Abstract
To assess the potential of domestic traffic for the regional spread of nonindigenous species (NIS), we surveyed the hull of an oceanographic vessel serving routes in the southwestern Atlantic and Southern Ocean. Sampling was performed while the vessel was in the water and in dry-dock in the Port of Mar del Plata, Argentina. We found 120 taxa belonging to 14 different invertebrate groups, including 53 species, 47 morphospecies, and 20 taxa identified at higher taxonomic levels. Ten of these species have not been reported for the Port of Mar del Plata and adjacent areas prior to the present study, and eight are new records for the entire Argentine coast. While both in-water and dry-dock sampling allowed for the detection of native, non-native, and cryptogenic fauna, more samples and species were obtained in dry-dock. Dry-dock richness estimates amounted to up to ~ 110 hull fouling species. Despite specific logistic challenges, dry-dock sampling should be considered by managers assessing vector strength due to its greater species detection power. The present results highlight the potential for domestic vessel spread of hull fouling marine NIS, and pinpoint likely future additions to the non-native fauna inventory in the southwestern Atlantic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vessel ballast water and hull fouling are well-known vectors for the transport and introduction of marine organisms across port hubs (López-Legentil et al., 2015; Ruiz et al., 2015). While large, commercial, transoceanic vessels can mediate primary introductions across continents (Sylvester et al., 2011), domestic and short-sea shipping boats, tugs, and medium-size vessels can mediate the scape of nonindigenous species (NIS) confined to one port or marina into new hubs and contribute to their regional spread (Clarke Murray et al., 2011; Zabin et al., 2014). The potential for ballast water in short-sea shipping vessels for secondary spread of marine NIS has been established (Rup et al., 2010; Kelly et al., 2013), but similar hull fouling studies are required.
Since the last quarter of the past century, a number of studies have assessed biofouling communities attached to large commercial vessels (Carlton & Hodder, 1995; Gollasch, 2002; Coutts & Taylor, 2004; Sylvester & MacIsaac, 2010) and, more recently, recreational boats in different ports and marinas worldwide (Clarke Murray et al., 2011; Zabin et al., 2014; Martínez-Laiz et al., 2019). The large potential of this vector for the transport and introduction of animals and plants into marine ecosystems (Davidson et al., 2009; Sylvester et al., 2011) has spurred research (Ammon et al., 2018) and regulatory efforts aimed at mitigating this risk nationally and internationally (e.g., IMO Biofouling Guidelines adopted in 2011 and GloFouling partnership between IMO, UNDP, and GEF initiated in 2017 to promote widespread guideline implementation). Small and medium size crafts can mediate secondary dispersal of organisms from initial points of introduction (typically main international port hubs) into smaller ports and marinas (Clarke Murray et al., 2011). While recreational boats have been the target of several recent studies (Zabin et al., 2014; Martínez-Laiz et al., 2019), the role of mid-sized domestic vessels such as fishing, Navy, and oceanographic vessels has thus far received less attention. These vessels often have operational and maintenance patterns (e.g., long stationary port periods, long intervals between successive paintings of the hull) sharply contrasting those of large commercial vessels, and which may be associated to extensive extents of hull fouling that should be investigated (Sylvester et al., 2011).
The assessment of hull fouling communities has been performed in various ways in the literature, including questionnaire-based collection of information from vessel owners (Clarke Murray et al., 2013), the analysis of visual records (e.g., dock-level observation of biofouling at the water-line, underwater photography, and video taken by remote-operated vehicles or divers) (Brine et al., 2013), and inspection of biological samples (Sylvester et al., 2011). While each method presents its own assets and problems, the latter method has been proved to be the most reliable having the highest detection power (Zabin et al., 2014). Biological samples from hull fouling communities are obtained by scrape sampling, either in-water using divers or while the vessel is out of the water for maintenance or other purposes in a dry-dock. Both methods, however, are logistically demanding and present their own specific challenges (Sylvester & Floerl, 2014). Previous studies suggest that in-water sampling may underestimate hull fouling extent due to flotation and loss of organisms in the water during the scraping of the hull surface, compared with dry-dock sampling where losses can be kept to a minimum (Kalaci, 2011; McCollin & Brown, 2014). Comparative assessment of methods is needed to evaluate compliance with developing regulations (Zabin et al., 2018). Unfortunately, direct comparison between hull fouling assessment methods are rare (but see Floerl & Coutts, 2013), and the relative performance of in-water and dry-dock sampling remains unknown.
Despite research efforts conducted during the past decades, the current knowledge on the biodiversity of the marine ecosystems in the southwestern Atlantic is poor, and species inventories still remain fragmentary or outdated compared to other regions (Schwindt & Bortolus, 2017). Although it could be perceived as a remote location to main global trade and economic hubs, the Atlantic coast of South America experiences an increasing maritime commerce with other regions of the world (Castro et al., 2017). As a likely consequence, the Argentine coast hosts many introduced species of recent discovery, species of uncertain non-native status (Schwindt et al., 2014), and possibly a potentially substantial number of yet undiscovered introduced NIS. The classical comprehensive study by Orensanz et al. (2002) constituted the first large scale update of the NIS flora and fauna of coastal and shelf areas of Uruguay and Argentina. The impressive number of new species found by this and subsequent studies (Schwindt et al., 2014, 2020; Rumbold et al., 2018) suggests these regions are little known and more NIS species will continue to be discovered as research continues.
In the present work, we sampled the hull fouling communities on the oceanographic vessel R/V Puerto Deseado in the Port of Mar del Plata. The prime objectives of the current study are: (1) To assess the potential for hull fouling on medium-sized domestic vessels to transport and secondary spread marine invertebrate NIS; (2) To compare the efficiency and detection powers of in-water and dry-dock hull fouling sampling methods; and (3) To monitor vessel hull fouling communities for the presence of invertebrate NIS unreported in Mar del Plata and on the whole Argentine coast, and thus make a potential contribution to NIS faunal inventories in the southwestern Atlantic.
Methods
Study vessel
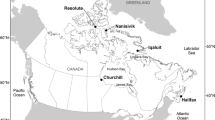
R/V Puerto Deseado is an Argentine Navy-operated vessel primarily based in the Port of Mar del Plata, a main shipping, fishing, recreational hub, and a hot-spot for marine research, but also calling other Argentine ports including Puerto Belgrano, Puerto Madryn, and Ushuaia (Fig. 1). Prior to the present survey, the vessel went out of the water into dry-dock at the Tandanor shipyard in the Port of Buenos Aires from 15 April through 2 June 2009. At the time of our first sampling event (see below), it had uninterruptedly been in the water for 754 days. During that time, the vessel conducted research activities related with 14 sailing campaigns between latitudes − 30.5° and − 65.4°, and longitudes − 44.0° to − 68.3°, encompassing the continental shelf and slope of Argentina, Uruguay and southern Brazil (Argentine and Magellan biogeographic provinces, southwestern Atlantic Ocean) as well as several sites in Antarctic waters (Southern Ocean) (Fig. 1). In total, the vessel spent 450 days in port and 304 days sailing during this period. In the 75 days elapsed between the first and second sampling events (see below), the vessel spent 12 more days at sea on a campaign over the continental shelf and slope in front of Uruguay and Southern Brazil (Fig. 1). In the 5 days between the second and third sampling events (see below), the vessel was stationarily moored at port, with the exception of its relocation from the Navy Base where it was docked to the SPI dry-dock within the same port facility (distance ~ 1800 m) immediately before dry-docking.
Sailing area of R/V Puerto Deseado in the last 2 years prior to conducting underwater survey and sampling of hull fouling communities in the Port of Mar del Plata (star point) in June and September 2011. The area (shading) was drawn based on specific operation sites reported by the vessel in the same period (circle points). The area is approximate and no attempt was made to circumscribe it to exact vessel’s routes or sea contours. Ports mentioned in the main text and principal geographical references are indicated. The inset shows the location of the study area within South America and Antarctica
Sample collection
Sampling was conducted in three separate events, two in the water and one in dry-dock. In-water sampling of R/V Puerto Deseado was conducted by divers of the Servicio de Hidrografía Naval, belonging to the Argentine Ministry of Defence, during stationary mooring at the Navy Base of Mar del Plata (coordinates − 38.0348°, − 57.5352°) on 26 June and 9 September 2011 (see Rumbold et al. (2018) for a map of the sampling sites in the Port of Mar del Plata). On each occasion, two scuba divers in the water, supervised by a scientific team at the dock or a support boat, conducted a thorough inspection of the submerged surfaces of the hull looking for biofouling. The dive team was composed of professional Navy divers experienced with underwater hull inspections and port work. Prior to sampling, divers received training on the use of sampling tools and sampling tips based on previous experience of the scientific team. Divers collected samples scraping hull fouling organisms within 20 × 20 cm quadrats or equivalent surface area into zip-lock plastic bags, which were sealed and brought to the surface. A manual suction device was used to sample quadrats containing soft organisms which would otherwise disperse in the water (Sylvester & Floerl, 2014).
Dry-dock sampling was conducted by the scientific team in the Mar del Plata SPI Shipyard dry-dock (coordinates − 38.0504°, − 57.5364°) on 14 September 2011. Dry-dock collection of samples started 1–2 h after the vessel was out of the water and lasted 6 h. Dry-dock entrance and sampling took place entirely during night hours (i.e., surfaces and organisms sampled did not get sun exposition throughout the whole process). Sampling was conducted in essentially the same way as in the water, except that the scientific team (rather than the divers) conducted the work. The use of the suction tool was unnecessary in dry-dock. Both in-water and dry-dock samplings were opportunistic and divers or the scientific team collected samples from accessible spots where biofouling was present, avoiding spots previously sampled. Hull locations sampled both in the water and dry-dock included the rudder leading, trailing edges, and sides, propeller nose-cone and blades, rope guard, bow-thrusters, bulbous bow, keel, bilge keels, dry-docking support strips (ddss; rectangular patches on the hull’s bottom where dry-dock blocks supporting the vessel while out of the water were located, and which therefore were devoid of antifouling coatings), and the main hull shell. In dry-dock, gratings covering sea-chest inlets were also sampled. Divers and scientific team members were the same throughout all sampling events. All samples were immediately preserved in 90% ethanol.
Sample processing, taxonomic identification, and species non-native status
In the laboratory, samples were processed under a stereoscopic microscope to sort the invertebrates. All invertebrates > 2 mm were picked from the samples, while for invertebrates < 2 mm, 50 individuals belonging to each major taxonomic group or half of the sample (whichever happened first) were isolated for subsequent identification. The organisms were identified to the lowest taxonomic level possible (species, morphospecies, or higher taxonomic level (here-in referred to as higher level taxa)) by taxonomic experts in each group. Based on exhaustive revisions of the available literature by the expert in each group, species were classified into one of the following five categories: Native: species that have presumably originated in the area without human involvement or which could reasonably be assumed to have arrived there without the aid of human activities (modified from Pysek et al., 2004); Non-native reported: species that were not native for which there were previous reports in the area; Non-native not reported: species that were not native that had never been reported in the area prior to the present study; Cryptogenic reported: species that were not demonstrably native or non-native (adapted from Carlton, 1996) for which there were previous reports in the area; and Cryptogenic not reported: species that were not demonstrably native or non-native that had never been reported in the area prior to the present study. For each species, non-native status (native, non-native, or cryptogenic; and for the latter two, reported or not reported) was determined both for Mar del Plata, understood as the area comprising the port and its adjacent natural coastal habitats (status in Mar del Plata), and for the whole Argentine coast from Río de la Plata down to Tierra del Fuego excluding Mar del Plata (status on the Argentine coast). Organisms that could not be identified to the species level were not included in the categories above.
Species richness estimation and assessment of sampling efficiency
Sample-based rarefaction and extrapolation curves based on Hill numbers (Chao et al., 2016; Hsieh et al., 2016) were constructed to compare hull fouling richness between sampling dates at a comparable sampling effort (Gotelli & Colwell, 2011). Sample-based curves offered a more reliable estimation than individual-based curves, and were used here, as individuals exhibited aggregation within or between species (Gotelli & Colwell, 2011). Because the estimation of individual abundances could not be reliably achieved for some taxa (e.g., colonial taxa), richness estimations were based on incidence data. Given that the average number of species varied across sampling events, we rescaled the x-axis of rarefaction graphs to a common scale of species occurrences to make data comparable across sampling events (Gotelli & Colwell, 2001).
In order to estimate asymptotic richness, we calculated Chao2-bc values corresponding to each of the curves above. Chao2 is a non-parametric, incidence-data based estimator of species richness recommended when groups of multiple individuals (as opposed to individual specimens) are collected randomly, as in quadrat sampling (Gotelli & Colwell, 2011). Its more generally obtainable (e.g., when the data have no duplicates) biased-corrected form, Chao2-bc, was used to increase the amount of data available and comparability with other studies (Chao & Shen, 2010; Gotelli & Colwell, 2011).
While the identifications at the morphospecies level do not resolve the identity of the organisms, they represent distinct taxonomic units that carry information on total species richness. To have the most realistic estimation possible of hull fouling species richness in our samples, we calculated Chao2-bc based on the combination of species and morphospecies incidences for each sampling date. Despite the confidence of the identifications, morphospecies identifications might in some cases have a degree of uncertainty that positively identified species do not have. As this may vary across groups and (along with abundances) across sampling events, these estimations may not be adequate for comparisons among sampling events; for which aim, species-based estimations were considered better suited. When different sampling methods are used, the pooling of data from different methods has been suggested as a way to maximize the kinds of species that may be sampled (Gotelli & Colwell, 2011). To estimate the asymptotic value of richness including all sampling methods used, we calculated Chao2-bc on incidence based on the two (i.e., in-water and dry-dock) September datasets pooled. While this estimation is clearly not comparable with any of the others, it is here proposed as the most complete and realistic estimation of the true total hull fouling species richness that may be achieved with the data in hand for September.
Significant differences were tested using 95% confidence intervals calculated based on 50 (rarefaction curves) and 100 (Chao2-bc) random bootstrap samples. Analyses were performed using the online versions of iNEXT and SpadeR programs, available from Dr. Anne Chao’s website at http://chao.stat.nthu.edu.tw. Hydrozoa and Nematoda could not be assessed in some of the samples, and thus these taxa were excluded from all richness analyses (even when included in the taxa lists).
Results
Hull fouling taxa observed
We obtained a total of 39 hull fouling samples containing members of seven invertebrate Phyla: Cnidaria (Class Hydrozoa), Bryozoa (Class Gymnolaemata), Mollusca (Class Bivalvia), Annelida (Class Polychaeta), Nematoda (Classes Chromadorea and Enoplea), Arthropoda (Class Pycnogonida; Class Malacostraca, Orders Amphipoda, Tanaidacea, Cumacea, Isopoda, and Decapoda; Subclass Copepoda; Infraclass Cirripedia) and Chordata (Class Ascidiacea). Within them, 53 taxa were identified at the species level, 47 as morphospecies, and 20 at a higher taxonomic level (Tables 1 and 2). Bryozoans, polychaetes, amphipods, cirripeds, and hydroids were the taxa that included the most frequent species (average incidence 9–15), followed by decapods, bivalves, and pycnogonids (4–6), while isopod, ascidian, and copepod species were the least frequent (< 3) in our samples (Table 1). Nematodes, cumaceans, and tanaidaceans were also found in the samples, but no species could be identified (Table 2).
Non-native status of hull fouling species
Ten (19%) of the species found associated with underwater surfaces of R/V Puerto Deseado have never been reported in Mar del Plata prior to the present study (Table 1). These species are either previously unreported NIS (i.e., non-native not reported), or first reports of species of unknown non-native status (cryptogenic not reported) in this area (Table 1). About another 60% of the species found are either known resident NIS (non-native reported; 21 species) or previously reported cryptogenics (cryptogenic reported; 10 species) (Table 1). Less than a quarter (12 out of 53 species) of the species found on the hull of R/V Puerto Deseado are members of the confirmed native fauna in Mar del Plata (Table 1). In total, 13 (25%) of the species found would be altogether new reports for the Argentine coast outside of Mar del Plata (eight cryptogenic not reported and five non-native not reported; Table 1).
Observed taxonomic richness across sampling events
Only about a quarter of the species and 13% of the remaining taxa (morphospecies and higher level taxa) found were observed in all three sampling events (Tables 1 and 2). In general, the percentage of shared species between sampling events was low. Thus, the two in-water sampling events shared 43% of the species and 21% of the remaining taxa. The two consecutive (September) in-water and dry-dock sampling events shared 42% of the species and 26% of the morphospecies and higher level taxa (Tables 1 and 2). Most of the taxa collected in this study were observed in the dry-dock sampling. Only two species (the amphipod Caprella dilatata and the isopod Sphaeroma serratum) and eight of the remaining taxa identified (one each of cumacean, cirriped, nematode, polychaete, and tanaidacean, and three copepod taxa) were present in the samples collected in-water but absent from those collected in dry-dock (Tables 1 and 2). The dry-dock sampling yielded a larger number of samples (19) and observed taxa (51 species, 41 morphospecies, and 18 higher level taxa) than either in-water sampling (June sampling: 13 samples, 20 species, 22 morphospecies, and 12 higher level taxa; September sampling: seven samples, 23 species, 13 morphospecies, and five higher level taxa) (Tables 1 and 2; see Electronic Supplementary Materials 1 and 2 for incidence data break-down by sampling event). In contrast, in-water samplings did not capture 23 species, 18 morphospecies, and six higher level taxa, which were exclusively detected in dry-dock (Tables 1 and 2).
Estimated species richness and comparative sampling efficiency
Species accumulation curves suggested a sharp difference between the efficiency of in-water and dry-dock samplings (Fig. 2). While both September in-water and dry-dock samplings yielded saturated curves, in principle suggesting that a large proportion of the species present was captured, a significantly larger asymptotic diversity of organisms was obtained from the dry-dock samples (Fig. 2). In contrast, the June and September in-water species accumulation curves were generally similar, although the September curve grew faster (Fig. 2). Chao2-bc estimations confirmed these results (Fig. 3). Based solely on species, the dry-dock sampling yielded a significantly higher species richness estimate than any of the in-water samplings (65 species in dry-dock vs. 23–25 species in the in-water samplings), while there was no difference between hull fouling richness estimated during in-water sampling in June and September (Fig. 3a). Estimations based on the species and morphospecies present in our samples also yielded a higher species richness in dry-dock than the in-water sampling in September; these estimations indicate that the total hull fouling species richness approached 65 and 110 species in June and September, respectively (Fig. 3b). In September, the combination of in-water and dry-dock samples did not yield a species richness estimation significantly higher than using dry-dock samples only (Fig. 3b).
Species accumulation curves of hull fouling invertebrate communities sampled from submerged surfaces of R/V Puerto Deseado while the vessel was docked in the water and in dry-dock in the Port of Mar del Plata in June and September 2011. Curves are based solely on organisms identified at the species level. Shading indicates 95% confidence intervals. Solid lines are interpolated values and dotted lines are extrapolated values. The x-axis has been rescaled to a common axis of species occurrences (see “Methods” section for analysis details and references)
Chao2-bc estimated richness of hull fouling invertebrate communities based on invertebrate species (a) and on species and morphospecies (b) sampled from underwater surfaces of R/V Puerto Deseado while the vessel was docked in the water and in dry-dock in the Port of Mar del Plata in June and September 2011. Error bars are 95% confidence intervals. Non-overlapping intervals indicate significant differences between estimates (see “Methods” section for analysis details and references)
Discussion
We have conducted a thorough assessment of the invertebrate biofouling communities associated with the submerged hull of an oceanographic vessel, and for the first time, attempted a direct comparison between the performance of sampling in the water and in dry-dock. We found that the hull fouling communities transported by this vessel were lavish and largely nonindigenous, including many species previously unreported for Mar del Plata and the whole Argentine coast. This finding clearly highlights the potential of hull fouling on regional vessels for secondary spread of marine NIS. Dry-dock inspections detected a significantly higher number of species than in-water surveys, which has methodological implications for biosecurity monitoring. The present study substantially increases the list of known introduced species in the southwestern Atlantic, suggesting that hull surveys constitute a powerful tool to detect resident species and complete NIS marine fauna inventories.
Hull fouling risk for regional dispersal of marine NIS
Reported, non-reported NIS, and cryptogenic invertebrates were found in all three sampling events performed and accounted for three quarters of the species obtained from the hull of R/V Puerto Deseado in the Port of Mar del Plata. Our Chao2-bc richness estimate of 65 species based solely on organisms identified at the species level in dry-dock is clearly an underestimation. Estimations based on the species and morphospecies present in our samples suggest that true hull fouling invertebrate richness on this vessel was likely to be closer to 110 species. A number of higher level taxa found in the samples might contain additional species that could not be identified, and thus even this estimation is likely to be underestimated and should be considered a lower bound. Furthermore, samples contained organisms that are currently considered a single species but might cover species complexes. This is the case of ascidians of the genera Ascidiella (Nishikawa et al., 2014), Ciona (Brunetti et al., 2015), Diplosoma (Pérez-Portela et al., 2013), and Botryllus (Brunetti et al., 2017). The clarification of the taxonomic statuses of these species complexes is out of the scope of the present paper, but most likely additional native and non-native species will be incorporated to our list, and richness estimates will increase, when these are resolved. The present results clearly indicate a high potential of hull fouling on domestic vessels for regional dispersal of marine organisms.
In-water vs. dry-dock assessments
Dry-dock sampling yielded significantly higher hull fouling richness estimates than in-water sampling. Over 95% of the species detected in our study have been observed during dry-dock sampling, including 23 species (43% of total observed diversity) that were not recorded in either of the preceding in-water samplings. Six of these, Bugulina simplex (Bryozoa), Helmutkunzia variabilis, Pholenota spatulifera, Schizopera carolinensis, Parapseudoleptomesochra dubia, and Drescheriella glacialis (Copepoda), are potentially new records for the Port of Mar del Plata and adjacent areas. The single specimen of the isopod marine invader Dynamene edwardsi present in our samples has been the first record of this species across the Americas (this occurrence has been reported and analyzed, in conjunction with harbor populations, in a separate paper (Rumbold et al., 2018)). Another 12 species detected during dry-dock sampling and unseen during in-water sampling are cryptogenic or reported NIS in Mar del Plata. Thus, in-water samplings missed at least 19 species potentially posing a biosecurity risk to Mar del Plata coastal ecosystems. While in-water samplings also yielded species absent in the dry-dock samples (the reported cryptogenic amphipod Caprella dilatata, the reported NIS isopod Sphaeroma serratum, one unidentified cumacean, and notably the probably new tanaidacean Hexapleomera sp. 1; see Electronic Supplementary Material 3), the efficiency of dry-dock sampling was clearly higher. The fact that adding in-water samples to those retrieved in dry-dock did not significantly increase the hull fouling richness estimated in September, suggests that the diversity that can typically be detected during in-water survey of a vessel, would essentially be seen in a dry-dock inspection of the same vessel; while the reverse does not seem to be true.
The September dry-dock sampling was carried out only 5 days after sampling in the water, which precludes the possibility of significant community changes between the two, including a ca. threefold estimated diversity increase. In roughly the same time (a full 8-h working day from the time the sampling team arrived in the port facility until we left with the samples), we were able to collect almost twice as many samples in dry-dock as in the water. Importantly, divers were not able to sample sea-chest gratings in either in-water sampling, while they were sampled in dry-dock. These locations constitute important refugia for hull fouling fauna (Coutts & Dodgshun, 2007; Frey et al., 2014) and may conceivably account for a part of the higher diversity observed in dry-dock. Still, the magnitude of the observed differences between both samplings cannot be solely explained by the number of locations sampled, but are most likely also due to the fact that in-water sampling is less efficient than dry-dock sampling also in terms of both the total number of samples obtained and the number and diversity of organisms present in those samples (Sylvester & Floerl, 2014).
Low efficiency of in-water dive samplings is most probably associated with difficulties related to working underwater. The ability to maintain a stable position relative to the hull and movement-precision are greatly reduced underwater as compared with conducting the work firmly and comfortably standing on a crane, ladder, or other surface in the dry-dock. Flotation and dispersal of organisms in the water is also a common source of loss when divers scrape buoyant biofouling (authors’ personal observation). Diver travels to the surface to deliver samples slow down sample collection. Wave, current action, water turbidity, and cumbersome diving gear aggravate these difficulties. To mitigate them, we used divers that were experienced with underwater hull surveys, familiar with the vessel sampled, and supervised by a team experienced with hull fouling sampling, and avoided diving in rough weather. A syringe sampling device allowed us to efficiently sample soft, small, and buoyant organisms. While sampling efficiency likely varies according to the sampling tools and protocols used, our results suggest that the loss of fouling organisms can be significant in the water. On top of this, properly trained and licensed dive teams are costly (Sylvester & Floerl, 2014). The main drawbacks of dry-dock sampling include that it obviously requires a dry-dock entry of the vessel, which being often short-notice internal decisions not always permit the organization of a sampling campaign (Sylvester & Floerl, 2014).
Seasonal species turnaround in hull fouling communities
The most remarkable temporal pattern we found was the high taxonomic turnover rate observed between the June and September samplings. In barely 75 days, ca. 60% of the species and almost 80% of the morphospecies and higher level taxa observed in in-water sampling were replaced. It is not totally clear whether overall richness remained virtually unchanged (Fig. 3a) or decreased (Fig. 3b) between sampling periods. The shape of the rarefaction curves suggests that species were spatially more evenly distributed in September than June, as in the former month species accumulated faster in the samples (Gotelli & Colwell, 2011). It is known that sailing, even at moderate speeds, can shift the abundance (percentage cover) and composition of hull fouling communities (Davidson et al., 2008; Coutts et al., 2010). Twelve-day continuous sailing without port stops at a typical speed of 10 knots with peaks of up to 13 knots (see “Methods” section) could have caused some of the compositional faunal changes observed between periods. Stationary permanence in port for the remaining time opened a window for recolonization by the same or new species, as well as for additional losses to predation. The seasonal change (southern hemisphere winter to early spring) likely also had an effect. Unfortunately, barely two points in time do not warrant further analysis of the mechanisms underlying the compositional changes observed.
Marine invasions in the southwestern Atlantic
The present findings are unfortunately spectacular, and echo those of the classic review paper by Orensanz et al. (2002) that, based on the finding of 31 NIS, concluded that the Patagonian coast could no longer be considered a remote, pristine region. A recent review substantially updates these figures to 129 introduced and 72 cryptogenic marine species (of which 146 are invertebrates) in the southwestern Atlantic (Schwindt et al., 2020). The present study has found (living on the vector) 10 additional non-native and cryptogenic invertebrates not previously reported in Mar del Plata, eight potential new records for the whole Argentine coast, and one species likely altogether new to science (see detailed considerations and references pertaining to these species in Electronic Supplementary Materials 3 and 4). Recent comprehensive sampling aiming to monitoring marine bioinvasions using colonization plates in combination with scuba-dive rapid-assessment surveys in six Patagonian ports found a single new NIS (Schwindt et al., 2014). Interestingly, the vessel presently surveyed serves routes that, with the exception of the sails between Ushuaia and Antarctica, largely match the geographic range of this multi-port, plate survey (Schwindt et al., 2014). As opposed to immobile plates, an active vessel can integrate hull fouling organisms across several ports. This might explain the relative large amount of species detected by vessel hull surveys (Castro et al., 2020; current results). Consequently, hull surveys should be considered a powerful complementary tool for the detection of introductions at both small and large geographical scales. The drawbacks of this method are that the presence of a species on the vector does not guarantee its occurrence in coastal habitats (Marchini et al., 2015) nor provides conclusive information on the location of the populations sampled by it, and it thus does not replace detailed port studies (e.g., Schwindt et al., 2014).
Conclusions
A first obvious conclusion of our study is that dry-dock sampling is preferable when NIS detection is the goal (Smith et al., 2016), although it is not always achievable. Furthermore, prior in-water assessments based on similar methods could have underestimated hull fouling diversity (Davidson et al., 2009; Sylvester & MacIsaac, 2010; Sylvester et al., 2011; Peters et al., 2019) as compared to dry-dock estimations (Gollasch, 2002; Drake & Lodge, 2007). As a result, even if hull fouling has long been a well-recognized vector for marine introductions, its potential for secondary introductions had not been clearly ascertained until now, and its strength may have been underestimated in the past. Our results forecast a substantial further increase in the list of known NIS in the Argentine coast. Yet more research is needed before we have a complete picture of native and introduced fauna in the southwestern Atlantic, and are in a position to answer questions such as whether mounting trends in NIS discovery is due to accelerating invasion rates fuelled by increasing pathways and vector strength, or to accelerating discovery rates resulting from growing scientific studies.
References
Ammon, U., L. Swift, S. Brand, A. Jeffs, S. Swift & B. Dunphy, 2018. Review of in-water hull encapsulation and enclosure treatments for eliminating marine biofouling. Technical Report, Ministry for Primary Industries, New Zealand Government.
Brine, O., L. Hunt & M. J. Costello, 2013. Marine biofouling on recreational boats on swing moorings and berths. Management of Biological Invasions 4: 327–341.
Brunetti, R., C. Gissi, R. Pennati, F. Caicci, F. Gasparini & L. Manni, 2015. Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis. Journal of Zoological Systematics and Evolutionary Research 53: 186–193.
Brunetti, R., L. Manni, F. Mastrototaro, C. Gissi & F. Gasparini, 2017. Fixation, description and DNA barcode of a neotype for Botryllus schlosseri (Pallas, 1766) (Tunicata, Ascidiacea). Zootaxa 4353: 29–50.
Carlton, J. T., 1996. Biological invasions and cryptogenic species. Ecology 77: 1653–1655.
Carlton, J. T. & J. Hodder, 1995. Biogeography and dispersal of coastal marine organisms: experimental studies on a replica of a 16th-century sailing vessel. Marine Biology 121: 721–730.
Castro, M. C. T. D., T. W. Fileman & J. M. Hall-Spencer, 2017. Invasive species in the Northeastern and Southwestern Atlantic Ocean: A review. Marine Pollution Bulletin 116: 41–47.
Castro, K. L., C. B. Giachetti, N. Battini, A. Bortolus & E. Schwindt, 2020. Cleaning by beaching: introducing a new alternative for hull biofouling management in Argentina. Aquatic Invasions 15: 63–80.
Chao, A. & T.-J. Shen, 2010. Program SPADE (Species Prediction and Diversity Estimation). Program and User’s Guide. http://chao.stat.nthu.edu.tw.
Chao, A., K. H. Ma & T. C. Hsieh, 2016. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. http://chao.stat.nthu.edu.tw/wordpress/software_download/.
Clarke Murray, C., E. A. Pakhomov & T. W. Therriault, 2011. Recreational boating: a large unregulated vector transporting marine invasive species. Diversity and Distributions 17: 1161–1172.
Clarke Murray, C., T. W. Therriault & E. Pakhomov, 2013. What lies beneath? An evaluation of rapid assessment tools for management of hull fouling. Environmental Management 52: 374–384.
Coutts, A. D. M. & T. J. Dodgshun, 2007. The nature and extent of organisms in vessel sea-chests: a protected mechanism for marine bioinvasions. Marine Pollution Bulletin 54: 875–886.
Coutts, A. D. M. & M. D. Taylor, 2004. A preliminary investigation of biosecurity risks. New Zealand Journal of Marine and Freshwater Research 38: 215–229.
Coutts, A. D. M., R. F. Piola, M. D. Taylor, C. L. Hewitt & J. P. A. Gardner, 2010. The effect of vessel speed on the survivorship of biofouling organisms at different hull locations. Biofouling 26: 539–553.
Davidson, I. C., L. D. McCann, P. W. Fofonoff, M. D. Sytsma & G. M. Ruiz, 2008. The potential for hull-mediated species transfers by obsolete ships on their final voyages. Diversity and Distributions 14: 518–529.
Davidson, I. C., C. W. Brown, M. D. Sytsma & G. M. Ruiz, 2009. The role of containerships as transfer mechanisms of marine biofouling species. Biofouling 25: 645–655.
Drake, J. M. & D. M. Lodge, 2007. Hull fouling is a risk factor for intercontinental species exchange in aquatic ecosystems. Aquatic Invasions 2: 121–131.
Farinati, E. A., 1985. Paleontología de los sedimentos marinos holocenos de los alrededores de Bahía Blanca, Provincia de Buenos Aires. Ameghiniana 21: 211–222.
Floerl, O. & A. D. M. Coutts, 2013. Feasibility of using remote-operated vehicles (ROVs) for vessel biofouling inspections. Fisheries Occasional Publication 117: 54.
Frey, M. A., N. Simard, D. D. Robichaud, J. L. Martin & T. W. Therriault, 2014. Fouling around: vessel sea-chests as a vector for the introduction and spread of aquatic invasive species. Management of Biological Invasions 5: 21–30.
Gollasch, S., 2002. The importance of ship hull fouling as a vector of species introductions into the North Sea. Biofouling 18: 105–121.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Gotelli, N. J. & R. K. Colwell, 2011. Estimating species richness. In Magurran, A. E. & B. J. McGill (eds.), Biological Diversity. Frontiers in Measurement and Assessment. Oxford University Press, Oxford: 39–54.
Hsieh, T. C., K. H. Ma & A. Chao, 2016. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods in Ecology and Evolution 7: 1451–1456.
Kalaci, O., 2011. Hull fouling as an invasion vector: comparison of in-water and dry-dock sampling methods. Honors Thesis, University of Windsor, Canada.
Kelly, N. E., K. Wantola, E. Weisz & N. D. Yan, 2013. Recreational boats as a vector of secondary spread for aquatic invasive species and native crustacean zooplankton. Biological Invasions 15: 509–519.
López-Legentil, S., M. L. Legentil, P. M. Erwin & X. Turon, 2015. Harbor networks as introduction gateways: contrasting distribution patterns of native and introduced ascidians. Biological Invasions 17: 1623–1638.
Martínez-Laiz, G., A. Ulman, M. Ros & A. Marchini, 2019. Is recreational boating a potential vector for non-indigenous peracarid crustaceans in the Mediterranean Sea? A combined biological and social approach. Marine Pollution Bulletin 140: 403–415.
Marchini, A., B. S. Galil & A. Occhipinti-Ambrogi, 2015. Recommendations on standardizing lists of marine alien species: lessons from the Mediterranean Sea. Marine Pollution Bulletin 101: 267–273.
McCollin, T. & L. Brown, 2014. Native and non native marine biofouling species present on commercial vessels using Scottish dry docks and harbours. Management of Biological Invasions 5: 85–96.
Nishikawa, T., I. Oohara, K. Saitoh, Y. Shigenobu, N. Hasegawa, M. Kanamori, K. Baba, X. Turon & J. D. D. Bishop, 2014. Molecular and morphological discrimination between an invasive ascidian, Ascidiella aspersa, and its congener A. scabra (Urochordata: Ascidiacea). Zoological Science 31: 180–185.
Orensanz, J. M., E. Schwindt, G. Pastorino, A. Bortolus, G. Casas, G. Darrigran, R. Elías, J. López Gappa, S. Obenat, M. Pascual, P. Penchaszadeh, M. L. Piriz, F. Scarabino, E. D. Spivak & E. A. Vallarino, 2002. No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biological Invasions 4: 115–143.
Pérez-Portela, R., V. Arranz, M. Rius & X. Turon, 2013. Cryptic speciation or global spread? The case of a cosmopolitan marine invertebrate with limited dispersal capabilities. Scientific Reports 3: 1–10.
Peters, K., K. J. Sink & T. B. Robinson, 2019. Sampling methods and approaches to inform standardized detection of marine alien fouling species on recreational vessels. Journal of Environmental Management 230: 159–167.
Pysek, P., D. M. Richardson, M. Rejmanek, G. L. Webster, M. Williamson & J. Kirschnerl, 2004. Alien plants in checklists and floras: towards better communication between taxonomists and ecologists. Taxon 53: 131–143.
Ruiz, G. M., P. W. Fofonoff, B. P. Steves & J. T. Carlton, 2015. Invasion history and vector dynamics in coastal marine ecosystems: a North American perspective. Aquatic Ecosystem Health and Management 18: 299–311.
Rumbold, C., M. Meloni, B. Doti, N. Correa, M. Albano, F. Sylvester & S. Obenat, 2018. Two new nonindigenous isopods in the Southwestern Atlantic: simultaneous assessment of population status and shipping transport vector. Journal of Sea Research 138: 1–7.
Rup, M. P., S. A. Bailey, C. J. Wiley, M. S. Minton, A. W. Miller, G. M. Ruiz & H. J. MacIsaac, 2010. Domestic ballast operations on the Great Lakes: potential importance of Lakers as a vector for introduction and spread of nonindigenous species. Canadian Journal of Fisheries and Aquatic Sciences 67: 256–268.
Schwindt, E. & A. Bortolus, 2017. Aquatic invasion biology research in South America: geographic patterns, advances and perspectives. Aquatic Ecosystem Health & Management 20: 322–333.
Schwindt, E., J. T. Carlton, J. M. Orensanz, F. Scarabino & A. Bortolus, 2020. Past and future of the marine bioinvasions along the Southwestern Atlantic. Aquatic Invasions 15: 1–19.
Schwindt, E., J. López Gappa, M. P. Raffo, M. Tatián, A. Bortolus, J. M. Orensanz, G. Alonso, M. E. Diez, B. Doti, G. Genzano, C. Lagger, G. A. Lovrich, M. L. Piriz, M. M. Mendez, V. Savoya & M. C. Sueiro, 2014. Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Marine Environmental Research 99: 60–68.
Smith, M., G. J. Inglis, S. Wilkens & S. McDonald, 2016. Emergency surveillance for marine pests after the grounding of the container vessel, MV Rena. New Zealand Journal of Marine and Freshwater Research 50: 42–55.
Sylvester, F. & O. Floerl, 2014. Assessment of in-service vessels for biosecurity risk. In Dobretsov, S., J. C. Thomason & D. N. Williams (eds.), Biofouling Methods. Wiley, Chichester: 271–280.
Sylvester, F. & H. J. MacIsaac, 2010. Is vessel hull fouling an invasion threat to the Great Lakes? Diversity and Distributions 16: 132–143.
Sylvester, F., O. Kalaci, B. Leung, A. Lacoursière-Roussel, C. C. Murray, F. M. Choi, M. A. Bravo, T. W. Therriault & H. J. Macisaac, 2011. Hull fouling as an invasion vector: can simple models explain a complex problem? Journal of Applied Ecology 48: 415–423.
Zabin, C. J., G. V. Ashton, C. W. Brown, I. C. Davidson, M. D. Sytsma & G. M. Ruiz, 2014. Small boats provide connectivity for nonindigenous marine species between a highly invaded international port and nearby coastal harbors. Management of Biological Invasions 5: 97–112.
Zabin, C. J., I. C. Davidson, K. K. Holzer, G. Smith, G. V. Ashton, M. N. Tamburri & G. M. Ruiz, 2018. How will vessels be inspected to meet emerging biofouling regulations for the prevention of marine invasions? Management of Biological Invasions 9: 195–208.
Acknowledgements
We are thankful to the Argentine Navy (Armada Argentina, Ministerio de Defensa de la Nación Argentina), R/V Puerto Deseado’s crews and divers for their assistance and support during planning and conduction of sampling. Rosana Rossini, Aurora Matsubara, Silvia Rivero, and Claudia Bremec helped with sample processing and taxonomic identification of organisms. We thank Anne Chao for kindly making freely available the statistical programs used in this work and providing statistical advice. Demetrio Boltovskoy and two anonymous reviewers provided most valuable suggestions to the original study design and to earlier versions of this manuscript. Funding was provided by PICT 0729 research grant from the Argentine Agencia Nacional de Promoción Científica y Tecnológica to F. S. and an Estímulo research scholarship from Universidad de Buenos Aires awarded to M. M. during the conduction of the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Katya E. Kovalenko, Fernando M. Pelicice, Lee B. Kats, Jonne Kotta & Sidinei M. Thomaz / Aquatic Invasive Species III
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meloni, M., Correa, N., Pitombo, F.B. et al. In-water and dry-dock hull fouling assessments reveal high risk for regional translocation of nonindigenous species in the southwestern Atlantic. Hydrobiologia 848, 1981–1996 (2021). https://doi.org/10.1007/s10750-020-04345-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04345-4