Abstract
The economically important marine red macroalga Gracilariopsis lemaneiformis was cultured for 2 weeks at two different temperatures, ambient and elevated CO2 levels, and two fertilizer levels. After the experimental period, growth, photosynthesis, and biochemical components were determined. Higher pCO2 significantly increased relative growth rates and nitrate reductase activity with the combination of higher temperature (HT) and/or N enrichment, whereas photosynthetic rates were rapidly reduced at the same condition. Both Q10 values for photosynthetic- (Pm) and respiratory (Rd) rates were higher in algae grown at 24 than at 20°C. This enhancement of Pm rate by HT was much more pronounced than that of the Rd rate. As a result, soluble carbohydrates produced by photosynthesis accumulated to higher levels at the higher temperature. Most of fluorescence variables and antioxidant enzyme activities (super oxide dismutase, catalase, peroxidase, malondialdehyde) did not differ among treatments. Additionally, higher pCO2 reduced chlorophyll a, carotenoids, phycoerythrin, and phycocyanin contents by 48.6, 41.7, 24.7, and 47.8% under LTLN condition, respectively, compared with the control treatment. We propose that high pCO2 would depress the carbon acquisition but increase N assimilation with higher temperature and/or coastal eutrophication, thereby exerting a positive effect on algal growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The atmospheric and oceanic concentrations of carbon dioxide have rapidly increased recently at an approximate rate of 2 ppm per year (Moreira & Pires, 2016), mainly due to the burning of fossil fuels combined with changes in land use. Our CO2-enriched world has significantly contributed to global warming and ocean acidification (OA), both of which have directly and indirectly had a strong impact on a wide range of organisms within the biosphere (Joos, 2015). Moreover, the global mean sea-surface temperature had already increased since the beginning of the industrial era, and has been projected to increase by 1.9°C by the end of the 21st century, and even by as much as 4°C in high northern latitudes (Bartsch et al., 2012; Alexander et al., 2013). It is necessary for the proposition of the Paris Agreement to set the goal of maintaining global temperature rise within 2°C above preindustrial levels, which is considered to be a sweeping-program of CO2 capture and storage (Archer et al., 2009; Schreurs, 2016), while biological approaches to CO2 capture remain research and policy priorities.
Apart from the immense challenges posed by rising global CO2 and temperature levels, eutrophication is another serious threat to environmental equilibrium. The rate and extent of eutrophication have been accelerated by anthropogenic activities (Taranu et al., 2015). In fact, several reasons (the expansion of urban populations and the increasing use of fertilizers in agriculture, etc.) ultimately enhance nutrient availability in coastal waters, which have rapidly developed contraries with increasing coastal populations (Liu & Wang, 2016). Excessive nutrient enrichment causes alternating cycles of algal flourishing and collapsing (i.e., red and green tides) (Farmaki et al., 2014). Moreover, changes in nutrient availability can alter the physiological properties of macroalgae (Hernández et al., 2006; Xu et al., 2010; Kang et al., 2017), even affect the production of primary and secondary metabolites. For instance, enrichment of the growth medium with nutrients effectively promoted the growth and photosynthesis in Gracilariopsis lemaneiformis (Bory) Dawson, Acleto et Foldvik (Xu et al., 2010), or changed the flow of C among compounds from carbohydrates to organic N compounds (Vergara et al., 1995). In addition, the carbohydrates of macroalgae have been displayed to be inversely related to nutrient concentrations (Marinho-Soriano et al., 2006), on account of carbohydrates from storage reserves toward the production of energy and carbon skeletons necessary to reduce nitrate and form amino acids, which both can cause starch degradation (Gouvêa et al., 2017).
Despite algae proliferation (microalgae and macroalgae) being a symptom of the eutrophication dilemma, algae have gained increasing attention as CO2 and nutrient bio-capture candidates. Due to their large biomass and relatively long turnover time, marine macroalgae are more effective carbon sinks than microalgae (Smith, 1981). Species of G. lemaneiformis exhibited high growth rates with strong CO2 capture abilities and a high affinity for nutrients (Xu et al., 2010). However, the responses of Gracilariopsis to a sustained increase of carbon and nutrients in the ocean in the future are not well known.
In coastal areas, global change (an interactive effect of elevated CO2 and temperature) and eutrophication may occur simultaneously, rather than individually. The interactive effects of two or three separate factors were possibly quite strong as compared to the independent effects of any separate factor by itself. To the best of our knowledge, no study has yet investigated the interactive effects of ocean warming and eutrophication on G. lemaneiformis from the perspective of their potential for carbon and nutrient capture for PSII, as well as their antioxidant enzyme activities and metabolic production but on other macroalga species (Binzer et al., 2016; Ontoria et al., 2019; Schulhof et al., 2019). Therefore, in this study we cultured G. lemaneiformis at current and simulated future conditions in the ocean. The objectives of the present study were to investigate: (1) whether there is an acclimation of photosynthesis in response to higher pCO2 and temperature, and if so, whether the degree of such acclimation is affected by N availability; (2) whether short-term (or long-term) exposure to higher temperature inhibits photosynthetic- and respiratory rates, and if so, whether this direct (or indirect) effect varies with growth, CO2 and N availability.
Materials and methods
Sample collection and culture conditions
Gracilariopsis lemaneiformis was collected in November 2017 from the cultivation field at Shenao Bay, Nanao Island, Shantou, in southern China (23.5° N, 117.1° E). Thalli were placed in a zip-lock plastic bag and transported to the laboratory within 1 h, where they were gently rinsed in one-micron filtered natural seawater to remove visible epiphytes and any accumulated sediments. Healthy thalli were randomly assigned to nine plastic tanks (10 L) for maintenance. Each tank contained 8 L of natural seawater. Thalli in the tanks were irradiated with cool-white light at 150 μmol photons m−2 s−1, under a 12/12 h light/dark regime, at 15°C for 2 days. The interactive effects of ocean warming, elevated CO2 and eutrophication were investigated using a fully crossed factorial design. Thalli were incubated under combinations of two pCO2 levels (400, 1000 μl L−1; designated as lower pCO2, LC and higher pCO2, HC, respectively), two temperatures (20, 24°C; designated as LT and HT, respectively), and two nutrient conditions (25 μM N as nitrate and 2.5 μM P as phosphate, or 500 μM N and 50 μM P; designated as LN and HN, respectively). These treatments under which G. lemaneiformis was grown were representative of current ambient and simulated, future ocean conditions. Experimental treatments started when 2.5 g fresh weight (FW) thalli were introduced into each of 24 flasks containing 2 L of filtered seawater. Three replicates were set up for each treatment. The flasks were placed in two temperature-controlled chambers; temperature inside the chambers was controlled at either 20 or 24°C, respectively. In each chamber, six flasks were aerated with atmospheric CO2, and the remaining flasks were aerated with elevated CO2. Three of the six flasks under either CO2 treatment were incubated under the HN condition, and the other three were incubated under the LN condition. Before measurement of experimental indictors, total alkalinity (TA) was measured by titration. Carbonated system values not directly measured were calculated using CO2SYS with the carbonic equilibrium constants (K1, K2) and KSO4− dissociation constant (Dickson, 1990; Pierrot et al., 2006). Carbonate chemical changes are shown in Table 1. Nutrient concentrations were maintained relatively constant by changing fresh seawater every 2 days. Culture temperatures and CO2 levels were controlled using a microcomputer system embedded in the laboratory incubators. The thalli were illuminated with a light intensity of 150 μmol photons m−2 s−1 under a 12/12 h light/dark photoperiod. Algae were cultured for 2 weeks during which seawater was fully renewed every 2 days.
Relative growth rate (RGR)
Biomass of thalli was measured in each treatment to estimate growth. The RGR was estimated as follows: RGR (% d−1) = (lnWt − lnW0) × t−1 × 100%, where W0 was the initial fresh weight, and Wt was the culture weight after t days of cultivation. Thalli were gently blotted on filter paper before FW weighing.
Determination of respiration and photosynthesis
Net photosynthetic rate (Pn) of the thalli was measured by a Clark-type oxygen electrode (YSI 5300, USA) held in a circulating water-bath to keep the desired temperature. Approximately 0.05 g FW algae sampled from each flask was introduced into the electrode chamber together with 8 mL of reaction seawater, same as their respective culture medium, and magnetically stirred. Algae were allowed to equilibrate in darkness until the rate of oxygen consumption was constant, usually for 4–6 min, prior to determination of respiration rate (Rd). Sequentially, oxygen fluxes were measured at light irradiances from 0 to 700 μmol photons m−2 s−1 to ensure active photosynthesis. Rd and Pn (Pg) rates were recorded to examine the instantaneous effect of temperature (measured temperature: 20, 24 and 28°C) on the algae with their own grown culture medium. Photosynthetic efficiency (α) was estimated from the slope of the initial linear region of photosynthesis-irradiation (P–I) curves. Light saturation (Ek) was calculated as Ek = Pm/α (Henley, 2010), where Pm was the maximum net photosynthesis. Gross photosynthetic rate (Pg), was obtained from the sum of Rd plus Pn. In turn, Rd and Pn (Pg) rates were measured at different temperatures (20, 24 and 28°C) to examine temperature effects on photosynthetic and respiratory flux. Different measured temperatures were adjusted in the oxygen electrode chamber.
Q10 values, which were termed as the proportional change in photosynthesis and respiration per 10°C rise, were calculated for photosynthesis and respiration over the measured temperature interval of 20–28°C using the following equation according to Atkin et al. (2010): Q10 = 10(slope·10), where the slope was the regression slope of log10-transformed photosynthetic- and respiratory rates verse temperature plot.
Measurement of fluorescence variables
Approximately 0.03 g FW thalli sampled from each culture flask was transferred to the cuvette with 2 mL of sterilized seawater. Thalli were firstly dark-adapted for 10 min and then the index of chlorophyll a fluorescence transient was measured using an Aqua Pen fluorometer-100-PC (PSI, Photon Systems Instruments, Czech Republic), which supplied a saturating light pulse of 3000 μmol m−2 s−1 during the measurement. Fluorescent transients were recorded over the time-span from 10 μs to 1 s, at 10-μs intervals. The resultant O-J-I-P transients were analyzed according to the JIP-test. Meanwhile, several relative variables were also calculated to explain PSII behavior, such as phenomenological fluxes/activities (effective antenna size of an active RC, ABS/RC; maximal trapping rate of PSII, TRo/RC; electron transport in an active RC, ETo/RC; effective dissipation of an active RC, DIo/RC), flux ratios/yields (maximum quantum yield of primary photochemistry, ψPo; electron transport probability, ψo; and quantum yield of electron transport, ψEo), and density of reaction centers (RC/CS).
With the Junior-PAM, rapid light curves (RLCs; so-called “short-term” (rapid) photosynthesis-irradiance curves) were measured yielding Ek (light saturation value) and rETRm (relative maximum electron transport rate) values. These were measured with light over the 400–700 nm wavelength interval while increasing from 0 to 820 μmol photons m−2 s−1, with an exposure time of 20 s at each photon flux density (PFD). Total in vivo Chl fluorescence signal was also recorded from PAM.
Physiological and biochemical variables
Estimation of antioxidant characteristics
Antioxidant activities, superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) are important. We used the SOD assay kit, the CAT kit and POD kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for each enzyme assay, respectively. The SOD assay was based on SOD ability to oxidize hydroxylamine by the xanthine–xanthine oxidase system (Oyanagui, 1984). One unit (U) of SOD activity was calculated as the inhibition by 50% of the oxidation of hydroxylamine without an enzyme source. The CAT assay was according to the method of Góth (1991). The POD assay was based on the method of Lawrence et al. (1974), after minor changes. In addition, we used a malondialdehyde (MDA) assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the method of Ohkawa et al. (1979).
Biochemical composition
Approximately 0.2 g FW thalli from each treatment were ground thoroughly in 10 mL methanol and placed in a refrigerator at 4°C for 24 h in total darkness. Then, the homogenates were centrifuged for 10 min at 5000 g and the supernatants were used to determine chlorophyll a (Chl a) and carotenoids (Car) content spectrophotometrically, according to the method of Wellburn (1994). The concentrations of phycobiliprotein (PB) in the supernatants were determined spectrophotometrically according to Beer and Eshel (1985). For extraction of soluble carbohydrates (SC) and soluble protein (SP), about 0.2 g of FW thalli from each treatment were ground in a mortar with distilled water and extraction buffer (0.1 M phosphate buffer, pH 6.8); SC were determined as sucrose equivalents, by the phenol–sulphuric acid method. SP was estimated from the supernatants using the Bradford (1976) assay with bovine serum albumin as a standard.
Nitrate reductase activity (NRA)
The NRA in the thalli was assayed according a modified in situ method (Corzo & Niell, 1991). Due to a circadian periodicity of the NRA, such as a maximum during the light period and a minimum in darkness (Whitaker, 1989; Deng et al., 1991), here the measurement was conducted during local noon. Approximately 0.1 g FW thalli from each treatment was incubated for 30 min at 30°C in darkness in 10 mL of a buffer (pH 8.1) containing 0.1 M phosphate buffer, 50 mM KNO3, 10 μM glucose, and 0.5 mM EDTA. The mixture was flushed with pure N2 gas (99.999%) for 2 min to attain anaerobic conditions before incubation. The concentration of NO2− produced from NO3− was determined colorimetrically at 543 nm; NRA was expressed as μmol NO2− g−1 FW h−1.
Data analysis
Data were expressed as means of three replicates ± standard deviation (SD). The statistical (multifactorial ANOVAs and complemented with Duncan test) was performed to assess the effects of pCO2 (400 μatm, LC; 1000 μatm, HC), temperature (20°C, LT; 24°C, HT) and nutrients (low (LN)-25 μM N as nitrate and 2.5 μM P as phosphate; high (HN)-500 μM N and 50 μM P) on physiologic and biochemical characteristics of G. lemaneiformis, using IBM SPSS Statistics 23.0 software with a significance level of 5% (P < 0.05). The pCO2, temperature, and nutrient levels were considered as fixed factors. The normality and assumption of homogeneity of variances were checked during the process of multifactor ANOVA. Where necessary, data were transformed in order to reduce structure in analytical residuals and homogenize variances. The graphs in present study were made using Origin 8.5 software and Microsoft Office 2016.
Results
Relative growth rates (RGR)
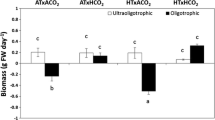
The temperature and pCO2 (T× C) showed interactive effects (double effect) on RGR of G. lemaneiformis (ANOVA, F(1,16) = 11.560, P = 0.004). The pCO2 also showed a main effect (ANOVA, F(1,16) = 44.737, P = 0.000) on RGR. At HTLN condition, higher pCO2 increased RGR by 31.3% (Fig. 1, P < 0.05). Furthermore, with the combination of higher T and N-enriched conditions, higher pCO2 enhanced RGR by 58.1% (F(1,16) = 11.828, P = 0.003).
The relative growth rates (RGR) of Gracilariopsis lemaneiformis grown at the experimental conditions during the cultivation. The error bars indicated the standard deviations (n = 3). LT lower temperature (20°C); HT higher temperature (24°C); LC lower pCO2 (400 μl L−1 CO2); HC higher pCO2 (1000 μl L−1 CO2); LN lower nutrients (25 μM N as nitrate and 2.5 μM P as phosphate); HN higher nutrients (500 μM N and 50 μM P)
Respiration and photosynthesis
The fitting values (Pm and α) derived from gross photosynthetic rates (Pg; i.e., Pn plus Rd, shown in Fig. 2a–f) are displayed in Table 2. All factors (temperature, T; pCO2 level, C; nutrient supply, N; measuring temperature, MT), as well as the interactive effects among these factors, almost had main effects on irradiance-saturated maximum photosynthetic rates (Pm) and apparent photosynthetic efficiency (α) (Table 3, P < 0.05). A Duncan comparison (P = 0.05) showed that higher pCO2 did not always have an impact on Pm at the lower temperature (20°C), while it reduced Pm rates by 20.0% (30.7%) under HTLN condition (or HTHN condition, 24°C) (Table 3, P < 0.05). In contrast, higher pCO2 increased the α by 41.5% under LTLN condition (F(1,48) = 21.40, P = 0.000), while it decreased the α by 17.5% with increased growth temperature (HTHCLN, F(1,48) = 49.72, P = 0.000). As for respiratory rates (Rd, Fig. 3), both T (ANOVA, F(1,48) = 23.799, P = 0.000) and pCO2 (ANOVA, F(1,48) = 9.434, P = 0.004) had main effects on them. However, there seemed no significant changes of Rd rates together with the interaction of higher T and N or enriched C and N (Fig. 3a, b).
The fitting curves of gross photosynthetic rates (Pg) verse irradiances for Gracilariopsis lemaneiformis grown at the experimental conditions during the cultivation. LT lower temperature (20°C); HT higher temperature (24°C); LC lower pCO2 (400 μl L−1 CO2); HC higher pCO2 (1000 μl L−1 CO2); LN lower nutrients (25 μM N as nitrate and 2.5 μM P as phosphate); HN higher nutrients (500 μM N and 50 μM P). The Pg rates were measured at 20°C, 24°C and 28°C, respectively. Vertical bar represented ± SD of the means (n = 3)
Respiratory rates (Rd) of Gracilariopsis lemaneiformis exposure to combined conditions of growth temperature (20 and 24°C, T), CO2 levels (400 and 1000 μl L−1), nutrient levels (25 μM N as nitrate and 2.5 μM P as phosphate, LN; 500 μM N and 50 μM P, HN), and measured temperature (20, 24 and 28°C). The approaches were conducted with samples for measuring at three temperatures. Data were pooled means ± SD, in accordance with significant effects obtained by ANOVA: interactive effects of T and N (a, n = 18), interactive effects of C and N (b, n = 18), interactive effects of T and MT (c, n = 12). Different letters above the histograms indicate significant difference (P < 0.05, Duncan post hoc test)
The instantaneous response of photosynthesis to changes in temperature showed that Pm rates increased with increasing measurement temperature up to 28°C (Table 2). The Q10 values of Pm rates indeed increased with an enhanced temperature interval (24–28°C, Table 4, P < 0.05). For 24°C-grown G. lemaneiformis, high pCO2 significantly (P < 0.05) reduced the Q10 values of Pm rates when measured at the higher temperature interval under evaluation. The MT and T had an interactive effect on Rd rates of G. lemaneiformis (ANOVA, F(1,48) = 4.584, P = 0.037). A Duncan comparison (P = 0.05) showed that higher measured temperature significantly decreased Rd rates of algae grown at low temperature (20 oC, Fig. 3c, P < 0.05), together with the higher Q10 values at two temperature intervals (20–24°C and 24–28°C, Table 4, P < 0.05). Moreover, the Q10 values of the Rd rates were much higher in low-C and low-N (LCLN) than high-C and/or high-N conditions, regardless of temperature (P < 0.05).
OJIP fluorescence curves and rapid light curves (RLCs)
Figures showed fluorescence induction curves for dark-adapted G. lemaneiformis (see supplementary materials). The highest polyphasic chlorophyll fluorescence transients (OJIP) intensities showed for the control treatment (LTLCLN). High culture temperature (24°C) increased OJIP intensities of G. lemaneiformis during the whole duration of the experiment. Selected variables of Chl a fluorescence and variables derived by the JIP-test are shown in Fig. 4a–h. All factors (T, C, N), as well as the interactive effects among these factors, almost had no significant effects on those variables (Table 5). The differences in patterns displayed by these variables indicated the extent of the impact of elevated temperature/CO2/nutrients in G. lemaneiformis. The ABS/RC (Fig. 4a), TRo/RC (Fig. 4b), ETo/RC (Fig. 4c), DIo/RC (Fig. 4d), ψPo (Fig. 4e), ψEo (Fig. 4f), and ψo (Fig. 4g) did not increase at high temperature, pCO2, and nutrient level (P > 0.05). In contrast, compared to low CO2, higher pCO2 reduced RC/CS by 84.6% (80.9%) at 20°C (Fig. 4h, P < 0.05), and this decrease was alleviated when temperature increased (24°C), for example, higher pCO2 reduced RC/CS by 24.8% with higher T and N enrichment, compared to the control.
The phenomenological fluxes of log scale OJIP chlorophyll fluorescence transients (ABS/RC = effective antenna size of an active RC, aTRo/RC maximal trapping rate of PSII, bETo/RC electron transport in an active RC, cDIo/RC effective dissipation of an active RC, d flux ratios or yields (ψPo = maximum quantum yield of primary photochemistry, eψEo = the quantum yield of electron transport, f and ψo = electron transport probability, g and density of reaction centers (RC/CS, h and RLCs variables (maximum relative transport rates, rETRm, i the light saturation point, Ek, μmol photos m−2 s−1, j and the initial photosynthetic efficiency, α, k of Gracilariopsis lemaneiformis grown at the experimental conditions during the cultivation. The error bars indicated the standard deviations (n = 3). LT = lower temperature (20 oC); HT higher temperature (24°C); LC lower pCO2 (400 μl L−1 CO2); HC higher pCO2 (1000 μl L−1 CO2); LN lower nutrients (25 μM N as nitrate and 2.5 μM P as phosphate); HN higher nutrients (500 μM N and 50 μM P)
Maximum relative electron transport rates (rETRm), half-light saturation (Ek) and photochemical yield (α) obtained from the RLCs of G. lemaneiformis are shown in Fig. 4i–k. The T and N had an interactive effect (ANOVA, F(1, 80) = 5.814, P = 0.018) on rETRm of G. lemaneiformis. Likewise, T × C (ANOVA, F(1,80) = 8.250, P = 0.005), T × N (ANOVA, F(1,80) = 11.528, P = 0.001) and C × N (ANOVA, F(1,80) = 8.789, P = 0.004) had the most double interactive effect on Ek. In contrast, no separate factor had a main effect on α (P > 0.05). A Duncan comparison (P = 0.05) showed that the rETRm (Fig. 4i), Ek (Fig. 4j) and α (Fig. 4k) remained constant under all treatments in the present study (P > 0.05), with the exception of reduction of rETRm and Ek at higher T and N conditions.
Physiological and biochemical variables
Antioxidant characteristics
The extent of SOD (Fig. 5a), CAT (Fig. 5b), and POD (Fig. 5c) activities was generally not affected by higher temperature, elevated CO2, and nutrient levels (P > 0.05). Likewise, significant differences in MDA concentration were not found among treatments (Fig. 5d, P > 0.05).
The antioxidant characteristics (activities of SOD, a CAT, b POD, c MDA contents, d biochemical compositions (the contents of chlorophyll a, Chl a, e carotenoids, Car, e phycoerythrin, PE, f phycocyanin, PC, f soluble carbohydrates, SC, g soluble proteins, SP, h, and nitrate reductase activity (NRA, i of Gracilariopsis lemaneiformis grown at the experimental conditions during the cultivation. The error bars indicated the standard deviations (n = 3). LT lower temperature (20°C); HT higher temperature (24°C); LC lower pCO2 (400 μl L−1 CO2); HC higher pCO2 (1000 μl L−1 CO2); LN lower nutrients (25 μM N as nitrate and 2.5 μM P as phosphate); HN higher nutrients (500 μM N and 50 μM P)
Biochemical composition
Higher pCO2 decreased Chl a and Car contents by 48.6% and 41.7% under LTLN condition, respectively (Fig. 5e). Likewise, higher pCO2 reduced PE and PC contents by 24.7% and 47.8% under LTLN condition, respectively (Fig. 5f). There were no obvious variations in PC among other treatments. Soluble carbohydrates (SC, Fig. 5g) and protein (SP, Fig. 5h) were estimated to understand the effects of ocean warming and eutrophication on the products of carbon and nitrogen assimilation in G. lemaneiformis. The T × C had an interactive effect on the SC (ANOVA, F(1,16) = 7.333, P = 0.016). Both T and C had a main effect on the SC (ANOVA, F(1,16) = 135.076, P = 0.000 for T; ANOVA, F(1,16) = 52.274, P = 0.000 for C). At 20°C, thalli under high pCO2 showed a slight change of SC compared with the control. However, high pCO2 combined with high T exhibited a positive effect on SC (P < 0.05, Fig. 5g). Additionally, the interactive effect of the three factors under study (T × C × N) was detected on SP content in G. lemaneiformis (ANOVA, F(1,16) = 4.657, P = 0.046). The higher N level increased SP content by 19.4% at HTLC, and increased it by 20.4% at HTHC, respectively, compared with the control (Fig. 5h).
Nitrate reductase activity (NRA)
The three factors showed an interaction effect on NRA of G. lemaneiformis (ANOVA, F(1,16) = 15.386, P = 0.001) and T/pCO2/N had a main effect (ANOVA, F(1,16) = 7.902, P = 0.013 for T; ANOVA, F(1,16) = 11.675, P = 0.004 for C; ANOVA, F(1,16) = 31.816, P = 0.000 for N). Higher pCO2 increased NRA by 145.6% under HTLN (compared with that at ambient CO2 level), while higher T decreased NRA by 53.1% under LCLN (compared with that at low temperature) and higher N decreased NRA by 44.7% (59.1%) under LTLC (under HTHC, compared with that at low nutrient level), respectively (Fig. 5i, P < 0.01).
Discussion
In the present study, higher pCO2 reduced gross photosynthetic rate in Gracilariopsis lemaneiformis at higher temperature and nutrient level. This observation is consistent with our previous study (Liu et al., 2016). The negative effects or the absence of any effects of elevated CO2 on photosynthesis were also reported in other macroalgal species, such as Ulva conglobate Kjellman (Deng & Zou, 2014), and Phaeocystis spp (Keys et al., 2018). In contrast, a promoting effect of elevated CO2 on photosynthesis was demonstrated in Ulva linza Linnaeus [Enteromorpha linza (L.) J. Ag.] (Gao et al. 1999), Pyropia haitanensis Chang et Zheng (Zou & Gao, 2002), Petalonia binghamiae (J. Agaradh) Vinogradova (Zou & Gao, 2010), and Sargassum muticum (Yendo) Fensholt (Xu et al., 2017).
Generally, the long-term response of photosynthesis to CO2 enrichment shows as a decreasing or even an inhibitory tendency in most plants (Porras et al., 2017). In the present study, the possible explanation for a decrease in photosynthesis in G. lemaneiformis might involve an excess accumulation of carbohydrates or the decline of NR activity (responsible for the reduction in N content), together with a negligible consumption of photosynthetic products through respiration. However, the obvious enhancement of growth was shown in G. lemaneiformis as a result of an interaction between higher pCO2 and higher temperature. This indicted that elevated CO2 might stimulate other assimilation processes that support growth. In this study, elevated CO2 did enhance N assimilation of the algae under higher temperature and lower nutrient levels.
Photosynthetic rates of G. lemaneiformis were significantly affected by the instantaneous change in temperature, termed temperature sensitivity, which could be described by the Q10 value. Our results showed that the values of Q10 for photosynthesis significantly increased with higher temperature. However, they were reduced when the temperature raised to 33°C (Zou & Gao, 2013). Indeed, previous studies demonstrated that the Q10 response of gross photosynthetic capacity was heterogeneous among terrestrial species and other macroalgae, upon exposure to high temperature (Gerard, 1997; Tjoelker et al., 2001; Atkin & Tjoelker, 2003). Likewise, changes in temperature also resulted in an immediate alternation in the rate of Rd. The calculated Q10 value for Rd was generally greater when temperature increased, and the value of Q10 increased significantly under HTLCLN (resulting in an increased temperature sensitivity for Rd at ambient CO2 level), which was higher than that often assumed in plants (Tjoelker et al., 2001). These results also exhibited similar Q10 values for respiration under two different culture temperatures at elevated CO2. Furthermore, the extent of acclimation of respiration and photosynthesis of G. lemaneiformis to a new culture temperature had been confirmed in previous reports, which might suggest possible large variations in acclimation potential to thermal environment (Zou & Gao, 2013; Liu et al., 2016). Additionally, our results showed that Q10 values were higher for photosynthesis than for respiration, implying that photosynthetic processes in G. lemaneiformis had a higher degree of temperature dependence than respiratory processes.
The effect of interaction of environmental factors on PSII photosynthetic performance in G. lemaneiformis, are just obtained from the JIP-test. The ψPo was used to evaluate PSII photosynthetic performance of G. lemaneiformis mainly due to a good correlation between this method and results obtained by the Junior-PAM. Similar studies have reported a high correlation between flash fluorescence induction and Phyto-PAM measurements (Pierangelini et al., 2014a, b). The ABS/RC, which refers to the photons absorbed per active reaction center, responded to different environmental factors similarly in G. lemaneiformis, indicting the quantity of antenna chlorophyll played similar functions regardless of treatment, so that light was harvested to support the subsequent photochemical process based on the integrity of the photosynthetic apparatus of the thalli (the antioxidant defense system functioned well and resulted in no lipid peroxidation events). The main photosynthetic inhibition targets by high CO2 on G. lemaneiformis were a decrease in the number of active reaction centers (RC/CS) at lower temperature (20°C). This implied that the density of photosynthetic units was reduced, thereby reducing photochemical transfer of harvested and excited photons. However, at higher nutrient level and higher temperature, increased excitation energy was transferred to the reaction centers (RCs) and converted in the RCs to redox energy by reducing the electron acceptor QA to Q −A , which was then re-oxidized to QA leading to electron transport. Nitrogen assimilation was a competing sink for electrons to CO2 fixation. The lower proportion of electrons distributed to NRA ultimately resulted in lower nitrogen metabolism (Figueroa et al., 2003; Liu & Zou, 2015). Our observations about NRA also confirmed this point. Ultimately, as the photochemical flux of photons and further metabolic events in the photosystem of G. lemaneiformis were maintained, the growth increase of the algae was expressed.
It has been suggested that CO2-induced global warming will lead to increases in mean global sea-surface temperatures by 1.0–4.4°C by the end of this century (Solomon et al., 2007); further, there is increasing concern over the potential consequences of anthropogenic climate change on marine systems and organisms (Helmuth et al., 2013). In order to predict the effects of climate change on mariculture systems of seaweeds in a warmer and eutrophicated ocean, it is imperative to understand the performance of algal photosynthesis, respiration and biochemical components during the period of algal cultivations at sea. Our results suggest that photosynthesis of G. lemaneiformis was favored over respiration as temperatures increased, yet nutrient assimilation decreased as a result of elevated CO2 and temperature. Therefore, seemingly, ocean warming may benefit photosynthetic carbon metabolism and may in fact enhance mariculture productivity of G. lemaneiformis. An increased capacity for carbon sequestration may be a negative feedback reaction to the environmental changes caused by human activities and would thus alleviate some aspects of global climate change.
Conclusions
It is essential to further investigate the antioxidant and gene expression characteristics of G. lemaneiformis in order to evaluate the possible consequences of ocean warming, increased atmospheric CO2 level, and increased eutrophication. Our results suggest that photosynthesis of G. lemaneiformis had a higher degree of temperature dependence than respiratory processes. Furthermore, high pCO2 would depress the carbon acquisition but increase N assimilation with higher temperature and/or coastal eutrophication, thereby exerting a positive effect on algal growth. Anyway, G. lemaneiformis could optimize metabolic balance by adjustment of carbon and nutrient capture in harmony with future global climate changes, which would have important ecophysiological implications for sea cultivation of this species.
References
Alexander, L., S. Allen & L. Nathaniel, 2013. Climate change 2013: the physical science basis summary for policymakers. Intergovernmental Panel on Climate Change, Geneva.
Archer, D., M. Eby, V. Brovkin, A. Ridgwell, L. Cao, U. Mikolajewicz, K. Caldeira, K. Matsumoto, G. Munhoven & A. Montenegro, 2009. Atmospheric lifetime of fossil fuel carbon dioxide. Annual Review of Earth and Planetary Sciences 37(1): 117–134.
Atkin, O. K. & M. G. Tjoelker, 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8(7): 343–351.
Atkin, O. K., E. J. Edwards & B. R. J. Loveys, 2010. Response of root respiration to changes in temperature and its relevance to global warming. New Phytologist 147(1): 141–154.
Bartsch, I., C. Wiencke & T. Laepple, 2012. Global seaweed biogeography under a changing climate: the prospected effects of temperature. Seaweed Biology. https://doi.org/10.1007/978-3-642-28451-9_18.
Beer, S. & A. Eshel, 1985. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Australian Journal of Marine and Freshwater Research 36(6): 785–792.
Binzer, A., C. Guill, B. C. Rall & U. J. G. C. B. Brose, 2016. Interactive effects of warming, eutrophication and size structure: impacts on biodiversity and food-web structure. Global Change Biology 22(1): 220–227.
Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(s1–2): 248–254.
Corzo, A. & F. X. Niell, 1991. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J Exp Mar Biol Ecol 146(2): 181–191.
Deng, Y. Y. & D. H. Zou, 2014. Effects of elevated atmospheric CO2 on photosynthesis of Gracilaria lemaneiformis and Ulva conglobata grown at low and high N supplies. Chinese Journal of Ecology 33(6): 1520–1527.
Deng, M. D., T. Moureaux, I. Cherel, J. P. Boutin & M. Caboche, 1991. Effects of nitrogen metabolites on the regulation and circadian expression of tobacco nitrate reductase. Plant Physiology and Biochemistry 29(3): 239–247.
Dickson, A. G., 1990. Standard potential of the reaction: ja:math, and and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. The Journal of Chemical Thermodynamics 22(2): 113–127.
Farmaki, E. G., N. S. Thomaidis, I. N. Pasias, C. Baulard, L. Papaharisis & C. E. Efstathiou, 2014. Environmental impact of intensive aquaculture: Investigation on the accumulation of metals and nutrients in marine sediments of Greece. Science of the Total Environment 485–486(3): 554–562.
Figueroa, F. L., R. Conde-Álvarez & I. Gómez, 2003. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynthesis Research 75(3): 259–275.
Gao, K., J. Yan & Y. Aruga, 1999. Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia 398–399(3): 355–359.
Gerard, V. A., 1997. The role of nitrogen nutrition in high-temperature tolerance of the kelp, laminaria saccharina (chromophyta). Journal of Phycology 33(5): 800–810.
Góth, L., 1991. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta 196(2–3): 143.
Gouvêa, L. P., N. Schubert, C. D. L. Martins, M. Sissini, F. Ramlov, E. R. D. O. Rodrigues, E. O. Bastos, V. C. Freire, M. Maraschin & J. J. L. Carlos Simonassi, 2017. Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnology and Oceanography 62(5): 2056–2075.
Helmuth, B., E. Babij, E. Duffy, D. Fauquier, M. Graham, A. Hollowed, J. Howard, D. Hutchins, L. Jewett & N. Knowlton, 2013. Impacts of Climate Change on Marine Organisms. Island Press/Center for Resource Economics, Washington.
Henley, W. J., 2010. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. Journal of Phycology 29(6): 729–739.
Hernández, I., A. Pérez-Pastor, J. J. Vergara, J. F. Martínez-Aragón, M. Á. Fernández-Engo & J. L. Pérez-Lloréns, 2006. Studies on the biofiltration capacity of Gracilariopsis longissima : From microscale to macroscale. Aquaculture 252(1): 43–53.
Joos, F., 2015. Global warming: growing feedback from ocean carbon to climate. Nature 522(7556): 295–296.
Kang, J. W., C. Kambey, Z. Shen, Y. Yang & I. K. Chung, 2017. The short-term effects of elevated CO2 and ammonium concentrations on physiological responses in Gracilariopsis lemaneiformis (Rhodophyta). Fisheries and Aquatic Science 20(1): 18.
Keys, M., G. Tilstone, H. S. Findlay, C. E. Widdicombe & T. Lawson, 2018. Effects of elevated CO2 and temperature on phytoplankton community biomass, species composition and photosynthesis during an experimentally induced autumn bloom in the western English Channel. Biogeosciences 15(10): 3203–3222.
Lawrence, R. A., R. A. Sunde, G. L. Schwartz & W. G. Hoekstra, 1974. Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Experimental Eye Research 18(6): 563–569.
Liu, C. X. & D. H. Zou, 2015. Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Marine Biology Research 11(10): 1043–1052.
Liu, X. J. & H. L. Wang, 2016. Dianchi Lake, China: geological formation, causes of eutrophication and recent restoration efforts. Aquatic Ecosystem Health & Management 19(1): 40–48.
Liu, C. X., D. H. Zou, Y. F. Yang, B. B. Chen & H. Jiang, 2016. Temperature responses of pigment contents, chlorophyll fluorescence characteristics, and antioxidant defenses in Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) under different CO2 levels. Journal of Applied Phycology 29(2): 1–9.
Marinho-Soriano, E., P. Fonseca, M. Carneiro & W. J. B. T. Moreira, 2006. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresource Technology 97(18): 2402–2406.
Moreira, D. & J. C. Pires, 2016. Atmospheric CO2 capture by algae: negative carbon dioxide emission path. Bioresource Technology 215: 371–379.
Ohkawa, H., N. Ohishi & K. Yagi, 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95(2): 351–358.
Ontoria, Y., E. Gonzalez-Guedes, N. Sanmartí, J. Bernardeau-Esteller, J. M. Ruiz, J. Romero & M. J. M. E. R. Pérez, 2019. Interactive effects of global warming and eutrophication on a fast-growing. Mediterranean Seagrass. 145: 27–38.
Oyanagui, Y., 1984. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Analytical Biochemistry 142(2): 290–296.
Pierangelini, M., S. Stojkovic, P. T. Orr & J. Beardall, 2014a. Elevated CO2 causes changes in the photosynthetic apparatus of a toxic cyanobacterium, Cylindrospermopsis raciborskii. The Journal of Plant Physiology 171(12): 1091–1098.
Pierangelini, M., S. Stojkovic, P. T. Orr & J. Beardall, 2014b. Photosynthetic characteristics of two Cylindrospermopsis raciborskii strains differing in their toxicity. Journal of Phycology 50(2): 292.
Pierrot, D., E. Lewis, R. Wallace, D. Wallace, W. Wallace & D. W. R. Wallace, 2006. MS excel program developed for CO2 system calculations. Carbon Dioxide Information Analysis Center. https://doi.org/10.3334/cdiac/otg.co2sys_xls_cdiac105a.
Porras, M. E., P. Lorenzo, E. Medrano, M. J. Sánchez-González, G. Otálora-Alcón, M. C. Piñero, F. M. del Amor & M. C. J. F. P. B. Sánchez-Guerrero, 2017. Photosynthetic acclimation to elevated CO2 concentration in a sweet pepper (Capsicum annuum) crop under Mediterranean greenhouse conditions: influence of the nitrogen source and salinity. Functional Plant Biology 44(6): 573–586.
Schreurs, M. A., 2016. The Paris Climate Agreement and the Three Largest Emitters: China, the United States, and the European Union. Politics and Governance 4(3): 219.
Schulhof, M. A., J. B. Shurin, S. A. Declerck & D. B. J. G. C. B. Van de Waal, 2019. Phytoplankton growth and stoichiometric responses to warming, nutrient addition and grazing depend on lake productivity and cell size. Global Change Biology 25(8): 2751–2762.
Smith, S. V., 1981. Marine macrophytes as a global carbon sink. Science 211(4484): 838–840.
Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, H. L. Miller, S. Solomon & D. Qin, 2007. Climate change 2007: synthesis report. Contribution of Working Group I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers.
Taranu, Z. E., I. Gregory-Eaves, P. R. Leavitt, L. Bunting, T. Buchaca, J. Catalan, I. Domaizon, P. Guilizzoni, A. Lami & S. J. E. L. McGowan, 2015. Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecology Letters 18(4): 375–384.
Tjoelker, M. G., J. Oleksyn & P. B. Reich, 2001. Modelling respiration of vegetation: evidence for a general temperature-dependent Q10. Global Change Biology 7(2): 223–230.
Vergara, J., K. Bird & F. Niell, 1995. Nitrogen assimilation following NH4+ pulses in the red alga Gracilariopsis lemaneiformis: effect on C metabolism. The Marine Ecology Progress Series 122(122): 253–263.
Wellburn, A. R., 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144(3): 307–313.
Whitaker, J. R., 1989. Synthesis and degradation of nitrate reductase during the cell cycle of Chlorella Sorokiniana. Plant Physiology 89(1): 220–224.
Xu, Z. G., D. G. Zou & K. S. Gao, 2010. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Botanica Marina 53(2): 123–129.
Xu, Z. G., G. Gao, J. T. Xu & H. Y. Wu, 2017. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences 14(3): 1–36.
Zou, D. H. & K. S. Gao, 2002. Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. British Phycological Journal 37(4): 587–592.
Zou, D. & K. Gao, 2010. Acquisition of inorganic carbon by Endarachne binghamiae (Scytosiphonales, Phaeophyceae). European Journal of Phycology 45(1): 117–126.
Zou, D. H. & K. S. Gao, 2013. Thermal Acclimation of Respiration and Photosynthesis in the Marine Macroalga Gracilaria lemaneiformis (Gracilariales, Rhodophyta). Journal of Phycology 49(1): 61–68.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Nos. 41706136 and 31741018), the Natural Science Foundation of Guangdong Province (2018B030311029) and Science and Technology Planning Project of Guangzhou (201904010287 and 201804010494), the Natural Science Foundation of Anhui Province (1808085MC81), and the Research Foundation of Education Bureau of Anhui Province, China (KJ2017A384).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Zou, D., Liu, Z. et al. Ocean warming alters the responses to eutrophication in a commercially farmed seaweed, Gracilariopsis lemaneiformis. Hydrobiologia 847, 879–893 (2020). https://doi.org/10.1007/s10750-019-04148-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04148-2