Abstract
Morphological approaches may not provide sufficient resolution for species delineation. Thus, we used an integrated approach that included molecular and ecological characters as well as morphological features to gain a better estimate of species diversity and to improve our understanding of the speciation process within rotifers. Previously, seven putative cryptic species were found within Euchlanis dilatata complex based on a nuclear marker. Here, we investigated reproductive isolation, variation in trophi morphology, and life history characteristics among representatives of some of these species. Mating success between each cryptic species was 0–1.1%; lower than that of positive controls (intra-clonal: 15.6–43.9%). SEM trophi images representing individuals from all seven lineages were used for morphometric analyses. Using Discriminant Analysis, 64% of individuals were correctly assigned to cryptic species. Five clonal lineages, each representing a putative species, were used in life table experiments with varying temperature and conductivity. Age-specific fecundity increased under high temperature and life expectancy decreased under high temperature and high conductivity. There was variation among cryptic species in some life table characteristics, such as life expectancy and generation time. Applying an integrative taxonomy approach, we examined the boundaries between E. dilatata cryptic species and described four of them as new species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reliable species delineation can be obtained using integrative taxonomy that implements various complementary approaches (Dayrat, 2005); this is especially true for understudied taxa such as microorganisms. Relying solely on morphology to designate species of microorganisms can be misleading and likely does not reflect true levels of species richness and biodiversity as there may not be sufficient recognizable morphological variation among them (Kaya et al., 2009). As a consequence, morphologically similar forms and cryptic species are often lumped together taxonomically (Finlay, 2002).
Rotifers are ubiquitous microorganisms inhabiting various aquatic habitats, making them one of the major constituents of freshwater zooplankton communities (Segers, 2008; Wallace & Snell, 2014). Cryptic diversity can be high in rotifers as they lack easily recognizable morphological characters (Segers, 2008; Fontaneto et al., 2009). Cryptic species in most taxa, including rotifers, are primarily found using molecular analyses (Fontaneto et al., 2015). Most cryptic species within Rotifera have remained at the putative species level and have not been established as new species. Some exceptions include members of the Epiphanes senta species complex. Epiphanes hawaiiensis Schröder & Walsh, 2007, E. ukera Schröder & Walsh, 2007, and E. chihuahuaensis Schröder & Walsh, 2007 were described using cross-mating experiments, and morphological and molecular analyses (Schröder & Walsh, 2007). Similarly, Brachionus manjavacas Fontaneto, Giordani, Melone & Serra, 2007 (Fontaneto et al., 2007a) and B. koreanus Hwang, Dahms, Park & Lee, 2013 (Hwang et al., 2013) in the B. plicatilis species complex were described based on molecular and morphological analyses. Integrating molecular and morphological approaches (Papakostas et al., 2016; Michaloudi et al., 2018) resulted in description of Brachionus elevatus Michaloudi, Papakostas, Stamou, Neděla, Tihlaříková, Zhang, Declerck, 2018 and B. fernandoi Michaloudi, Papakostas, Stamou, Neděla, Tihlaříková, Zhang, Declerck, 2018.
Although DNA taxonomy is not always sufficient to define species boundaries, it can be considered an effective tool to complement morphology-based taxonomy (Packer et al., 2009). DNA taxonomy provides hypotheses about species that can be tested with morphological, behavioral, ecological, and mate choice studies, and to eventually describe cryptic species. Using several lines of evidence to describe cryptic species aids in substantiating the taxonomic status of new species.
Genetic studies are only capable of indicating whether or not cryptic species have exchanged genetic materials. However, it is not possible to determine whether they have lost the ability for such exchange (Bickford et al., 2007). Mate choice experiments can examine the strength of reproductive isolation among cryptic species (e.g., in rotifers: Gómez et al., 1995; Gilbert & Walsh, 2005; Schröder & Walsh, 2007, 2010; Wiwegweaw et al., 2009). Although mating experiments have the potential to confirm whether genetic variation among cryptic species of monogonont rotifers is accompanied by reproductive isolation (Rico-Martinez & Snell, 1995; Schröder & Walsh, 2007), few studies have focused on examining this among rotifer cryptic species. For example, Xiang et al. (2011) carried out cross-mating experiments among cryptic species of Brachionus calyciflorus Pallas, 1766 and considered the production of resting eggs as the indicator of successful mating. Others have studied pre- and post-mating reproductive isolation among cryptic species (e.g., B. plicatilis species complex: Gómez et al., 1995; Rico-Martinez & Snell, 1995; Gómez & Snell, 1996; Suatoni et al., 2006; B. calyciflorus species complex: Gilbert & Walsh, 2005; E. senta species complex: Schröder & Walsh, 2007, 2010). Examining reproductive isolation and its drivers can increase confidence in delimiting species boundaries and furthermore describing them as new species.
Studying morphological variation is another approach that can complement results obtained through DNA taxonomy. For rotifers, morphological variation among cryptic species has been found in some complexes. For example in the B. plicatilis complex, variation in lorica size was used to differentiate between B. plicatilis and B. rotundiformis Tschugunoff, 1921 (Fu et al., 1991). Similarly, lorica size and spine patterns vary between B. rotundiformis and B. ibericus Ciros-Pérez, Gómez & Serra, 2001 (Ciros-Pérez et al., 2001). Hwang et al. (2013) showed that B. koreanus has a smaller lorica with shorter distances between pairs of inner and outer anterior spines as compared to B. plicatilis. In the E. senta complex, morphological variation in trophi and resting egg features, along with DNA evidence and cross-mating experiments, led to the designation of new species (Schröder & Walsh, 2007, 2010). In bdelloid rotifers, Fontaneto et al. (2007b) found variation in trophi size and shape within seven bdelloid morphospecies. However in other studies, no robust morphological variation was found among cryptic species. For instance, Fontaneto et al. (2007a) noted there are no robust morphological differences between B. plicatilis and B. manjavacas based on geometric morphometric analyses of lorica and trophi. Leasi et al. (2013) did not find any significant variation in morphological features of lorica and body size among cryptic species of Testudinella clypeata (Müller, 1786). In a study by Malekzadeh-Viayeh et al. (2014), there was no significant variation in lorica morphology between B. plicatilis ‘Tiscar’ and a putative new lineage from Iran. Although morphological analyses may not always be sufficient to represent all of the diversity that is identified through molecular methods. However in some cases, they can be applied to distinguish among and to help describe some cryptic species of rotifers.
Ecological niche differentiation can promote reproductive isolation and consequently speciation (Schluter, 2001). By incorporating physical and chemical features of the habitat—in the study of cryptic species, it is possible to examine whether occurrence of a cryptic species is correlated to specific environmental factors (e.g., Ortells et al., 2003; Dennis & Hellberg, 2010; Obertegger et al., 2012; Papakostas et al., 2013; Zhang et al., 2015), and beyond that, whether ecological differentiation is a driver of speciation (e.g., Liu et al., 2013; Fouet et al., 2017). Moreover, investigating ecological adaptation among cryptic species can test the species boundaries specified by molecular methods (Rissler & Apodaca, 2007). Temperature is an environmental factor that affects age-specific survivorship and reproduction rate of zooplankton, including rotifers (e.g., Galkovskaja, 1987; Ma et al., 2010; Johnston & Snell, 2016). Furthermore, variation in response to temperature has been documented among some cryptic species from a variety of protists and rotifers (e.g., 15 protist species: Rose et al., 2008; dinoflagellate: Akashiwo sanguinea species complex: Luo et al., 2017; rotifers: B. ibericus, B. plicatilis, and B. rotundiformis: Gómez et al., 1995; B. calyciflorus: Li et al., 2010). While the relationship between conductivity and zooplankton species composition and abundance has also been well studied (e.g., Bos et al., 1996; Soto & Rios, 2006; Çelik & Ongun, 2007; Sousa et al., 2008; Walsh et al., 2008; Fatema et al., 2016; Celewicz-Goldyn & Kuczynska-Kippen, 2017), few studies have addressed the differential response to conductivity among cryptic species. Pfenninger & Nowak (2008) showed that two cryptic species of the midge Chironomus piger Strenzke, 1956 can tolerate higher levels of conductivity compared to C. riparius (Meigen, 1804). Obertegger et al. (2014) found that the occurrence of cryptic species in the rotifer Polyarthra dolichoptera Bartoš, 1951 in high-altitude lakes was related to conductivity as well as to longitude, and silica concentrations. Differential response of cryptic species to conductivity levels can contribute to ecological niche differentiation, thus providing information on ecological variation among them.
Despite the observed reproductive isolation and variation in ecological adaptation among cryptic species of rotifers, many of them are not described. In a few studies where cryptic species were described (see above), morphological variation has been found among them. Cryptic species are likely to be left unnamed if they are not morphologically distinguishable. This cryptic diversity can be described based on their diagnostic nucleotides. This method has been successfully used to describe cryptic species of other taxa (e.g., skipper butterfly Astraptes fulgerator (Walch, 1775), Brower, 2010; sea snail Alviniconcha hessleri Okutani & Ohta, 1988, Johnson et al., 2015; amphipod Niphargus stygius (Schiödte, 1847), Delic et al. 2017) and has been recommended for this purpose by Jörger & Schrödl (2013).
In this study, we investigated reproductive isolation and variation in trophi morphology among members of the Euchlanis dilatata Ehrenberg, species complex. In addition, we conducted life table experiments to examine variation in survivorship, fecundity, generation time, net reproductive rate, and intrinsic rate of population increase among five cryptic species of the E. dilatata complex in response to water temperature and conductivity. We used diagnostic nucleotides in the ITS region to describe four E. dilatata cryptic species. Finally, a neotype for the species was designated.
Materials and methods
In a study by Kordbacheh et al. (2017), ITS region and COI gene sequences from 62 populations of the Euchlanis dilatata species complex were analyzed to measure genetic differentiation and identify cryptic species. Molecular species delimitation was based on ITS region sequences and at least seven putative cryptic species were found. Clonal isolates derived from populations representing selected cryptic species (previously included in the molecular analysis) were used in the following experiments. Populations were selected based on availability of laboratory cultures. Additional details are available in Supplemental Document S1 (Figure S1.1, Table S1.2) and Kordbacheh et al. (2017).
Cross-mating experiments
Clonal lineages from 11 populations of the Euchlanis dilatata complex were used in mating experiments within and between six putative cryptic species (Table 1). Each clonal lineage was initiated using one asexual female. Clonal lineages were cultured in modified MBL media (Stemberger, 1981) at room temperature and were fed a mixture of the algae Rhodomonas minuta Skutja, 1948, Chlorella vulgaris Berijerinck, 1890 (Culture Collection of Algae at the University of Texas at Austin [UTEX] strain 30) and Chlamydomonas reinhardtii Dangeard, 1888 (UTEX strain 90).
To conduct mating experiments, female embryos and males were isolated from cultures that were mictic under laboratory conditions. We did not control for male age, however slow swimming males (indicative of old age) were not used. Two female embryos and two males were used in each mating trial. Females and males obtained from the same clonal lineage (intra-clonal) were included as positive controls. For cross-mating experiments (inter-clonal), females and males were obtained from different lineages. Trials were checked after 48 h and the success of mating (e.g., the production of resting eggs) was recorded. If the female embryos did not hatch or the neonate produced amictic eggs, the trial was excluded from statistical analyses. Cross-mating experiments were reciprocal, for example between males from lineage ‘A’ and females from lineage ‘B’ and vice versa. We conducted six combinations of mating experiments between putative cryptic species that were either sister groups (e.g., species ‘A’ and ‘B’, see Supplemental Document S1, Figure S1.1) or had low genetic distance based on the ITS region sequences (≤ 3.2% e.g., species ‘C’ and ‘D’, Supplemental Document S1, Table S1.2). The genetic distance in ITS region sequences was substantial between cryptic species ‘F’ and the other five cryptic species, ranging from 9.7 to 10.3%. We crossed this species with species ‘E’ (genetic distance: 10.3%) and species ‘D’ (genetic distance: 9.7%). Species ‘E’ was crossed with species ‘C’ and ‘D’ as well. We also used four inter-clonal combinations of mating experiments within cryptic species. Moreover, 11 intra-clonal combinations were used as positive controls (Tables 2, 3). Success of each mating experiment was determined by dividing the number of successful trials (e.g., fertilized resting egg produced) by the total number of trials. Inter-clonal mating success was compared to success found in positive controls. To test whether there was a significant difference in mating success between inter-clonal and intra-clonal trials, Pearson’s χ2 test was implemented in RStudio v 1.1.383 (RStudio Team, 2016). Clonal lineages were considered to be reproductively isolated when inter-clonal mating success rate was significantly lower than intra-clonal mating success rate.
Trophi morphology
Trophi, frontal and caudal view, for populations representing all seven putative cryptic species from the E. dilatata complex were prepared for scanning electron microscopy (SEM) by dissolving rotifer tissue in ~ 5% sodium hypochlorite, extracting the trophi and rinsing them with deionized water approximately 15 times, and air-drying them on circular cover slips at room temperature (modified from Segers, 1993). Trophi were coated with gold/palladium using a Gatan 682 PECS sputter coater. A Hitachi S-4800 scanning electron microscope located in UTEP’s Metallurgical, Materials and Biomedical Engineering department was used to obtain SEM images at 20 kV.
Images of trophi were observed and examined for features to distinguish among putative cryptic species. Length of rami, manubria, and fulcrum were measured for 175 trophi representing seven putative cryptic species (‘A’ (n = 32), ‘B’ (n = 4), ‘C’ (n = 44), ‘D’ (n = 23), ‘E’ (n = 32), ‘F’ (n = 36), and ‘G’ (n = 4)) using a Zeiss Axioscope equipped with a SPOT camera and Basic Software v 5.2 (Diagnostic Instruments, Inc). Discriminant Analysis in SPSS v 24.0 (IBM Corp, 2016) was used to determine the percentage of individuals that were correctly assigned to cryptic species based on the size of measured trophi elements.
Life table experiments
Five clonal lineages representing five putative cryptic species from the E. dilatata complex collected from the Rio Grande at Williamsburg, Sierra Co., NM (cryptic species ‘A’), the Rio Grande at American Dam, El Paso Co., TX (cryptic species ‘C’), a former cattle tank, White Sands National Monument, Doña Ana Co., NM (cryptic species ‘D’), a small temporary pond at Cinnamon Bay Beach, St. John, U.S. Virgin Islands (cryptic species ‘E’), and a pond alongside Krome Ave., Miami-Dade Co., FL (cryptic species ‘F’) were selected for life table experiments. Populations from cryptic species ‘B’ and ‘G’ were not available in the laboratory at the time of the experiments. Age-specific survivorship (lx), fecundity (mx), generation time (T), net reproductive rate (R0), and the intrinsic rate of population increase (r) were examined under four conditions: (1) Low temperature, low conductivity (tc: 20°C, conductivity at 180 µS/cm), (2) Low temperature, high conductivity (tC: 20°C, conductivity at 1800 µS/cm), (3) High temperature, low conductivity (Tc: 27°C, conductivity at 180 µS/cm), and (4) High temperature, high conductivity (TC: 27°C, conductivity at 1800 µS/cm). The high values for water temperature and conductivity in these treatments were greater than what was recorded in the field for most studied populations. In field collections where members of the E. dilatata species complex were found, the highest recorded conductivity was in the Rio Grande at Williamsburg, NM (~ 1,600 uS/cm); this population was also found at the highest water temperature, 27.7°C. The conductivity of modified MBL medium was 180 µS/cm. To raise the conductivity to 1800 µS/cm, 0.73 g NaCl was added to 1 L of MBL medium. Each treatment initially consisted of four replicates, each with nine females. Female neonates (< 6 h old) were placed into nine well culture plates with 0.8 ml of modified MBL medium containing the alga C. reinhardtii (500,000 ± 50,000 cell/ml) and were incubated using a 18:6 h light:dark photoperiod. Females were checked every 12 h to determine survival and the number of eggs and offspring that were produced until the death of the last individual. Offspring were removed from the culture plates as they hatched. Females were moved to new food-containing medium every 24 h. Mictic females, females lost during the experiment, or those that died in the first 24 h were excluded from the analyses, as were replicates with fewer than five individuals. Therefore in the final analysis, the number of females in each replicate ranged from 5 to 9. The following formulae were used to calculate age-specific survivorship (lx), fecundity (mx), net reproductive rate (R0), generation time (T), and intrinsic rate of population increase (r):
where x is time (day), nx is the number of females, sx is the number of survivors, and bx is the number of offspring produced each day.
We used a Linear Mixed-Effects Model in RStudio v 1.1.383 (RStudio Team, 2016) with fixed effects of temperature, conductivity, cryptic species, and their interactions to determine differences in lxmx and ex; variation among individuals was considered as a random effect. To determine significant differences in generation time (T), net reproductive rate (R0), and intrinsic rate of population increase (r) among cryptic lineages we used the exact permutation test in R v 3.4.3 (R core team, 2017).
Species accounts
We described cryptic species ‘B’, ‘C’, ‘D’, and ‘F’ from the E. dilatata complex (Kordbacheh et al., 2017) following Jörger & Schrödl (2013). In the study by Kordbacheh et al. (2017) mitonuclear discordance was found, thus species delimitation was based on the ITS marker as it yielded more conservative results as compared to COI gene sequences. Moreover, ITS region sequences have been found to be a more reliable marker for species delimitation in the B. plicatilis and B. calyciflorus species complexes (Mills et al., 2017 and Papakostas et al., 2016, respectively). Based on these findings, in this study the position and character state of diagnostic nucleotides corresponding to the alignment of ITS sequences in MAFFT v 7 (Katoh & Standley, 2013) were used for species diagnosis (849 bp including insertions; Supplemental Document S2).
Because there is no record of a type specimen for Euchlanis dilatata corresponding to its original description of (Ehrenberg, 1830), a specimen from the most widespread species in the complex (species ‘A’) was selected as a neotype. Following the recommendation of Jörger and Schrödl’s (2013), species ‘E’ and ‘G’ were not described because they were each represented by only one population in the phylogenetic analysis by Kordbacheh et al. (2017).
Results
Cross-mating experiments
The intra-clonal mating success rate (positive controls) ranged from 15.6 to 43.9%. Within cryptic species mating success rate was 1.2 to 17.9% and between cryptic species success rate was 0 to 1.1% (Table 2). Within cryptic species, the probability of inter-clonal mating success was lower than intra-clonal mating success (χ2 value = 0.04–47.4, P < 0.001). The exception was crosses between Cattail Falls pool C’ and G from cryptic species ‘F’. In this case mating success was not different from that of positive controls (χ2 = 0.035, df = 1, P = 0.8). Further, resting eggs were produced in all mating experiments within cryptic species (Table 2). However, it should be noted that the viability of embryos was not determined.
For inter-clonal crosses between cryptic species, the success rate was 0 for all crosses except for the crosses between individuals from Cattail Falls pool C’, TX (cryptic species ‘F’) and Cinnamon Bay Beach pond, USVI (cryptic species ‘E’). In this cross, one resting egg was produced out of 40 trials between males from cryptic species ‘F’ and females from cryptic species ‘E’. However, no resting eggs were produced in 49 trials when females from cryptic species ‘F’ were crossed with males from cryptic species ‘E’. The genetic variation between these cryptic species was 10.3% in the ITS region and 13.5% in the COI gene sequences (Kordbacheh et al., 2017).
Based on χ2 test results, the probability of interbreeding between cryptic species was significantly lower than the probability of inbreeding within clonal lineages (Tables 2, 3).
Trophi morphology
Discriminant Analysis showed that only 64% of individuals were assigned correctly to their cryptic species based on trophi measurements (Wilks’ Lambda = 0.08, χ2 = 78, P < 0.001, Fig. 1). Species ‘A’ was the most distinguishable, with 83% of individuals assigned correctly. Moreover, all trophi analyzed from individuals representing four populations of species ‘A’ had a small projection on the left ramus, which was not observed in the other putative cryptic species (Fig. 2). In Discriminant Analysis, Function 1 (71.3% of variation) was associated with fulcrum length and Function 2 (14.8% of variation) was associated with manubria length showing most variation in trophi size was related to fulcrum and manubria length.
Trophi of the Euchlanis dilatataa cryptic species ‘A’ frontal view, b cryptic species ‘A’ caudal view, c cryptic species ‘B’ frontal view, d cryptic species ‘B’ caudal view, e cryptic species ‘C’ frontal view, f cryptic species ‘C’ caudal view, g cryptic species ‘D’ frontal view, h cryptic species ‘D’ caudal view, i cryptic species ‘E’ frontal view, j cryptic species ‘E’ caudal view, k cryptic species ‘F’ frontal view, l cryptic species ‘F’ caudal view, m cryptic species ‘G’ frontal view, n cryptic species ‘G’ caudal view. Arrows indicate the projection on left ramus of cryptic species ‘A’ trophi
Life table experiments
Age-specific survivorship (l x), fecundity (m x), and life expectancy (e x)
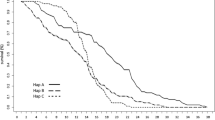
Lineage, water temperature, and the interaction between temperature and lineage and the interaction between temperature, lineage and conductivity had significant effects on lxmx (Linear Mixed-Effects Model, P < 0.001). Lineage (P < 0.01), water temperature (P < 0.01), conductivity (P = 0.01), and the interaction between temperature and lineage (P = 0.01) had significant effects on ex (for more details, see Supplemental Document S3, Table S3.1). Higher values of lxmx were observed at temperature 27°C (Fig. 3, Table 4). Life expectancy decreased under high temperature and high conductivity (Table 4).
Age-specific fecundity (lxmx) of five Euchlanis dilatata cryptic species under four conditions: (1) Low temperature, low conductivity (tc: temperature: 20°C, conductivity: 180 µS/cm), (2) Low temperature, high conductivity (tC: temperature: 20°C, conductivity: 1800 µS/cm), (3) High temperature, low conductivity (Tc: temperature: 27°C, conductivity: 180 µS/cm), and (4) High temperature, high conductivity (TC: temperature: 27°C, conductivity: 1800 µS/cm). Each line on the graph represents a cryptic lineage
Only significant differences among cryptic species in each treatment are reported below. In all cases, the P value was 0.03, unless otherwise specified (Supplemental Document S3, Table S3.2).
Generation time (T)
For all cryptic species, generation time (T) was the longest under tc conditions. Cryptic species ‘A’ and ‘E’ had the shortest generation time under TC treatments while cryptic species ‘C’, ‘D’, and ‘F’ had the shortest generation time under Tc treatments (Table 4).
In tc treatments, generation time ranged from 4.8 to 5.6 days, but there was no significant difference among cryptic species. Under tC, generation time ranged from 3.3 to 5.4 days. In these treatments, cryptic species ‘E’ had the longest generation time (mean ± standard error: 5.4 ± 0.06) and was significantly different from cryptic species ‘A’ (3.9 ± 0.75) and ‘D’ (4.6 ± 0.17). Under Tc, generation time ranged from 2.2 to 3.2 days. Cryptic species ‘A’ (3.1 ± 0.18) and ‘E’ (3.2 ± 0.17) had longer generation time compared to cryptic species ‘C’ (2.2 ± 0.09) and ‘F’ (2.7 ± 0.15). Under TC, generation time ranged from 2.7 to 3.3 days. In these treatments, generation time of cryptic species ‘E’ (3.1 ± 0.06) and ‘C’ (3.3 ± 0.22, P = 0.05) was longer than that of cryptic species ‘D’ (2.7 ± 0.09) (Table 4 and Supplemental Document S3, Table S3.2).
Net reproductive rate (R 0)
Cryptic species ‘C’, ‘D’, and ‘F’ showed the highest net reproductive rate (R0) under TC. Cryptic species ‘A’ had the highest R0 under Tc. Cryptic species ‘E’ had the highest R0 under tc. For cryptic species ‘A’ and ‘F’, the lowest R0 was under tC conditions. Cryptic species ‘D’ and ‘E’ had their lowest R0 under Tc. Cryptic species ‘C’ showed the lowest value of R0 under tc and tC (Table 4 and Supplemental Document S3, Table S3.2).
Under tc, R0 ranged from 1.0 to 9.2. Cryptic species ‘F’ showed the lowest R0 (1.0 ± 0.17) that was significantly lower than the other four cryptic species. Net reproductive rate of cryptic species ‘D’ (9.2 ± 1.54) was significantly higher than that of ‘A’ and ‘C’. Cryptic species ‘C’ (3.7 ± 0.39) had a lower R0 compared to cryptic species ‘E’ (6.3 ± 0.68). Under tC, R0 ranged from 0.5 to 10.9. Net reproductive rate of cryptic species ‘D’ (10.9 ± 0.96) was the highest, and that of cryptic species ‘F’ (0.5 ± 0.15) was the lowest in these treatments. Species ‘A’ had a higher R0 than that of species ‘F’ (P = 0.05). Under Tc, R0 ranged from 0.7 to 9.4. Net reproductive rate of cryptic species ‘A’ (9.4 ± 1.04) was the highest and R0 of cryptic species ‘F’ (0.7 ± 0.25) was the lowest in these treatments. Net reproductive rate of cryptic species ‘C’ (5.2 ± 0.73) was lower than that of cryptic species ‘A’ (P = 0.05). Cryptic species ‘D’ (3.1 ± 0.60) had lower R0 than cryptic species ‘C’. Under TC, R0 ranged from 1.2 to 11.6. Net reproductive rates of cryptic species ‘E’ (1.2 ± 0.43) and ‘F’ (1.3 ± 0.40) were significantly lower compared to the other three cryptic species (Table 4 and Supplemental Document S3, Table S3.2).
Intrinsic rate of population increase (r)
Cryptic species ‘A’ had the highest intrinsic rate of population increase (r) under Tc treatments. Cryptic species ‘C’ showed the highest r under Tc and TC. Cryptic species ‘E’ had the highest value of r under tc and Tc. Cryptic species ‘D’ and ‘F’ had the highest r under TC conditions. Intrinsic rate of population increase was the lowest under tC conditions for cryptic species ‘A’. Cryptic species ‘F’ had the lowest values of r under tC and Tc. Cryptic species ‘C’ showed the lowest value of r under tc and tC conditions. For cryptic species ‘D’, the lowest value of intrinsic rate of population increase was under tc treatments. Cryptic species ‘E’ had the lowest r under TC (Table 4 and Supplemental Document S3, Table S3.2).
Under tc, r was − 0.02 to 0.4. Cryptic species ‘D’ (0.4 ± 0.02) had the highest and cryptic species ‘F’ (− 0.02 ± 0.04) showed the lowest values of r in these treatments. The r of cryptic species ‘A’ (0.3 ± 0.01) and ‘E’ (0.3 ± 0.01) were larger than cryptic species ‘C’ (0.2 ± 0.01). Under tC, r increase ranged from − 0.3 to 0.5. The r of cryptic species ‘D’ (0.5 ± 0.03) was higher than the other cryptic species (between cryptic species ‘A’ and D, P = 0.05). Cryptic species ‘F’ (− 0.3 ± 0.15) had a lower r than cryptic species ‘A’ (0.2 ± 0.11) (P = 0.05), ‘C’ (0.2 ± 0.03), and ‘E’ (0.2 ± 0.04). Under Tc, r was − 0.3 to 0.7. The r of cryptic species ‘A’ (0.7 ± 0.05) and ‘C’ (0.7 ± 0.04) were higher than the other three cryptic species. Cryptic species ‘D’ (0.4 ± 0.08) showed a higher r than ‘F’ (− 0.3 ± 0.22). Under TC, r ranged from − 0.04 to 0.90. The r of cryptic species ‘E’ (− 0.04 ± 0.17) and ‘F’ (0.01 ± 0.09) were lower than that of cryptic species ‘A’ (0.6 ± 0.02), ‘C’ (0.7 ± 0.08), and ‘D’ (0.9 ± 0.05) (between cryptic species ‘A’ and ‘E’, P = 0.05). Cryptic species ‘A’ had a lower r than cryptic species ‘D’ (P = 0.05) (Table 4; Supplemental Document S3, Table S3.2). Negative value of r indicates population size was decreasing.
Discussion
Euchlanis dilatata is a species complex with at least seven cryptic species (Kordbacheh et al., 2017). In this study, we observed reproductive isolation among six and ecological differentiation among five of them. Moreover, one of the cryptic species (cryptic species ‘A’) showed morphological differences in trophi shape and size from other cryptic species within the complex.
Reproductive isolation can be mediated through pre-mating and/or post-mating reproductive barriers. Reproductive isolation has been examined among cryptic species of many aquatic invertebrates [e.g., Lee (2000); Addison & Kim (2018)]. In rotifers, pre-mating barriers include differentiation in timing and cues of shifting from asexual to sexual reproduction (e.g., B. plicatilis: Carmona et al., 1995; E. hawaiiensis and E. chihuahuaensis: Schröder & Walsh, 2010), and failure to recognize individuals of other species as potential mates (e.g., B. plicatilis species complex: Kotani et al., 1997; Gribble & Mark Welch, 2012). Post-mating barriers include the lack of resting egg production after copulation, production of unviable resting eggs, and/or female mortality after copulation (e.g., Asplanchna brightwelli Gosse, 1850: King, 1977; E. ukera and E. chihuahuaensis: Schröder & Walsh, 2007) and unviable and/or sterile F1 females (e.g., B. plicatilis species complex: Suatoni et al., 2006). In this study, resting eggs were not produced in cross-mating trials between cryptic species, with one exception. A single resting egg was produced in a mating between a female of cryptic species ‘E’ and a male of cryptic species ‘F’, despite high genetic differentiation between them based on both markers. We did not determine whether this resting egg was viable. Therefore, we cannot be certain whether gene flow between these two species occurs. On the other hand, cryptic species ‘A’ and ‘B’ were reproductively isolated based on our results. The genetic distance between cryptic species ‘A’ and ‘B’ in the ITS region was 1.2% and 11.1% in the COI gene. It is possible that when there is no or limited gene flow between cryptic species of the E. dilatata complex, the COI gene accumulates genetic variation at a higher rate compared to the ITS region due to faster rate of evolution (Cruickshank, 2002; Vilas et al., 2005). Overall, the inter-clonal mating success rates within cryptic species were lower than the positive controls, and all the within cryptic species cross-mating experiments were successful. Thus, suggesting gene flow occurs within cryptic species although at a lower rate than between species.
True cryptic species are not easily distinguishable based on morphology (Bickford et al., 2007). However, morphological variation among cryptic species of rotifers has been reported (see Fu et al., 1991; Ciros-Pérez et al., 2001; Campillo et al., 2005; Anitha & George, 2006; Schröder & Walsh, 2007; Fontaneto et al., 2007b; Hwang et al. 2013; Michaloudi et al., 2016). Here, we examined trophi morphology of representatives of populations from the E. dilatata. Based on trophi size, in general only 64% of individuals were assigned correctly to cryptic species. Individuals of cryptic species ‘A’ had the highest rate of accurate assignment to their cryptic species (83%). In addition to variation in trophi size for cryptic species ‘A’, we observed a projection on the left ramus of trophi that was specific to this cryptic species. A population of the E. dilatata species complex collected in Italy had a similar projection (Parise, 1966). Molecular analyses would be necessary to determine whether that population is assigned to species ‘A’. Based on our results, at least six cryptic species of the E. dilatata complex are indistinguishable based on trophi morphology. Lack of variation in trophi among these cryptic species may represent morphological stasis despite high genetic variation. Yet, differentiation in body size related to ploidy level has been reported within E. dilatata populations (Walsh & Zhang, 1992). Life history characteristics under high and low temperatures also varied with ploidy level (Walsh, 1992). It is possible that body size as well as other morphological features vary among cryptic species.
We observed variation in life table parameters among five examined cryptic species that may stem from genetic variation among them as noted for net reproductive rate of B. calyciflorus cryptic species (Wang et al., 2014) and some life history features such as age-specific fecundity for lineages of Keratella cochlearis (Gosse, 1851) (Cieplinski et al., 2018). For example in our study, cryptic species ‘D’ had the highest age-specific fecundity (lxmx) under all experiment conditions except Tc, and species ‘F’ had the lowest values of lxmx and net reproductive rate under all experiment conditions. This variation indicates that E. dilatata lineages are evolving independently and can be treated as species.
Life history parameters of rotifers can be affected by many factors (Table 5). In this study, temperature had a significant effect on age–specific fecundity (Ixmx), and life expectancy (ex), while conductivity only significantly impacted ex. As poikilotherms, rotifers are known to exhibit a positive correlation between development rates and temperature, while life expectancy tends to be negatively correlated with temperature (e.g., Miracle & Serra, 1989; Pavón-Meza et al., 2005; Ogello et al., 2016; Saucedo-Ríos et al., 2017). Pavón-Meza et al. (2005) recorded lower life expectancy and shorter generation time, and higher net reproductive rate and population growth at a temperature 25°C as compared to 15°C for B. havanaensis Rousselet, 1911. Similarly, Ogello et al. (2016) observed longer life expectancy at a lower temperature for a Kenyan strain of the rotifer Brachionus angularis Gosse, 1851. In our study, all five cryptic species from the E. dilatata complex tested had higher age-specific fecundity and lower life expectancy at temperature 27°C as compared to 20°C.
Generation time decreases under high temperatures for many poikilotherms (Gillooly, 2000) including rotifers (e.g., Galkovskaja, 1987; Pavón-Meza et al., 2005; Xiang et al., 2010; Wang et al., 2014). We observed the longest generation time for all cryptic species under tc. With an increase of temperature, the generation time for all species decreased. Increase of temperature affects the rate of metabolic activities including developmental processes and thus results in increased r (Miracle & Serra, 1989). We also noted all cryptic species had higher R0 and r at 27°C. For cryptic species ‘D’ and ‘F’, the highest R0 and r were under TC. Cryptic species ‘C’ had its highest R0 under TC and the highest r under Tc and TC. In this study, under high temperatures, cryptic species showed variation in generation time, reproduction rate, and population growth in response to water conductivity. Because we used NaCl to increase the conductivity of MBL to 1,800 µS/cm2, conductivity in our experiments represents salinity. In a review by Miracle and Serra (1989), it was noted that for Brachionus dimidiatus Bryce, 1931 and B. plicatilis, r decreases as the salinity concentration deviates from the optimal level. Differential response to water salinity among cryptic species of rotifers has been addressed. Brachionus plicatilis had higher reproduction rate compared to B. manjavacas in lower salinities (Gabaldón et al., 2015). On the other hand, Leasi et al. (2013) did not find variation in the occurrence of cryptic species of the Testudinella clypeata species complex in relation to salinity.
Life history characteristics of E. dilatata has been studied, for instance in response to competition and/or food type (Espinosa-Rodríguez et al., 2012; Farhadian et al., 2013), combined effects of methyl parathion and food density (Sarma et al., 2001), cadmium, lead, mercury, and methyl parathion concentration (Arias-Almeida & Rico-Martínez, 2011), and predation (Nandini et al., 2011). Moreover, Walsh & Zhang (1992) found that a large morphotype of E. dilatata was triploid and had a longer time to maturity as compared to that of a smaller diploid morphotype. Here, we provided evidence of variation in survivorship, reproduction rate, and population growth in response to water temperature and conductivity among genetically distinct isolates. Kordbacheh et al. (2017) reported co-occurrence of cryptic species ‘C’ and ‘D’ in Laguna Prieta, El Paso Co., TX. Cryptic species ‘C’ was hatched from rehydrated sediments and an active population representing cryptic species ‘D’ was collected in 2013. These two species may be temporarily isolated, that could have resulted from the ecological differentiation between them. Cryptic species of B. plicatilis that show ecological variation have been found in sympatry by Montero-Pau et al. (2011) and Wang et al. (2014). Ecological niche differentiation has been recorded between E. dilatata and E. dilatata lucksiana (Hauer, 1930) where the latter, even in sympatry with E. dilatata, has been found only from planktonic habitats (Adamkiewicz-Chojnacka, 1988). It should be noted that E. dilatata cryptic species were collected from habitats with different pHs, temperatures, and conductivities. For example, species ‘C’ and ‘D’ were collected from habitats with water temperatures > 30°C and they showed the highest R0 under high temperature and high conductivity conditions. Species ‘F’ was found in habitats with pH and conductivity as low as 5.9 and 405 µS/cm, respectively. However, it did not show a significant difference in life history characteristics between high and low conductivity conditions. Species ‘F’ may be the most ecologically differentiated lineage because it had the lowest lxmx and R0 under all experiment conditions. The observed variation in life history parameters highlights potential ecological niche differentiation among E. dilatata cryptic species, and thus it may be a major contributor to the genetic divergence among cryptic species.
Cryptic species of several invertebrates have been described based on diagnostic nucleotides (e.g., Brower, 2010; Johnson et al., 2015; Delic et al. 2017). Although we did not find morphological variation in trophi features among cryptic species of the E. dilatata complex (except for cryptic species ‘A’), we documented reproductive isolation among six, and ecological differentiation among five cryptic species. We described four cryptic species from the E. dilatata complex using diagnostic nucleotides in the ITS region sequence following Jörger & Schrödl’s (2013) guidelines as well as designated a neotype. In a study with description of two new species within B. calyciflorus complex, Michaloudi et al. (2018) selected the lineage with highest variation in the morphology of the antero-dorsal spines as the neotype. Here, we designated the cryptic species (i.e., lineage ‘A’) with widest geographic distribution and that has a feature of the rami that was illustrated in a drawing of E. dilatata trophi based on a European population (Parise, 1966) as the neotype.
To better understand mechanisms involved in speciation within the E. dilatata complex and other rotifer species complexes, future studies should focus on identifying pre- and post-mating reproductive barriers among cryptic species within this species complex such as variation in ploidy level, mixis induction, differentiation in mating behaviors, and the viability of hybrids.
To further study the ecological niche differentiation, other environmental variables such as food density, food type, and competition should be included in life table experiments among cryptic species, and the genetic basis of variation in ecological adaptation should be explored. Biological and ecological species concepts can be used to delimit species within rotifers and other microorganisms by investigating reproductive isolation and ecological differentiation among them.
Species accounts
Euchlanis dilatata was first described by Ehrenberg (1830). However, there is no known type specimen (Segers et al., 2012) and the type locality was not specified (Ehrenberg, 1830). Therefore, following Article 75.3.4 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999), we designated a specimen from cryptic species ‘A’ and from the population collected from the Rio Grande, Sierra Co., NM, USA as a neotype for Euchlanis dilatata sensu stricto. A representative from this lineage was selected because it had the widest geographic distribution compared to other cryptic species analyzed this study. Moreover, the small projection on the ramus observed in this species was also depicted in a drawing of a specimen from a population collected in Italy (Parise, 1966).
Nomenclatural considerations: Because no type specimen is known for E. dilatata and we are describing new species within this species complex, we designate a neotype to stabilize the taxonomic identity of the name. The neotype is deposited in a public repository, the National Museum of Natural History, Smithsonian Institution (USNM), for future studies.
Euchlanis dilatata sensu stricto
Neotype locality: Rio Grande, Sierra Co., NM, USA, 33.110039, − 107.297839, 1288 m.
Diagnosis: This species is distinguished from other cryptic species of Euchlanis dilatata complex in this study based on the following character states: 156A; 350T (MAFFT alignment of ITS region sequences; Supplemental Document S2). The morphological diagnostic feature is a small projection on the left ramus (Fig. 2).
Neotype: The neotype is an amictic female on a permanent microscopic slide, deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532147). Additional materials consist of one permanent microscopic slide containing 3 amictic females and 30 amictic females preserved in 95% ethanol deposited in both the National Museum of Natural History, Smithsonian Institution (USNM 1532148 and USNM 1532149, respectively) and UTEP Biodiversity Collections (UTEP:Zoo:69), and the trophi specimen deposited in the UTEP Biodiversity Collections (UTEP:Zoo:69). All deposited specimens are from the same clonal lineage. ITS region and COI gene sequences are deposited in GenBank (accession numbers, ITS: KU665929-45, COI: KU665853-69).
Other examined populations: Detroit Lake State Park, Marion Co., OR, USA (44.700829, − 122.185241), Centinela Creek, Imperial Co., CA, USA (33.987892, − 118.406403), Chicken Creek Reservoir, Juab Co., UT, USA (39.49185, − 111.95760), Santaquin Reservoir, Juab Co., UT, USA (39.955778, − 111.780283), Alto Reservoir, Lincoln Co., NM, USA (33.395833, − 105.671944), Bear Canyon Lake, Grant Co., NM, USA (32.8846, − 107.998033), Mescalero Lake, Otero Co., NM, USA (33.296660, − 105.687849), Storrie Lake, San Miguel Co., NM, USA (35.6606694, − 105.2345833), Rio Grande, Fabens, El Paso Co., TX, USA (31.430121, − 106.142488), Walter E. Long Lake, Travis Co., TX, USA (30.284417, − 97.608383), Shafter stream, Presidio Co., TX, USA (29.8143166, − 104.3071333), Lake Travis, Travis Co., TX, USA (30.402417, − 97.947967), Oknoname 085006 Reservoir, Love Co., OK, USA (33.927583, − 97.301067), Lake Okeechobee, Glades Co., FL, USA (26.830456, − 80.941303), Lake Mendota, Dane Co., WI, USA (43.079504, − 89.419193), Madeleine Lake, Oneida Co., WI, USA (45.889733, − 89.643833), Schmitthenner Lake, Wyoming Co., PA, USA (41.43738, − 76.24304), Panther Hollow Lake, Allegheny Co., PA, USA (40.436856, − 79.948825), Silver Creek, Schuylkill Co., PA, USA (40.543206, − 75.304106), Presa de la Boquilla, Chihuahua, Mexico (27.5361333, − 105.4011333), Lago Colina, Chihuahua, Mexico (27.5724, − 105.4004666), Presa Chihuahua, Chihuahua, Mexico (28.5762166, − 106.1711833), Poza Azul, Coahuila, Mexico (26.926417, − 102.122538).
Morphology: Body is oval. Lorica is truncated at the anterior and rounded at the posterior part (Fig. 4). Cross-section of the lorica is arc of circle. Foot is slender with two joints, toes are parallel-sided with sharp tip. Trophi has four stout unci teeth, with inner denticulate comb at the tip of rami. Fulcrum is club shaped. There is a small projection on the left ramus (Fig. 2). Dorsal plate length 132 to 270 µm.
Ecology and distribution: In our study, this species was mostly found in streams, permanent lakes and reservoirs from Texas, Wisconsin, Oregon, California, Pennsylvania, New Mexico, Florida, and Mexico. pH ranged from 7.7 to 9.0, temperature ranged from 14.7 to 27.7°C and conductivity ranged from 230 to 1638 µS/cm.
New species descriptions
Euchlanis kingi sp. nov.
urn:lsid:zoobank.org:act:6E65C5BB-2046-4498-BE6D-891CD2F620B7
Type locality: Fish Pond, Nockamixon State Park, Bucks Co., PA, USA, 40.472567, − 75.224823, 131 m.
Diagnosis: This species is distinguished from other cryptic species of Euchlanis dilatata complex in this study based on following character states in the ITS region: 309C; a deletion at 350.
Holotype: An amictic female from cryptic species ‘B’ on a permanent microscopic slide is deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532150). Paratypes (one permanent microscopic slide of amictic females, and approximately 30 amictic females preserved in 95% ethanol) are deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532151 and USNM 1532152, respectively) and UTEP Biodiversity Collections (UTEP:Zoo:91). ITS region and COI gene sequences are deposited in GenBank (accession numbers, ITS: KX714930, COI: KX714920).
Etymology: This species is named after Charles E. King because he was the first to hypothesize that genetically distinct lineages of Euchlanis dilatata are temporally isolated because of differentiation in ecological adaptation.
Other examined populations: American River, San Joaquin Co., CA, USA (38.57221207, − 121.3531190), Echo Lake, El Dorado Co., CA, USA (38.834557, − 120.046143), Lodi Lake, San Joaquin Co., CA, USA (38.147222, − 121.292723), Timber Lake ditch, Mount Hood National Forest, Clackamas Co., OR, USA (45.083424, − 122.050234).
Morphology: Body is oval. Lorica is truncated at the anterior and rounded at the posterior part (Fig. 4). Cross-section of the lorica is arc of circle. Foot is slender with two joints, toes are parallel-sided with sharp tip. Trophi has four stout unci teeth, with inner denticulate comb at the tip of rami. Fulcrum is club shaped (Fig. 2). Dorsal plate length 140 to 200 µm.
Ecology and distribution: In our study, this species was found in ponds, lakes and streams from California, Pennsylvania, and Oregon. For one population that was collected from a drainage ditch near Timber Lake, OR: pH was 6.7, temperature was 17.1°C and conductivity was 104 µS/cm. Environmental information was not available for other populations representing this species.
Euchlanis texana sp. nov.
urn:lsid:zoobank.org:act:14D2DCB3-8037-4049-B6BB-DD69B9C02649
Type locality: Rio Grande, El Paso Co., TX, USA, 31.784234,
− 106.527845, 1136 m.
Diagnosis: This species is distinguished from other cryptic species of Euchlanis dilatata complex in this study based on following character states in the ITS region: 644C.
Holotype: An amictic female from cryptic species ‘C’ on a permanent microscopic slide is deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532153). Paratypes (one permanent microscopic slide of amictic females, and approximately 30 amictic females preserved in 95% ethanol) are deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532154 and USNM 1532155, respectively) and UTEP Biodiversity Collections (UTEP:Zoo:127). ITS region and COI gene sequences are deposited in GenBank (accession numbers, ITS: KU665946, COI: KU665851).
Etymology: The species name refers to the state of Texas because most populations representing this species are found in Texas, USA.
Other examined populations: Triangle Pond, Buenos Aires National Wildlife Refuge, Pima Co., AZ, USA (31.55, − 111.533889), Behind Mescalero Canyon playa, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.9190694, − 106.0408305), Corral Tank, Hudspeth Co., TX, USA (30.8237166, − 105.3155833), Feather Lake Wildlife Sanctuary, El Paso Co., TX, USA (31.6890972, − 106.3052666), Glenn Springs, pool 3, Big Bend National Park, Brewster Co., TX, USA (29.1744166, − 103.1575), Glenn Springs, pool 6, Big Bend National Park, Brewster Co., TX, USA (29.1744166, − 103.1575), Roadside Pond, Jefferson Co., TX, USA (29.834722, − 94.482222), Laguna Prieta, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.9246388, − 106.046675), Mesocosm, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.9188166, − 106.040366), Miller Ranch, Jeff Davis Co., TX, USA (30.623845, − 104.674005), Sam Rayburn State Park, Sabine Co., TX, USA (31.061244, − 94.106127), Palo Pinto Canyon stream, Presidio Co., TX, USA (30.0308666, − 104.4684333), Lake Okeechobee, Glades Co., FL, USA (26.830456, − 80.941303), Shorty Howell pond, Gwinnett Co., GA, USA (33.97455, − 84.14936), La Mesa Canyon Tule spring, Lower Canyons, Mexico (29.75111, − 102.58305).
Morphology: Body is oval. Lorica is truncated at the anterior and rounded at the posterior part (Fig. 4). Cross-section of the lorica is arc of circle. Foot is slender with two joints, toes are parallel-sided with sharp tip. Trophi has four stout unci teeth, with inner denticulate comb at the tip of rami (Fig. 2). Fulcrum is club shaped. Dorsal plate length 120 to 184 µm.
Ecology and distribution: In our study, this species was found in a variety of habitats from temporary ponds and playas to permanent lakes from Texas, Florida, Arizona and Mexico. pH ranged from 6.4 to 9.4, temperature ranged from 7.0 to 33.6°C and conductivity ranged from 179 to 1110 µS/cm.
Euchlanis chihuahuaensis sp. nov.
urn:lsid:zoobank.org:act:DDE39A67-7024-4FDA-B915-C2E76F52E043
Type locality: Former cattle tank, White Sands National Monument, Doña Ana Co., NM, USA, 32.67485, − 106.44345, 1213 m.
Diagnosis: This species is distinguished from other cryptic species of Euchlanis dilatata complex in this study based on following character states in the ITS region: 366A; 756T.
Holotype: An amictic female from cryptic species ‘D’ on a permanent microscopic slide is deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532159). Paratypes (one permanent microscopic slide of amictic females, and approximately 30 amictic females preserved in 95% ethanol) are deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532160 and USNM 1532161, respectively) and UTEP Biodiversity Collections (UTEP:Zoo:24). ITS region and COI gene sequences are deposited in GenBank (accession numbers, ITS: KU665978, COI: KU665883).
Etymology: The species name refers to Chihuahuan Desert in North America from where most populations of this species were collected.
Other examined populations: Mescalero Canyon playa, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.9188166, − 106.0403666), Red Tank, Hudspeth Co., TX, USA (30.7303083, − 104.9891083), Peccary Tank, Hudspeth Co., TX, USA (30.7555556, − 105.0041667), Behind Ranch House playa, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.924072, − 106.041589), Laguna Prieta rehydrated sediments, Hueco Tanks State Park and Historic Site, El Paso Co., TX, USA (31.9246388, − 106.046675), Paint Gap Cattle Tank, Big Bend National Park, Brewster Co., TX, USA (29.3878555, − 103.302675), Alazan Lake, Alazan Bayou Wildlife Management Area, Nacogdoches Co., TX, USA (31.5033389, − 094.7546583), Ojo de Santa Maria, Chihuahua, Mexico (31.1552777, − 107.3172222).
Morphology: Body is oval. Lorica is truncated at the anterior and rounded at the posterior part (Fig. 4). Cross-section of the lorica is arc of circle. Foot is slender with two joints, toes are parallel-sided with sharp tip. Trophi has four stout unci teeth, with inner denticulate comb at the tip of rami. Fulcrum is club shaped (Fig. 2). Dorsal plate length 150 to 230 µm.
Ecology and distribution: In our study, this species was found in various habitats including lakes, lagoons, artificial temporary tanks, temporary playas in Texas and Mexico. pH ranged from 8.4 to 9.3, temperature ranged from 23.6 to 34.3°C and conductivity ranged from 137 to 988 µS/cm.
Euchlanis floridensis sp. nov.
urn:lsid:zoobank.org:act:E0CEDD30-5CAA-4A78-ACC2-AF8DB1D77CE5
Type locality: Krome pond, Miami-Dade Co., FL, USA, 25.883615, − 80.484920, 3.3 m.
Diagnosis: This species is distinguished from other cryptic species of Euchlanis dilatata complex in this study based on following character states in the ITS region: 29T; 54T; 55T; 92C; 180C; 186G; 189G; 232T; 233C; 259A; 260C; 264T; 277T; 278G; 288A; 307G; 312T; 314A; 344C; 369T; 657G; 693G; 70A; 702A; 708G; 756C.
Holotype: An amictic female from cryptic species ‘F’ on a permanent microscopic slide is deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532156). Paratypes (one permanent microscopic slide of amictic females, and approximately 30 amictic females preserved in 95% ethanol) are deposited in the National Museum of Natural History, Smithsonian Institution (USNM 1532157 and USNM 1532158, respectively) and UTEP Biodiversity Collections (UTEP:Zoo:129). ITS region and COI gene sequences are deposited in GenBank (accession numbers, ITS: KU665985 and KX714931, COI: KU665896 and KX714921).
Etymology: The species named refers to the state of Florida, USA where the type specimen was collected.
Other examined populations: Buttrill Springs, Big Bend National Park, Brewster Co., TX, USA (29.54585, − 103.2738), Cattail Falls, pool C’, Big Bend National Park, Brewster Co., TX, USA (29.2731833, − 103.3361638), Cattail Falls, pool D, Big Bend National Park, Brewster Co., TX, USA (29.2731527, − 103.3358277), Cattail Falls, pool E, Big Bend National Park, Brewster Co., TX, USA (29.2731444, − 103.3361638), Cattail Falls, pool G, Big Bend National Park, Brewster Co., TX, USA (29.2731666, − 103.3361638), Cattail Falls, pool H, Big Bend National Park, Brewster Co., TX, USA (29.2731694, − 103.3362388), Duck Pond, Beaver Co., OK, USA (35.551944, − 97.574722), Lake June in the Winter, Highlands Co., FL, USA (27.30715, − 81.37542), Lake Jackson, Highlands Co., FL, USA (27.48775, − 81.47664).
Morphology: Body is oval. Lorica is truncated at the anterior and rounded at the posterior part (Fig. 4). Cross-section of the lorica is arc of circle. Foot is slender with two joints, toes are parallel-sided with sharp tip. Trophi has four stout unci teeth, with inner denticulate comb at the tip of rami. Fulcrum is club shaped (Fig. 2). Dorsal plate length 98 to 199 µm.
Ecology and distribution: In our study, this species was found in a variety of habitats that includes ponds, permanent lakes, and natural springs in Texas, Florida, and Oklahoma. pH ranged from 5.9 to 7.9, temperature ranged from 7.5 to 22.6°C and conductivity ranged from 405 to 523 µS/cm.
References
Adamkiewicz-Chojnacka, B., 1988. The genus Euchlanis (Rotatoria) in brackish waters of the Vistula Lagoon (Southern Baltic). Oceanologia 26: 7–103.
Addison, J. A. & J. H. Kim, 2018. Cryptic species diversity and reproductive isolation among sympatric lineages of Strongylocentrotus sea urchins in the northwest Atlantic. FACETS 3: 61–78.
Anitha, P. S. & R. M. George, 2006. The taxonomy of Brachionus plicatilis species complex (Rotifera: Monogononta) from the Southern Kerala (India) with a note on their reproductive preferences. Journal of the Marine Biological Association of India 48: 6–13.
Arias-Almeida, J. C. & R. Rico-Martínez, 2011. Toxicity of cadmium, lead, mercury and methyl parathion on Euchlanis dilatata Ehrenberg 1832 (Rotifera: Monogononta). Bulletin of Environmental Contamination and Toxicology 87: 138–142.
Bickford, D., D. J. Lohman, N. S. Sodhi, P. K. L. Ng, R. Meier, K. Winker, K. K. Ingram & I. Das, 2007. Cryptic species as a window on diversity and conservation. Trends in Ecology and Evolution 22: 148–155.
Bos, D. G., B. F. Cumming, C. E. Watters & J. P. Smol, 1996. The relationship between zooplankton, conductivity and lake-water ionic composition in 111 lakes from the Interior Plateau of British Columbia, Canada. International Journal of Salt Lake Research 5: 1–15.
Brower, A. V. Z., 2010. Alleviating the taxonomic impediment of DNA barcoding and setting a bad precedent: Names for ten species of ‘Astraptes fulgerator’ (Lepidoptera: Hesperiidae: Eudaminae) with DNA-based diagnoses. Systematics and Biodiversity 8: 485–491.
Campillo, S., E. M. García-Roger, D. Martínez-Torres & M. Serra, 2005. Morphological stasis of two species belonging to the L-morphotype in the Brachionus plicatilis species complex. Hydrobiologia 546: 181–187.
Carmona, M. J., A. Gómez & M. Serra, 1995. Mictic patterns of the rotifer Brachionus plicatilis in small ponds. Hydrobiologia 313(314): 365–371.
Celewicz-Goldyn, S. & N. Kuczynska-Kippen, 2017. Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 12: e0177317. .
Çelik, K. & T. Ongun, 2007. The relationships between certain physical and chemical variables and the seasonal dynamics of phytoplankton assemblages of two inlets of a shallow hypertrophic lake with different nutrient inputs. Environmental Monitoring and Assessment 124: 321–330.
Cieplinski, A., U. Obertegger & T. Weisse, 2018. Life history traits and demographic parameters in the Keratella cochlearis (Rotifera, Monogononta) species complex. Hydrobiologia 811: 325–338.
Ciros-Pérez, J., A. Gómez & M. Serra, 2001. On the taxonomy of three sympatric sibling species of the Brachionus plicatilis (Rotifera) complex from Spain, with the description of B. ibericus n. sp. Journal of Plankton Research 23: 1311–1328.
Cruickshank, R. H., 2002. Molecular markers for the phylogenetics of mites and ticks. Systematic & Applied Acarology 7: 3–14.
Dayrat, B., 2005. Towards integrative taxonomy. Biological Journal of the Linnean Society 85: 407–415.
Delić, T., P. Trontelj, M. Rendoš & C. Fišer, 2017. The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Scientific Reports 7: 3391.
Dennis, A. B. & M. E. Hellberg, 2010. Ecological partitioning among parapatric cryptic species. Molecular Ecology 19: 3206–3225.
Ehrenberg, C. G., 1830. Organisation, systematik und geographisches serhältnis der infusionsthierchen. Zwei vorträge in der Akademie der Wissenschaften zu Berlin gehalten in den Jahren 1828 [Die geographische verbreitung der infusionsthierchen in Nord-Afrika und West-Asien, beobachtet auf Hemprich und Ehrenbergs Reisen] und 1830 [Beiträge zur kenntnis der organisation der infusorien und ihrer geographischen verbreitung, besonders in Sibirien]. Druckerei der königlichen Akademie der Wissenschaften, Berlin. 108 pp. In German.
Espinosa-Rodríguez, C. A., S. S. S. Sarma & S. Nandini, 2012. Interactions between the rotifer Euchlanis dilatata and the cladocerans Alona glabra and Macrothrix triserialis in relation to diet type. Limnologica 42: 50–55.
Farhadian, O., L. Daghighi & E. E. Dorche, 2013. Effects of microalgae and alfalfa meal on population growth and production of a freshwater rotifer, Euchlanis dilatata (Rotifera: Mongononta). Journal of the World Aquaculture Society 44: 86–95.
Fatema, K., W. M. W. Omar, M. M. Isa & A. Omar, 2016. Effects of water quality parameters on abundance and biomass of zooplankton in Merbok Estuary Malaysia. Journal of Environmental Science and Natural Resources 9: 117–122.
Finlay, B. J., 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063.
Fontaneto, D., J. F. Flot & C. Q. Tang, 2015. Guidelines for DNA taxonomy, with a focus on the meiofauna. Marine Biodiversity 45: 433–451.
Fontaneto, D., I. Giordani, G. Melone & M. Serra, 2007a. Disentangling the morphological stasis in two rotifer species of the Brachionus plicatilis species complex. Hydrobiologia 583: 297–307.
Fontaneto, D., E. A. Herniou, T. G. Barraclough, C. Ricci & G. Melone, 2007b. On the reality and recognisability of asexual organisms: morphological analysis of the masticatory apparatus of bdelloid rotifers. Zoologica Scripta 36: 361–370.
Fontaneto, D., M. Kaya, E. A. Herniou & T. G. Barraclough, 2009. Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy. Molecular Phylogenetics and Evolution 53: 182–189.
Fouet, C., C. Kamdem, S. Gamez & B. J. White, 2017. Genomic insights into adaptive divergence and speciation among malaria vectors of the Anopheles nili group. Evolutionary Applications 10: 897–906.
Fu, Y., K. Hirayama & Y. Natsukari, 1991. Morphological differences between two types of the rotifer Brachionus plicatilis O.F. Müller. Journal of Experimental Marine Biology and Ecology 151: 29–41.
Gabaldón, C., M. Serra, M. J. Carmona & J. Montero-Pau, 2015. Life-history traits, abiotic environment and coexistence: the case of two cryptic rotifer species. Journal of Experimental Marine Biology and Ecology 465: 142–152.
Galkovskaja, G. A., 1987. Planktonic rotifers and temperature. Hydrobiologia 147: 307–317.
Gilbert, J. J., 2004. Females from resting eggs and parthenogenetic eggs in the rotifer Brachionus calyciflorus: lipid droplets, starvation resistance and reproduction. Freshwater Biology 49: 1505–1515.
Gilbert, J. J. & E. J. Walsh, 2005. Brachionus calyciflorus is a species complex: mating behavior and genetic differentiation among four geographically isolated strains. Hydrobiologia 546: 257–265.
Gillooly, J. F., 2000. Effect of body size and temperature on generation time in zooplankton. Journal of Plankton Research 22: 241–251.
Gómez, A. & T. W. Snell, 1996. Sibling species and cryptic speciation in the Brachionus plicatilis species complex (Rotifera). Journal of Evolutionary Biology 9: 953–964.
Gómez, A., M. Temprano & M. Serra, 1995. Ecological genetics of a cyclical parthenogen in temporary habitats. Journal of Evolutionary Biology 622: 601–622.
Gorokhova, E., 2017. Shifts in rotifer life history in response to stable isotope enrichment: testing theories of isotope effects on organismal growth. Royal Society Open Science 4: 160810.
Gribble, K. E. & D. B. Mark Welch, 2012. The mate recognition protein gene mediates reproductive isolation and speciation in the Brachionus plicatilis cryptic species complex. BMC Evolutionary Biology 12: 134.
Hwang, D. S., H. U. Dahms, H. Park & J. S. Lee, 2013. A new intertidal Brachionus and intrageneric phylogenetic relationships among Brachionus as revealed by allometry and CO1-ITS1 gene analysis. Zoological Studies 52: 13.
IBM Corp, 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
International Commission on Zoological Nomenclature, 1999. International Code of Zoological Nomenclature, 4th ed. International Trust for Zoological Nomenclature, London.
Johnson, S. B., A. Warén, V. Tunnicliffe, C. Van Dover, C. G. Wheat, T. F. Schultz & R. C. Vrijenhoek, 2015. Molecular taxonomy and naming of five cryptic species of Alviniconcha snails (Gastropoda: Abyssochrysoidea) from hydrothermal vents. Systematics and Biodiversity 13: 278–295.
Johnston, R. K. & T. W. Snell, 2016. Moderately lower temperatures greatly extend the lifespan of Brachionus manjavacas (Rotifera): thermodynamics or gene regulation? Experimental Gerontology 78: 12–22.
Jörger, K. M. & M. Schrödl, 2013. How to describe a cryptic species? Practical challenges of molecular taxonomy. Frontiers in Zoology 10: 59.
Katoh, K. & D. M. Standley, 2013. MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780.
Kaya, M., E. A. Herniou, T. G. Barraclough & D. Fontaneto, 2009. Inconsistent estimates of diversity between traditional and DNA taxonomy in bdelloid rotifers. Organisms Diversity and Evolution 9: 3–12.
King, C. E., 1977. Genetics of reproduction, variation and adaptation in rotifers. Archiv für Hydrobiologie 8: 187–201.
Kirk, K. L., 1997. Life-history responses to variable environments: starvation and reproduction in planktonic rotifers. Ecology 78: 434–441.
Kordbacheh, A., G. Garbalena & E. J. Walsh, 2017. Population structure and cryptic species in the cosmopolitan rotifer Euchlanis dilatata. Zoological Journal of the Linnean Society 181: 757–777.
Kotani, T., A. Hagiwara & T. Snell, 1997. Genetic variation among marine Brachionus strains and function of mate recognition pheromone (MRP). Hydrobiologia 358: 105–112.
Leasi, F., C. Q. Tang, W. H. De Smet & D. Fontaneto, 2013. Cryptic diversity with wide salinity tolerance in the putative euryhaline Testudinella clypeata (Rotifera, Monogononta). Zoological Journal of the Linnean Society 168: 17–28.
Lee, C. E., 2000. Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate “populations”. Evolution 54: 2014–2027.
Li, L., C. Niu & R. Ma, 2010. Rapid temporal succession identified by COI of the rotifer Brachionus calyciflorus Pallas in Xihai Pond, Beijing, China, in relation to ecological traits. Journal of Plankton Research 32: 951–959.
Liu, J., M. Möller, J. Provan, L. M. Gao, R. C. Poudel & D. Z. Li, 2013. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytologist 199: 1093–1108.
Luo, Z., W. Yang, C. P. Leaw, V. Pospelova, G. Bilien, G. R. Liow, P. T. Lim & H. Gu, 2017. Cryptic diversity within the harmful dinoflagellate Akashiwo sanguinea in coastal Chinese waters is related to differentiated ecological niches. Harmful Algae 66: 88–96.
Ma, Q., Y. L. Xi, J. Y. Zhang, X. L. Wen & X. L. Xiang, 2010. Differences in life table demography among eight geographic populations of Brachionus calyciflorus (Rotifera) from China. Limnologica 40: 16–22.
Malekzadeh-Viayeh, R., H. Mohammadi & A. B. Shafiei, 2010. Population growth of six Iranian Brachionus rotifer strains in response to salinity and food type. International Review of Hydrobiology 95: 461–470.
Malekzadeh-Viayeh, R., R. Pak-Tarmani, N. Rostamkhani & D. Fontaneto, 2014. Diversity of the rotifer Brachionus plicatilis species complex (Rotifera: Monogononta) in Iran through integrative taxonomy. Zoological Journal of the Linnean Society 170: 233–244.
Michaloudi, E., S. Mills, S. Papakostas, C. P. Stelzer, A. Triantafyllidis, I. Kappas, K. Vasileiadou, K. Proios & T. J. Abatzopoulos, 2016. Morphological and taxonomic demarcation of Brachionus asplanchnoidis Charin within the Brachionus plicatilis cryptic species complex (Rotifera, Monogononta). Hydrobiologia 796: 19–37.
Michaloudi, E., S. Papakostas, G. Stamou, V. Neděla, E. Tihlaříková, W. Zhang & S. A. Declerck, 2018. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re)description of four species. PLoS ONE 13: e0203168.
Mills, S., J. A. Alcantara-Rodriguez, J. Ciros-Pérez, A. Gόmez, A. Hagiwara, K. Hinson Galindo, C. Jersabek, R. Malekzadeh-Viayeh, F. Leasi, J. Lee, D. B. Mark Welch, S. Papakostas, S. Riss, H. Segers, M. Serra, R. Shiel, R. Smolak, T. W. Snell, C. Stelzer, C. Q. Tang, R. L. Wallace, D. Fontaneto & E. J. Walsh, 2017. Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 796: 39–58.
Miracle, M. R. & M. Serra, 1989. Salinity and temperature influence in rotifer life history characteristics. Hydrobiologia 52: 81–102.
Montero-Pau, J., E. Ramos-Rodríguez, M. Serra & A. Gómez, 2011. Long-term coexistence of rotifer cryptic species. PLoS ONE 6: e21530.
Nandini, S., S. S. S. Sarma & H. J. Dumont, 2011. Predatory and toxic effects of the turbellarian (Stenostomum cf leucops) on the population dynamics of Euchlanis dilatata, Plationus patulus (Rotifera) and Moina macrocopa (Cladocera). Hydrobiologia 662: 171–177.
Neil, W. E., 1984. Regulation of rotifer densities by crustacean zooplankton in an oligotrophic montane lake in British Columbia. Oecologia 61: 175–181.
Obertegger, U., D. Fontaneto & G. Flaim, 2012. Using DNA taxonomy to investigate the ecological determinants of plankton diversity: Explaining the occurrence of Synchaeta spp. (Rotifera, Monogononta) in mountain lakes. Freshwater Biology 57: 1545–1553.
Obertegger, U., G. Flaim & D. Fontaneto, 2014. Cryptic diversity within the rotifer Polyarthra dolichoptera along an altitudinal gradient. Freshwater Biology 59: 2413–2427.
Ogello, E. O., H. J. Kim, K. Suga & A. Hagiwara, 2016. Lifetable demography and population growth of the rotifer Brachionus angularis in Kenya: Influence of temperature and food density. African Journal of Aquatic Science 41: 329–336.
Ortells, R., A. Gomez & M. Serra, 2003. Coexistence of cryptic rotifer species: ecological and genetic characteristics of Brachionus plicatilis. Freshwater Biology 48: 2194–2202.
Packer, L., J. Gibbs, C. Sheffield & R. Hanner, 2009. DNA barcoding and the mediocrity of morphology. Molecular Ecology Resources 9: 42–50.
Pan, L., Y. L. Xi, Z. C. Li, Q. Q. Zhao & Z. Hu, 2016. Effects of mercury on the life table demography of the rotifer Brachionus calyciflorus under different algal food (Scenedesmus obliquus) densities. Acta Ecologica Sinica 36: 218–223.
Papakostas, S., E. Michaloudi, A. Triantafyllidis, I. Kappas & T. J. Abatzopoulos, 2013. Allochronic divergence and clonal succession: two microevolutionary processes sculpturing population structure of Brachionus rotifers. Hydrobiologia 700: 33–45.
Papakostas, S., E. Michaloudi, K. Proios, M. Brehm, L. Verhage, J. Rota, C. Peña, G. Stamou, V. L. Pritchard, D. Fontaneto & S. A. Declerck, 2016. Integrative taxonomy recognizes evolutionary units despite widespread mitonuclear discordance: evidence from a rotifer cryptic species complex. Systematic Biology 65: 508–524.
Parise, A., 1966. The genus Euchlanis (Rotatoria) in the marsh of Fucecchio (Central Italy) with description of a new species. Hydrobiologia 27: 328–337.
Pavón-Meza, E. L., S. S. S. Sarma & S. Nandini, 2005. Combined effects of algal (Chlorella vulgaris) food level and temperature on the demography of Brachionus havanaensis (Rotifera): a life table study. Hydrobiologia 546: 353–360.
Pfenninger, M. & C. Nowak, 2008. Reproductive isolation and ecological niche partition among larvae of the morphologically cryptic sister species Chironomus riparius and C. piger. PLoS ONE 3: e2157.
Ramírez-Pérez, T., S. S. S. Sarma & S. Nandini, 2004. Effects of mercury on the life table demography of the rotifer Brachionus calyciflorus Pallas (Rotifera). Ecotoxicology 13: 535–544.
Rico-Martinez, R. & T. W. Snell, 1995. Mating behavior and mate recognition pheromone blocking of male receptors in Brachionus plicatilis Müller (Rotifera). Hydrobiologia 313: 105–110.
Ríos-Arana, J. V., E. J. Walsh & M. Ortiz, 2007. Interaction effects of multi-metal solutions (As, Cr, Cu, Ni, Pb and Zn) on life history traits in the rotifer Plationus patulus. Journal of Environmental Science and Health Part A 42: 1473–1481.
Rissler, L. J. & J. J. Apodaca, 2007. Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Systematic Biology 56: 924–942.
Rose, J. M., N. M. Vora, P. D. Countway, R. J. Gast & D. A. Caron, 2008. Effects of temperature on growth rate and gross growth efficiency of an Antarctic bacterivorous protist. Multidisciplinary Journal of Microbial Ecology 3: 252–260.
R Core Team, 2017. R: A language and environment for statistical computing. https://www.R-project.org/.
RStudio Team, 2016. RStudio: integrated development for R. Rstudio. RStudio, Inc., Boston, MA. http://www.rstudio.com/.
Santos Medrano, G. E., D. Robles, S. Hernandez Flores & R. Rico Martinez, 2016. Life table demography of Asplanchna brightwellii Gosse, 1850 fed with five different prey items. Hydrobiologia 796: 169–179.
Sarma, S. S. S., S. Nandini, J. L. Gama-Flores & M. A. Fernandez-Araiza, 2001. Population growth of Euchlanis dilatata (Rotifera): combined effects of methyl parathion and food (Chlorella vulgaris). Journal of Environmental Science and Health - Part B Pesticides, Food Contaminants, and Agricultural Wastes 36: 43–54.
Saucedo-Ríos, S., G. E. Santos-Medrano & R. Rico-Martínez, 2017. Life table analysis reveals variation in thermal tolerance among three species of the Lecane genus (Rotifera: Monogononta). Annales de Limnologie-International Journal of Limnology 53: 253–259.
Schluter, D., 2001. Ecology and the origin of species. Trends in Ecology & Evolution 16: 372–380.
Schröder, T. & E. J. Walsh, 2007. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 593: 129–140.
Schröder, T. & E. J. Walsh, 2010. Genetic differentiation, behavioural reproductive isolation and mixis cues in three sibling species of monogonont rotifers. Freshwater Biology 55: 2570–2584.
Segers, H., 1993. Rotifera of some lakes in the floodplain of the River Niger (Imo State, Nigeria). I. New species and other taxonomic considerations. Hydrobiologia 250: 39–61.
Segers, H., 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595: 49–59.
Segers, H., W. H. D. Smet, C. Fischer, D. Fontaneto, E. Michaloudi, R. L. Wallace & C. D. Jersabek, 2012. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 3179: 61–68.
Soto, D. & P. Rios, 2006. Influence of trophic status and conductivity on zooplankton composition in lakes and ponds of Torres del Paine National Park (Chile). Biologia 61: 541–546.
Sousa, W., J. L. Attayde, E. D. S. Rocha & E. M. Eskinazi-Sant’Anna, 2008. The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. Journal of Plankton Research 30: 699–708.
Stelzer, C. P., 2002. Phenotypic plasticity of body size at different in a planktonic rotifer : mechanisms temperatures and adaptive significance. Functional Ecology 16: 835–841.
Stemberger, R. S., 1981. A general approach to the culture of planktonic rotifers. Canadian Journal of Fisheries and Aquatic Sciences 38: 721–724.
Suatoni, E., S. Vicario, S. Rice, T. Snell & A. Caccone, 2006. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: The rotifer Brachionus plicatilis. Molecular Phylogenetics and Evolution 41: 86–98.
Vilas, R., C. D. Criscione & M. S. Blouin, 2005. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131: 839–846.
Wallace, R. L. & T. W. Snell, 2014. Rotifera. In Thorp, J. H. & A. Covich (eds), Ecology and classifications of North American freshwater invertebrates. Academic Press, San Diego.
Walsh, E. J., 1992. Ecological and genetics aspects of the population biology of the littoral rotifer Euchlanis dilatata. Ph.D. Dissertation, University of Nevada, Las Vegas.
Walsh, E. J. & L. Zhang, 1992. Polyploidy and body size variation in a natural population of the rotifer Euchlanis dilatata. Journal of Evolutionary Biology 5: 345–353.
Walsh, E. J., T. Schröder, R. L. Wallace, J. V. Ríos-Arana & R. Rico-Martínez, 2008. Rotifers from selected inland saline waters in the Chihuahuan Desert of Mexico. Saline Systems 30: 1046–1050.
Wang, X. L., X. L. Xiang, M. N. Xia, Y. Han, L. Huang & Y. L. Xi, 2014. Differences in life history characteristics between two sibling species in Brachionus calyciflorus complex from tropical shallow lakes. Annales De Limnologie-International Journal of Limnology 50: 289–298.
Wiwegweaw, A., K. Seki, H. Mori & T. Asami, 2009. Asymmetric reproductive isolation during simultaneous reciprocal mating in pulmonates. Biology Letters 5: 240–243.
Xiang, X. L., Y. L. Xi, J. Y. Zhang, Q. Ma & X. L. Wen, 2010. Effects of temperature on survival, reproduction, and morphotype in offspring of two Brachionus calyciflorus (rotifera) morphotypes. Journal of Freshwater Ecology 25: 9–18.
Xiang, X. L., Y. L. Xi, X. L. Wen, G. Zhang, J. X. Wang & K. Hu, 2011. Genetic differentiation and phylogeographical structure of the Brachionus calyciflorus complex in eastern China. Molecular Ecology 20: 3027–3044.
Zhang, G., Y. Xi, Y. Xue, X. Xiang & X. Wen, 2015. Ecotoxicology and environmental safety Coal fly ash effluent affects the distributions of Brachionus calyciflorus sibling species. Ecotoxicology and Environmental Safety 112: 60–67.
Acknowledgements
This research was supported by Grants from the National Science Foundation (DEB 0516032, 1257068), Grant 2G12MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH), Sigma Xi Grants-in-Aid of Research (G2012162274) and UTEP’s Dodson Research Grant (2014, 2015). Howard Hughes Medical Institute SPN01136 (52008125) provided funding to AK during manuscript preparation. Special thanks to Marcela Rivero-Estens and Robert Walsmith for their help in conducting life table experiments. Carl S. Lieb, Robert L. Wallace, Kevin Floyd, and S. Nandini kindly provided rotifer samples. Soyoung Jeon, UTEP BBRC Statistical Consulting Laboratory provided assistance in statistical analyses of life table data. This manuscript benefited from comments provided by three reviewers, the editors, Hendrik Segers, Robert Wallace, Michael Moody and Arshad Khan. Rotifers were collected under permits to EJ Walsh unless otherwise noted. Permits: BIBE-2006-SCI-0003, BIBE-2013-SCI-0020, VIIS-2012-SCI-007, WHSA-2006-SCI-0004, TDPW 2013-01, TDPW 2014-01, OPRD-015-14, CPDCNBSP-2016-32 (P. Brown), and under a permit from the Secretario de Medio Ambiente y Recursos Naturales #09436 (M. Silva Briano).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Steven A. J. Declerck, Diego Fontaneto, Rick Hochberg & Terry W. Snell / Crossing Disciplinary Borders in Rotifer Research
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kordbacheh, A., Shapiro, A.N. & Walsh, E.J. Reproductive isolation, morphological and ecological differentiation among cryptic species of Euchlanis dilatata, with the description of four new species. Hydrobiologia 844, 221–242 (2019). https://doi.org/10.1007/s10750-019-3892-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3892-0