”. To analyze the relationship of the major food categories present in the fish diet, we performed a simple regression using the food categories as our response variable and the diversity of macrophytes as our explanatory variable, using the function “lm” in the package “vegan”. We log + 1 transformed the food items data for each stand (as we did in the PERMANOVA analysis).
To assess the third prediction, we addressed the trophic niche breadth at the population level by using the Levins Index (Krebs, 2014) according to the following formula: B = \(\frac{1}{i=\sum_{n}^{1}{pi}^{2}}\), where: B = trophic niche breadth, pi = proportion of prey i in the diet and n = number of food items. Niche breadth values vary from 1, when a species consumes only one type of food, to n, when a species similarly consumes all food items. The higher this value, the higher the niche breadth. Levins’ measure places more importance on abundant prey items and it’s commonly used for niche breadth (Krebs, 2014). To assess the relationship between the response variable (Levins index) and the explanatory variable (diversity of macrophytes) we modelled the trophic niche breadth using a Gamma distribution using the function “glmrob'' in the package “robustbase” in order to weight extreme values that have an influence in the explanatory variable after checking for outliers (Cantoni & Ronchetti, 2001).
Results
We sampled a total of 14 species of macrophytes in the nine selected stands (Fig. 2), with different forms: free-floating, emergent, epiphytic, and rooted with floating stems. The rooted with floating stems, Eichhornia azurea, was the only species present in seven out nine stands. The free-floating macrophytes occurred in seven stands and was comprised of different macrophyte species (Salvinia auriculata Aublet, Salvinia biloba Raddi, Salvinia minima Baker, Eichhornia crassipes, Limnobium laevigatum (Humb. & Bonpl. ex Willd.) Heine, Pistia stratiotes and Ricciocarops natans (L.) Corda) followed by the emergent (Paspalum repens, Polygonum acuminatum Kunth, Polygonum ferrugineum and Polygonum stelligerum) and the epiphytic form (Fig. 2). The only epiphytic species, Oxycaryum cubense Poepp. & Kunth, was most abundant in stands with higher diversity, accounting for 7.29% to 14.31% of biomass in these stands (Fig. 2). Macrophyte diversity, considered the explanatory variable in this study, ranged from H' = 0 to H' = 2.21 along the nine stands. Richness of macrophytes didn’t show an influence along the gradient (z-value = 2.07; P = 0.05) while evenness (J') showed a positive relationship (t-value = 7.13; P < 0.001) with increasing diversity.
The macrophyte diversity used as a categorical variable for the diet analysis, differed significantly between stands (F2, 6 = 49.47; P < 0.0001; low × medium: P = 0.01; medium × high: P = 0.003; high × low: P = 0.0001), confirming differences in macrophyte diversity among stands classified as low (H' = 0.37 ± 0.32), medium (H' = 1.12 ± 0.13), and high (H' = 2.09 ± 0.10) diversity. The average values of macrophyte biomass ranged from 132.06 ± 43.6 gDW/0.125 m3 to 210.44 ± 22.7 gDW/0.125 m3 and did not differ significantly between levels of macrophyte diversity (F2,6 = 0.53; P = 0.60).
The invertebrate community represented 31 taxa, including both aquatic and terrestrial invertebrates, belonging to the phylum Mollusca (Bivalvia and Gastropoda), two belonging to the phylum Annelida (Hirudinea and Oligochaeta) and 27 belonging to the phylum Arthropoda, further distributed among Crustacea (Amphipoda, Cyclopoida, Chydoridae, Daphniidae, Harpacticoida, and Ostracoda), Arachnida (Acarina and Araneae), Collembola, and Insecta (Table 1). Regarding taxonomic richness, the insects accounted for 10 orders, four of which were exclusive to stands with higher diversity (Homoptera, Plecoptera, Orthoptera, and Thysanoptera) (Table 1). Diversity of invertebrates increased with macrophyte diversity (z-value = 6.48; P < 0.0001), ranging from H' = 1.68 ± 0.05 to H' = 2.25 ± 0.04 (Fig. 3a). The taxonomic richness of invertebrates also increased with macrophyte diversity (z-value = 3.15; P = 0.001) (Fig. 3b). Regarding the abundance of invertebrates, we counted a total of 10,934 individuals (Table 1). Chironomidae larvae, followed by Ostracoda, Cyclopoida, and Amphipoda, were the taxa of greatest contribution regarding abundance in lower diversity stands (L1, L2, L3) (Table 1). In stands of higher diversity (L7, L8, L9), Amphipoda, Ceratopogonidae and Chironomidae larvae were the most abundant (Table 1). No significant relationship was observed between the abundance of invertebrates and macrophyte diversity (t-value = 1.48; P = 0.18) (Fig. 3c).
The abundance of fish (measured by 3 traps per 24 h in each stand) varied between 64 individuals/24 h and 533 individuals/24 h. Fish standard length ranged from 22.6 to 36.23 mm (Table S1). The diversity of macrophyte didn’t show a significant effect on fish abundance (z-value = 0.147; P = 0.88) and on fish length (t-value = − 0.454; P = 0.66). The composition of Moenkhausia forestii diet differed significantly between macrophyte diversity levels (pseudo-F2, 199 = 7.48, P = 0.001; low × medium: pseudo-F1, 132 = 3.52, P = 0.001; low × high: pseudo-F1, 132 = 13.6, P = 0.0001; medium × high: pseudo-F1, 132 = 5.37, P = 0.0001). The SIMPER analysis identified food items that were most responsible for distinctions between factor levels (Table S2). Between low and medium diversity levels, aquatic plants, Diptera pupae and Hemiptera, were responsible for dissimilarities in the diet (61.50% cumulative dissimilarity). For low and high diversity levels, aquatic plants, Diptera pupae, Hymenoptera and Araneae accounted for 66.47% of cumulative dissimilarities in the diet. Between medium and high diversity levels, aquatic plants, Diptera pupae, Hemiptera and Hymenoptera, accounted for 61.23% of cumulative dissimilarities (Table S2). In general, M. forestii diet was composed of aquatic plants and insects (Table 2). In low diversity stands, aquatic plants occurred in 94.03% of the stomachs and represented 51.26% of the volume, while Diptera pupae (O% = 64.17; V% = 16.77) and Araneae (O% = 16.41; V% = 7.90) were the most consumed invertebrates. With the increasing diversity of macrophytes, occurrence and the volume of aquatic plants consumed by M. forestii consistently decreased, representing 74.62% and 38.58%, respectively, of the diet in stands of medium diversity, and 52.23% and 13.23%, respectively, in stands of high diversity (Table 2). In stands of medium diversity of macrophytes, in addition to aquatic plants, Diptera pupae (O% = 67.16; V% = 14.47) and Hemiptera (O% = 25.37; V = 14.37) were the most consumed invertebrates. In high diversity stands, Diptera pupae (O% = 71.64; V% = 23.03%) was the most consumed item, followed by Hymenoptera (O% = 29.85; V% = 15.63), aquatic plants (O% = 52.23, V% = 13.23), and Araneae (O% = 17.91; V% = 9.16). Some taxa were consumed exclusively in stands of high diversity, such as Thysanoptera, Plecoptera, Coleoptera larvae, Lepidoptera, and Diptera (Sarcophagidae and Culicidae). The consumption of aquatic plants decreased with the increasing diversity of macrophytes (t-value = − 3.58; P = 0.008) while the consumption of invertebrates increased (t-value = 4.61; P = 0.002; Fig. 4). As predicted, trophic niche breadth increased with increasing diversity of macrophytes (z-value = 2.94; P = 0.003) (Fig. 5).
Discussion
The attributes of invertebrate communities were shown to be influenced by the diversity of macrophytes, since richer and more diverse communities were associated with more diverse stands of these plants. Thus, our prediction was partially corroborated considering that the abundance of invertebrates was consistently lower in stands of lower macrophyte diversity; however it was not statistically influenced by macrophyte diversity. Commonly, richness, abundance and the diversity of communities associated with macrophytes are correlated with measurements of plant biomass or density (Warfe & Barmuta, 2004; Nakamoto et al., 2018). Since all of the stands assessed in our study had similar biomasses, our results point to the importance of macrophyte diversity on the structure of associated communities. The components used to measure diversity, richness and evenness, are important when considered together but they do not play equal roles into diversity indexes (Strong, 2016). For example, even in stands of greater richness, as seen for L3 (Fig. 2), the diversity of invertebrates was lower than in stands of similar species richness, which can be a result of the high dominance of Eichhornia azurea in this location. Thus, evenness can play a significant role affecting communities, and it is possible that even with those additional macrophyte species, they were not sufficiently abundant to cause a significant change in plant composition and, therefore, to affect the invertebrate’s communities.
The presence of some frequent and abundant taxa in all stands, such as Amphipoda, Diptera larvae (Chironomidae and Ceratopogonidae), and Ostracoda, can be explained in part by the high availability of shared resources for these groups, which feed on particulate organic matter and periphyton. Their ability to provide refuge also favors the colonization of macrophytes by these groups (Mormul et al., 2006; Marçal & Callil, 2008; Tóth et al., 2012). With increasing diversity of macrophytes, other groups of invertebrates became present, which can be attributed to the plurality of microhabitat that these plants offer.
For that reason, more diverse macrophyte stands can effectively provide a higher diversity of food resources and microhabitat over monospecific stands, or those with one or few species, which favor the coexistence of different taxa (Choi et al., 2014; Celewicz-Goldyn & Kuczynska-Kippen, 2017). Our findings demonstrated that the composition of more diverse stands includes plants with different morphologies (free-floating, emergent, and epiphytic macrophytes), and that the invertebrate community may be distributed according to the different habitats provided by these plants, possibly being explained by niche complementarity (Bell et al., 2014; Boelter et al., 2018; Nakamoto et al., 2018). Free floating macrophytes, such as Salvinia, provide submerged and aerial habitat, which can sustain the presence of terrestrial invertebrates such as Coleoptera, Orthoptera, Thysanoptera, Hymenoptera and Collembola (Fontanarrosa et al., 2013; Gallardo et al., 2017). These invertebrates were found mainly or exclusively in stands of higher diversity, which were the same stands that were covered by a more expressive percentage of the free-floating Salvinia genus. However, not only the morphological form, but a combination of form and species may play a role into the distribution of invertebrates, since a stand covered mainly by the free-floating Limnobium laevigatum didn’t show the same trend as for the stands covered with Salvinia. This may be in part due to morphological differences of these plants. Salvinia presents characteristic trichomes that repel water and also maintain air layers under water which can favor the occurrence of terrestrial invertebrates, but these hairy surfaces aren’t found in Limnobium (Barthlottet al., 2009; Vermeij, 2016).
Different groups of invertebrates may have preferences for different species of macrophytes, and even structurally similar plants may not necessarily provide equivalent habitat for the invertebrates (Sipaúba-Tavares et al., 2017; Carniatto et al., 2020). For instance, similar morphological forms but with differing complexities (i.e. E. crassipes and E. azurea) has shown to alter assemblages of spiders associated with macrophytes, due to the intertwined leaves and stems (Cunha et al., 2012). The Eichhornia genus, in the absence of other species, can determine the structure of the invertebrate community and limit it to those that can efficiently use this plant as a resource. In this study, stands with dominance of Eichhornia presented lower richness and diversity of invertebrates, probably due to the lower oxygen concentration as well as a limited variety or inaccessibility of resources (Toft et al., 2003; Schultz & Dibble, 2012).
Our predicitions regarding the M. forestii diet were met given that changes in the diet were found to be associated with the diversity of macrophyte stands. In low diversity stands, M. forestii consumed aquatic plants and invertebrates in similar proportions. With increasing macrophyte diversity, consumption of aquatic plants decreased, while invertebrates consumption increased. Indeed, invertebrate consumption represented 87% of the diet in stands of high diversity. The presence of new invertebrates was reflected on M. forestii diet; ranging from an omnivorous diet (here defined as individuals feeding at contrasting trophic levels, such as primary producers and consumers of any kind in similar proportions) (Novakowski et al., 2008) in stands of low macrophyte diversity, to a predominantly invertivorous diet in stands of high macrophytes diversity. The difference in the composition of M. forestii diet may be associated with the fact that animals that are able to feed on multiple trophic levels, consuming both plant and animal resources, generally tend to prefer the consumption of animal prey over plants due to the higher concentration of nutrients (Dorenbosch & Bakker, 2011; Guinan et al., 2015; Zhang et al., 2018). In this scenario, the increase of richness, diversity and even abundance of invertebrates can explain changes in the diet (despite the latter not differing but having mean higher values in stands of medium and high diversity). For M. forestii, animal resources appear to be more important in these stands (of high diversity), while in less rich and diverse environments plant material seems to be more important.
In general, consumption of common invertebrates occurred among stands with different macrophyte diversity, such as Diptera pupae which had a considerable presence in all sampled stands. Diptera pupae were not highly abundant in the environment, though this could in part be due to sampling bias, which mainly captured organisms associated with the submerged portion of macrophytes (submerged stems and roots), and didn’t sample Diptera pupae that generally are found in contact with the surface of the water prior to emerging as adults (Adler & Courtney, 2019). In contrast, Diptera larvae (a previous life stage of Diptera), particularly the Chironomidae family, which are common in the submerged portion of macrophytes (Higuti et al., 2007) were abundant in our samples. Considering that larvae migrate to the surface before emerging as adults (Adler & Courtney, 2019), the high abundance of larvae can indicate a high abundance of other stages (pupae) and explain the high consumption of Diptera pupae. This result is similar to Quirino et al. (2017), who found a high abundance of Diptera larvae in M. forestii diet. In addition, this item may provide a greater nutritional benefit above other abundant taxa that are difficult to access or digest (Fall & Fiksen, 2019).
Other notable items in the diet were Araneae, Hemiptera, Coleoptera, Hymenoptera, and Orthoptera, depending on the diversity of the stands. Aquatic arthropods, such as Hemiptera, may represent a higher quality food source over terrestrial organisms, due to their lower amounts of chitin that requires less energy for digestion (Cauchie, 2002), while terrestrial arthropods (Hymenoptera and Orthoptera) have higher energy density (Francis & Schindler, 2009; Sullivan et al., 2014). The increase in terrestrial items in high diversity stand diets can be attributed to the presence of such groups, which facilitates the encounter between predator and prey. Diverse stands can harbour by chance plant species with more attractive characteristics, such as emergent parts (e.g. flowers, leaves), that have the potential to attract adult insects for oviposition, offer resources for larvae after eclosion, and provide food for host specific herbivores (Cronin et al., 1998; Lancaster & Downes, 2013).
According to our prediction, the trophic niche breadth of M. forestii increased with macrophyte diversity. Expansion of the niche breadth was promoted by the diversity of invertebrates in high diversity stands, that can be attributed to the diversity of ecologically accessible resources that may be exploited, that is, ecological opportunity (Araújo et al., 2011; Stroud & Losos, 2016). Our findings are in agreement with the study of Sánchez-Hernández et al (2020) that showed that for freshwater fish, prey diversity is the major factor shaping trophic niche rather than the constraining effects of resource abundance. The presence of exclusive items in the diet in stands of higher diversity may expand the populations foraging possibilities, allowing their niches to diverge.
We acknowledge that our results were from only nine stands of macrophytes in a floodplain. Despite this limitation, the conspicuous gradient in plant, invertebrate diversity and in the diet changes following these gradients, make us confident about the importance of the macrophytes taxonomic diversity for the associate organisms. Future studies should replicate the gradient, in order to include more spatial replicates to establish the generality of the observed patterns and also consider these patterns over the seasonality of this environment.
In summary, it was demonstrated that the diversity of macrophytes changes the invertebrate community and these changes have consequences for the M. forestii diet. The increasing macrophyte diversity has led to an increase in invertebrate consumption by M. forestii, changing it from an omnivorous diet in low diversity stands to a predominantly invertivorous diet in stands of high macrophyte diversity. Thus, the importance of the diversity of these plants is emphasized, as they structure the environment and can change the dynamics of the associated communities. In a scenario involving changes in the attributes of these plants, in which stands become dominated by one or a few species of macrophytes, it is expected that these results will be reflected on other trophic levels and even on a local scale.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adler, P. H., & G. W. Courtney, 2019. Ecological and societal services of aquatic Diptera. Insects 10: 1–23.
Anderson, M. J, 2005. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. Auckland, NZL: Department of Statistics, University of Auckland.
Anderson, M. J., 2017. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online 1–15.
Araújo, M. S., D. I, Bolnick & C. A. Layman, 2011. The ecological causes of individual specialisation. Ecology Letters 14: 948–958.
Ávila, A. C., C. Stenert & L. Maltchik, 2011. Partitioning macroinvertebrate diversity across different spatial scales in southern Brazil coastal wetlands. Wetlands 31: 459–469.
Barthlott, W., S. Wiersch, Z. Čolić, & K. Koch, 2009. Classification of trichome types within species of the water fern Salvinia, and ontogeny of the eggbeater trichomes. Botany 87: 830–836.
Bell, N., T. Riis, A. M. Suren & A. Baattrup-Pedersen, 2014. Distribution of invertebrates within beds of two morphologically contrasting stream macrophyte species. Fundamental and Applied Limnology / Archiv für Hydrobiologie 183: 309–321.
Boelter, T., C. Stenert, M. M. Pires, E. S. F. Medeiros & L. Maltchik, 2018. Influence of plant habitat types and the presence of fish predators on macroinvertebrate assemblages in southern Brazilian highland wetlands. Fundamental and Applied Limnology / Archiv für Hydrobiologie 192: 65–77.
Campos, R., E. C. Oliveira, M. B. O. Pinto, A. P. S. Bertoncin, J. Higuti & K. Martens, 2017. Evaluation of quantitative sampling methods in pleuston: an example from ostracod communities. Limnologica 63: 36–41.
Cantoni, E. & E. Ronchetti, 2001. Robust inference for generalized linear models. Journal of the American Statistical Association 96: 1022–1030.
Carniatto, N., R. Fugi, B. A. Quirino, E. R. Cunha & S. M. Thomaz, 2020. An invasive and a native macrophyte species provide similar feeding habitat for fish. Ecology of Freshwater Fish 29: 112–120.
Cauchie, H. M., 2002. Chitin production by arthropods in the hydrosphere. Hydrobiologia 470: 63–96.
Celewicz-Goldyn, S. & N. Kuczynska-Kippen, 2017. Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 12: 1–14.
Choi, J. Y., K. S. Jeong, S. K. Kim, G. H. La, K. H. Chang & G. J. Joo, 2014. Role of macrophytes as microhabitats for zooplankton community in lentic freshwater ecosystems of South Korea. Ecological Informatics 24: 177–185.
Clarke, K.R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117–143.
Cremona, F., D. Planas & M. Lucotte, 2008. Biomass and composition of macroinvertebrate communities associated with different types of macrophyte architectures and habitats in a large fluvial lake. Fundamental and Applied Limnology / Archiv für Hydrobiologie 171: 119–130.
Cronin, G., K. Wissing & D. Lodge, 1998. Comparative feeding selectivity of herbivorous insects on water lilies: aquatic vs semi-terrestrial insects and submersed vs floating leaves. Freshwater Biology 39: 243–257.
Cunha, E. R., S. M. Thomaz, R. P. Mormul, E. G. Cafofo & A. B. Bonaldo, 2012. Macrophyte structural complexity influences spider assemblage attributes in wetlands. Wetlands 32: 369–377.
Dibble, E. D. & F. M. Pelicice, 2010. Influence of aquatic plant-specific habitat on an assemblage of small neotropical floodplain fishes. Ecology of Freshwater Fish 19: 381–389.
Dorenbosch, M. & E. S. Bakker, 2011. Herbivory in omnivorous fishes: effect of plant secondary metabolites and prey stoichiometry. Freshwater Biology 56: 1783–1797.
Fall, J. & Ø. Fiksen, 2019. No room for dessert: a mechanistic model of prey selection in gut-limited predatory fish. Fish and Fisheries 21:63–79.
Farina, S., R. Arthur, J. F. Pagès, P. Prado, J. Romero, A. Vergés, G. Hyndes, K. L. Heck, S. Glenos & T. Alcoverro, 2014. Differences in predator composition alter the direction of structure-mediated predation risk in macrophyte communities. Oikos 123: 1311–1322.
Fisher, J. C., W. E. Kelso & D. A. Rutherford, 2012. Macrophyte mediated predation on hydrilla-dwelling macroinvertebrates. Fundamental and Applied Limnology 181: 25–38.
Fontanarrosa, M. S., G. N. Chaparro & I. O’Farrell, 2013. Temporal and spatial patterns of macroinvertebrates associated with small and medium-sized free-floating plants. Wetlands 33: 47–63.
Francis, T. B. & D. E. Schindler, 2009. Shoreline urbanization reduces terrestrial insect subsidies to fishes in North American lakes. Oikos 118: 1872–1882.
Gallardo, L. I., R. P. Carnevali, E. A. Porcel & A. S. G. Poi, 2017. Does the effect of aquatic plant types on invertebrate assemblages change across seasons in a subtropical wetland? Limnetica 36: 87–98.
Guinan, M. E., K. L. Kapuscinski & M. A. Teece, 2015. Seasonal diet shifts and trophic position of an invasive cyprinid, the rudd Scardinius erythrophthalmus (Linnaeus, 1758), in the upper Niagara river. Aquatic Invasions 10: 217–225.
Habib, S. & A. R. Yousuf, 2015. Effect of macrophytes on Phytophilous macroinvertebrate community: a review. Journal of Entomology and Zoology Studies 3: 377–384.
Hansen, J. P., S. A. Wikström, H. Axemar & L. Kautsky, 2011. Distribution differences and active habitat choices of invertebrates between macrophytes of different morphological complexity. Aquatic Ecology 45: 11–22.
Heino, J. & J. Soininen, 2007. Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biological Conservation 137: 78–89.
Hellawell, J. M. & R. Abel, 1971. A rapid volumetric method for the analysis of the food of fishes. Journal of Fish Biology 3: 29–37.
Henderson, P. A. & A. E. Magurran, 2014. Direct evidence that density-dependent regulation underpins the temporal stability of abundant species in a diverse animal community. Proceedings of the Royal Society B: Biological Sciences 281: 20141336.
Hyslop, E. J, 1980. Stomach contents analysis review of methods and their applications. Journal of Fish Biology 17: 411–429.
Jeffries, M., 1993. Invertebrate colonization of artificial pondweeds of differing fractal dimension. Oikos 67: 142–148.
Jiménez-Ramos, R., F. G. Brun, L. G. Egea & J. J. Vergara, 2018. Food choice effects on herbivory: Intra-specific seagrass palatability and inter-specific macrophyte palatability in seagrass communities. Estuarine, Coastal and Shelf Science 204: 31–39.
Kliemann, B. C. K., M. C. Baldasso, S. F. R. Pini, M. C. Makrakis, S. Makrakis & R. L. Delariva, 2019. Assessing the diet and trophic niche breadth of an omnivorous fish (Glanidium ribeiroi) in subtropical lotic environments: Intraspecific and ontogenic responses to spatial variations. Marine and Freshwater Research 70: 1116–1128.
Krebs, C.J, 2014. Niche measures and resource preferences. In: Krebs CJ (ed), Ecological Methodology. Harper & Row, New York: 597-653.
Lancaster, J. & B.J. Downes, 2013. Oviposition and eggs. In: Lancaster, J. & B.J. Downes (eds), Aquatic Entomology. Oxford University Press, Oxford, 173-190.
Layman, C. A., J. P. Quattrochi, C. M. Peyer & J. E. Allgeier, 2007. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecology Letters 10: 937–944.
Lopes, C. D. A., A. C. E. A. Faria, G. I. Manetta & E. Benedito-Cecilio, 2006. Caloric density of aquatic macrophytes in different environments of the Baía river subsystem, Upper Paraná river floodplain, Brazil. Brazilian Archives of Biology and Technology 49: 835–842.
Maechler M, P. Rousseeuw, C. Croux, V. Todorov, A. Ruckstuhl, M. Salibian-Barrera, T. Verbeke, M. Koller, E.L. Conceicao & M. Anna di Palma, 2020. robustbase: Basic Robust Statistics.
Marçal, S. & C. Callil, 2008. Structure of invertebrates community associated with Eichhornia crassipes Mart. (Solms-Laubach) after the introduction of Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) in the Upper Paraguay River, MT, Brazil. Acta Limnologica Brasiliensia 20: 359–371.
Marshall, J. C., A. L. Steward & B. D. Harch, 2006. Taxonomic resolution and quantification of freshwater macroinvertebrate samples from an Australian dryland river: the benefits and costs of using species abundance data. Hydrobiologia 572: 171–194.
McAbendroth, A. L., P. M. Ramsay, A. Foggo, S. D. Rundle, D. T. Bilton & L. McAbendroth, 2005. Does macrophyte fractal drive invertebrate complexity diversity, biomass and body size distributions? Oikos 111: 279–290.
McCafferty, W. P., 1983. Aquatic Entomology: The Fshermen’s and Ecologists’ Illustrated Guide to Insects and Their Relative. Jones and Bartlett Learning Publishers, Boston.
Moreno-Rueda, G., E. Melero, S. Reguera, F. J. Zamora-Camacho & I. Álvarez-Benito, 2018. Prey availability, prey selection, and trophic niche width in the lizard Psammodromus algirus along an elevational gradient. Current Zoology 64: 603–613.
Mormul, R. P., L. A. Vieira, S. Pressinatte, A. Monkolski & A. M. Dos Santos, 2006. Sucessão de invertebrados durante o processo de decomposição de duas plantas aquáticas (Eichhornia azurea e Polygonum ferrugineum). Acta Scientiarum - Biological Sciences 28: 109–115.
Nakamoto, K., J. Hayakawa, T. Kawamura, M. Kodama, H. Yamada, T. Kitagawa & Y. Watanabe, 2018. Phylogenetically diverse macrophyte community promotes species diversity of mobile epi-benthic invertebrates. Estuarine, Coastal and Shelf Science 207: 56–62.
Novakowski, G. C., N. S. Hahn & R. Fugi, 2008. Diet seasonality and food overlap of the fish assemblage in a pantanal pond. Neotropical Ichthyology 6: 567–576.
Ohtaka, A., T. Narita, T. Kamiya, H. Katakura, Y. Araki, S. Im, R. Chhay & S. Tsukawaki, 2011. Composition of aquatic invertebrates associated with macrophytes in Lake Tonle Sap, Cambodia. Limnology 12: 137–144.
Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., Minchin, P.R., O’hara, R.R., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E & Wagner, H, 2017. Vegan: community ecology package. R package version 2.4–0
Oliveira, S. S., J. C. G. Ortega, L. G. S. Ribas, V. G. Lopes & L. M. Bini, 2020. Higher taxa are sufficient to represent biodiversity patterns. Ecological Indicators 111: 105994.
Ota, R. R., G. de C. Deprá, W. J. da Graça & C. S. Pavanelli, 2018. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotropical Ichthyology 16: 1–111.
Padial, A. A., S. M. Thomaz & A. A. Agostinho, 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624: 161–170.
Pelicice, F. M. & A. A. Agostinho, 2006. Feeding ecology of fishes associated with Egeria spp. patches in a tropical reservoir, Brazil. Ecology of Freshwater Fish 15: 10–19.
Pérez, G. R., 1998. Guía para el estudio de los macroinvertebrados acuáticos del Departamento de Antioquia. Editorial Presencia, Colombia.
Petry, P., P. B. Bayley & D. F. Markle, 2003. Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. Journal of Fish Biology 63: 547–579.
Pielou, E. C, 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13: 131-144.
Prejs, A. & K. Prejs, 1987. Feeding of tropical freshwater fishes: seasonality in resource availability and resource use. Oecologia 71: 397–404.
Priyadarshana, T., T. Asaeda & J. Manatunge, 2001. Foraging behaviour of planktivorous fish in artificial vegetation: the effects on swimming and feeding. Hydrobiologia 442: 231–239.
Quirino, B. A., N. Carniatto, R. Guglielmetti & R. Fugi, 2017. Changes in diet and niche breadth of a small fish species in response to the flood pulse in a Neotropical floodplain lake. Limnologica 62: 126–131.
Rejmankova, E., 2011. The role of macrophytes in wetland ecosystems. Journal of Ecology and Environment 34: 333–345.
Rennie, M. D. & L. J. Jackson, 2005. The influence of habitat complexity on littoral invertebrate distributions: patterns differ in shallow prairie lakes with and without fish. Canadian Journal of Fisheries and Aquatic Sciences 62: 2088–2099.
Sánchez-Botero, J. I. & C. A. R. M. Araújo-lima, 2001. As macrófitas aquáticas como berçário para a ictiofauna da várzea do rio Amazonas. Acta Amazonica 31: 437–447.
Sánchez-Botero, J. I., C. A. R. M. Araujo-Lima & D. S. Garcez, 2008. Effects of types of aquatic macrophyte stands and variations of dissolved oxygen and of temperature on the distribution of fishes in lakes of the amazonian floodplain. Acta Limnologica Brasiliensia 20: 45–54.
Sánchez-Hernández, J., A. G. Finstad, J. V. Arnekleiv, G. Kjærstad & P. A. Amundsen, 2020. Beyond ecological opportunity: prey diversity rather than abundance shapes predator niche variation. Freshwater Biology 00: 1–18
Schneider, B., E. R. Cunha, M. Marchese & S. M. Thomaz, 2018. Associations between macrophyte life forms and environmental and morphometric factors in a large sub-tropical floodplain. Frontiers in Plant Science 9: 1–10.
Schultz, R. & E. Dibble, 2012. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: the role of invasive plant traits. Hydrobiologia 684: 1–14.
Shannon, C. E. & Weaver, W, 1949. The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press.
Sipaúba-Tavares, L. H., C. B. Anatriello, A. Milstein, R. N. Millan & B. Scardoeli-Truzzi, 2017. Macrophyte - environment relationships during a monospecific and a multispecific massive invasion in a fishpond. Tropical Plant Research 4: 471–479.
Stahr, K. J. & M. A. Kaemingk, 2017. An evaluation of emergent macrophytes and use among groups of aquatic taxa. Lake and Reservoir Management 33: 314–323.
Strayer, D. L. & H. M. Malcom, 2007. Submersed vegetation as habitat for invertebrates in the Hudson River estuary. Estuaries and Coasts 30: 253–264.
Strong, W. L., 2016. Biased richness and evenness relationships within Shannon-Wiener index values. Ecological Indicators 67: 703–713.
Stroud, J. T. & J. B. Losos, 2016. Ecological opportunity and adaptive radiation. Annual Review of Ecology, Evolution, and Systematics 47: 507–532
Sullivan, M. L., Y. Zhang & T. H. Bonner, 2014. Carbon and nitrogen ratios of aquatic and terrestrial prey for freshwater fishes. Journal of Freshwater Ecology 29: 259–266.
Svanbäck, R. & D. I. Bolnick, 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society B: Biological Sciences 274: 839–844.
Tang, Y., S. F. Harpenslager, M. M. L. Van Kempen, E. J. V. Verbaarschot, L. M. J. M. Loeffen, J. G. M. Roelofs, A. J. P. Smolders & L. P. M. Lamers, 2017. Aquatic macrophytes can be used for wastewater polishing but not for purification in constructed wetlands. Biogeosciences 14: 755–766.
Taniguchi, H., S. Nakano & M. Tokeshi, 2003. Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshwater Biology 48: 718–728.
Theel, H. J., E. D. Dibble & J. D. Madsen, 2008. Differential influence of a monotypic and diverse native aquatic plant bed on a macroinvertebrate assemblage; an experimental implication of exotic plant induced habitat. Hydrobiologia 600: 77–87.
Thomaz, S. M. & E. R. Cunha, 2010. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnologica Brasiliensia 22: 218–236.
Toft, J. D., C. A. Simenstad, J. R. Cordell & L. F. Grimaldo, 2003. The effects of introduced water hyacinth on habitat structure, invertebrate assemblages, and fish diets. Estuaries 26: 746–758.
Tóth, M., A. Móra, B. Kiss, G. Dévai & A. Specziár, 2012. Are macrophyte-dwelling chironomidae (Diptera) largely opportunistic in selecting plant species? European Journal of Entomology 109: 247–260.
Vermeij, G. J., 2016. Plant defences on land and in water: why are they so different? Annals of Botany 117: 1099–1109.
Vilmi, A., S. M. Karjalainen, T. Nokela, K. Tolonen & J. Heino, 2016. Unravelling the drivers of aquatic communities using disparate organismal groups and different taxonomic levels. Ecological Indicators 60: 108–118.
Warfe, D. M. & L. A. Barmuta, 2004. Habitat structural complexity mediates the foraging success of multiple predator species. Oecologia 141: 171–178.
Wickham, H, 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York.
Zhang, P., R. F. Van Den Berg, C. H. A. Van Leeuwen, B. A. Blonk & E. S. Bakker, 2018. Aquatic omnivores shift their trophic position towards increased plant consumption as plant stoichiometry becomes more similar to their body stoichiometry. PLoS ONE 13: 1–13.
Zhang, M., Y. Wang, B. Gu, Y. Li, W. Zhu, L. Zhang, L. Yang & X. Li, 2019. Resources utilization and trophic niche between silver carp and bighead carp in two mesotrophic deep reservoirs. Journal of Freshwater Ecology 34: 199–212.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Limitations of linear regression applied on ecological data. In: M. Gail, K. Krickeberg, J. M. Samet, A. Tsiatis & W. Wong (eds), Mixed Effects Models and Extensions in Ecology with R. Springer, Springer, New York: 11-31.
Acknowledgements
The authors thank S.M. Thomaz and M.S. Dainez for the help in sampling and identifying macrophytes. We also acknowledge Nupélia and its researchers, the Graduate Program in Ecology of Continental Aquatic Environments (UEM), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support and infrastructure provided for the development of this study. We also thank three anonymous reviewers for their helpful comments
Author information
Authors and Affiliations
Contributions
Conceived and designed the investigation: KYY, RF, BAQ, ALPC, MHFA. Performed field and/or laboratory work KYY, RF, BAQ, ALPC. Analyzed the data: KYY, RF, BAQ, ALPC, MHFA. Contributed materials, reagents, and/or analysis tools: KYY, RF, BAQ. Wrote the paper: KYY, RF.
Corresponding author
Additional information
Handling editor: Katya E. Kovalenko
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yofukuji, K.Y., Cardozo, A.L.P., Quirino, B.A. et al. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia 848, 913–927 (2021). https://doi.org/10.1007/s10750-020-04501-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04501-w