Abstract
Insecticides are broadly applied in many urban areas of the western United States to control mosquito populations and reduce the prevalence of diseases such as West Nile and Zika Virus. We assessed the extent to which incidental exposure to the insecticide Permethrin affected drifting and benthic invertebrates in Spring Creek, Laramie, WY, USA. We collected drift samples before, immediately after (~ 3–6 h), and 1 day after (~ 28 h) insecticide application. We collected benthic invertebrates with a Hess sampler twice before and twice after spraying began. We measured an 11 × increase in the density of drifting aquatic insects immediately after pesticide application. Additionally, we observed an increase in drifting aquatic invertebrates 2.25 km downstream from treatment. A decrease in the biomass of benthic invertebrates and an increase in taxa richness at the end of the summer suggested that invertebrates are colonizing the stream but may be unable to persist. Fewer aquatic invertebrates in the stream may have cascading effects on the ecosystem by altering resources available to fish and riparian insectivores (e.g., bats, spiders, and amphibians). Understanding the unintended ecological impacts of current pest control practices could lead to more effective and less detrimental management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquito (Order Diptera, Family Culicidae) abatement programs are common across the United States and throughout the world to prevent the spread of disease to humans, pets, and livestock as mosquitos are vectors for various diseases including West Nile virus, Zika virus, Malaria, and Dengue (Impoinvil et al., 2007). Various methods of prevention and control often target specific life stages; aerial fogging targets the adult stage of mosquitos while insecticides administered directly to bodies of water target the egg, larval, and pupal stages of mosquitoes. Chemical control methods are some of the most common, but many areas also effectively use biological controls or a combination of these methods. Common insecticides include: Pyrethroids, Organophosphates, Organochlorines, and Carbamates (Chang et al., 2014; Zika Virus, 2014). These chemicals may be applied via airplanes, vehicles, and hand-sprayers while bodies of water may also be directly injected with chemicals (Kreutzweiser & Sibley, 1991; Sundaram, 1991; Whiles &Wallace, 1992; Davies & Cook, 1993). Our study investigated the degree to which an aerial mosquito treatment program altered macroinvertebrate communities in an urban stream.
Permethrin is a hydrophobic and lipophilic member of the Pyrethroid family of synthetic insecticides with a high photo-stability (Sundaram, 1991). Permethrin inhibits voltage-gated sodium channels in the nervous system of arthropods through contact or consumption (Toynton et al., 2009; Currie & McCarthy, 2010). Once in an arthropod’s system, Permethrin can cause muscle spasms, paralysis, and death (Toynton et al., 2009). Permethrin is not only effective as an aerial insecticide, but also as a treatment for clothing, mosquito nets, and mattress covers (Kells & Hymel, 2016). Permethrin can eliminate scabies in humans, a skin infestation caused by the parasitic mite Sarcoptes scabiei (Linnaeus, 1758) without adverse effects because voltage-gated sodium channels differ between humans and arthropods (Currie & McCarthy, 2010). However, cases of irritation or toxicity in humans have been reported (Reactions Weekly, 2011; Reactions Weekly, 2015; Anziska & Rahman, 2016). The broad use of Permethrin has negative consequences in many cases. For example, veterinarians use products containing Permethrin to treat ticks on dogs, but Permethrin can cause poisoning in cats (Sparks et al., 2016). Permethrin is also widely used to treat lice, many of which are now resistant to the chemical (Kyong et al., 2008). Permethrin effectively kills many arthropods as a pest control or medical treatment but can have undesired effects in many circumstances.
Permethrin is commonly used to control mosquito populations but often affects non-target animals (Toynton et al., 2009), including terrestrial and aquatic organisms (Cox & Wilson, 1984; Sundaram, 1991; Peterson et al., 2016). For example, Permethrin can kill lady bugs when applied through an ultra-low volume fogger (Peterson et al., 2016) and can change honey bee behavior (Cox & Wilson, 1984). In water, Permethrin was toxic to rainbow trout [Oncorhynchus mykiss (Walbaum, 1792)] at concentrations as low as 6 μg/l (Baser et al., 2003). When Permethrin was applied to a Canadian stream directly, the stream bed, detritus, crayfish [Orconectes propinquus (Girard, 1852)], brook trout [Salvelinus fontinalis, (Mitchill, 1814)], and aquatic vegetation absorbed the chemicals, acting as sinks (Sundaram, 1991). The maximum Permethrin concentrations measured in plants and fish during that study surpassed the maximum concentrations of the stream water (Sundaram, 1991). Characteristics of streams including turbidity, primary production, and discharge can alter how Permethrin moves through the ecosystem (Sundaram, 1991; Moore et al., 2014). For example, a natural wetland reduced the concentration of Permethrin flowing through the site in a shorter distance from the source than an aquatic environment without plants (Moore et al., 2014). Like plants and sediment, aquatic invertebrates can act as sinks for chemicals and be negatively affected by Permethrin (e.g., Wallace & Hynes, 1981; Everts et al., 1983; Sundaram, 1991). For instance, Davies and Cook (1993) observed an increase in benthic invertebrate abundance over time in a control stream, but they did not observe the same increase in a stream treated with pyrethoids.
Sub-lethal effects from applying pyrethroid insecticides can alter stream ecosystems through changes in behavior or growth amongst biota without killing individuals. For example, caddisfly larva (Trichoptera) abandoned their cases after exposure to sub-lethal concentrations of pyrethroids, making them more vulnerable to predation (Johnson et al., 2008). Permethrin decreased the growth of the midge, Chironomus dilutus (Shobanov, 1999), at concentrations as low as 22.51 ng/l (Hasenbein et al., 2015). Further, pyrethroid concentrations that reduced C. dilutus mobility were eight times lower than those that decreased C. dilutus survival in lab experiments (Hasenbein et al., 2015). These results suggest that extremely low concentrations of Permethrin can impair the fitness of aquatic invertebrates living in aquatic ecosystems.
We studied the effect of Permethrin on non-target invertebrates in Spring Creek, an urban stream flowing through Laramie, Wyoming, during the typical mosquito abatement season. The questions we asked were: (1) To what degree does Permethrin application affect non-target aquatic invertebrates? (2) Did the density and biomass of invertebrates decrease throughout the summer during Permethrin application? (3) Did the responses of invertebrate taxa to Permethrin application differ? To address these questions, we sampled drifting and benthic aquatic macroinvertebrates in Spring Creek during the traditional mosquito control season in Laramie, Wyoming. Invertebrates can drift for a variety of reasons including to escape unfavorable conditions such as toxins (Wallace & Hynes, 1981; Brittain & Eikeland, 1988). We sampled drifting invertebrates to measure if invertebrate drift increased after spraying events. We sampled benthic invertebrates to investigate the degree to which their density or biomass decreased throughout the spraying season. Without chemical disturbance, we expect invertebrate drift to have small variance from day to day (Waters, 1972) and benthic invertebrate biomass should increase throughout the summer (Davies & Cook, 1993). Quantifying Permethrin’s effects on aquatic invertebrates in an urban stream may help managers reevaluate mosquito control methods to maximize control of mosquitos and mitigate negative effects on the stream ecosystem.
Study area
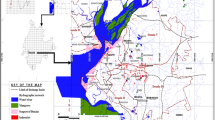
Spring Creek is a small (~ 5.6 km long, ~ 1.5 m wide) spring-fed urban stream, flowing entirely within the city limits of Laramie, Wyoming, USA (Fig. 1). Spring Creek supports a year-round population of brown trout (Salmo trutta Linnaeus, 1758), and provides an important habitat resource for spawning trout from the Laramie River (S. Gale, personal communication). Permethrin was applied immediately adjacent to Spring Creek for the stream’s entire length as an indiscriminate fog via an ultra-low volume fogger on a tank truck throughout the summer. To estimate the impact of Permethrin on Spring Creek we sampled two sites: LaPrele Park, which is typically fogged ~ 5 nights a week, and 9th Street Bridge (2.25 km downstream), which is fogged ~ 2 nights per week during the mosquito season (late May–August).
Methods
We measured Permethrin concentrations and water temperature to assess conditions at each site. We collected water samples to analyze the concentration of Permethrin in the stream on each sampling day and immediately transported them to the Wyoming Department of Agriculture’s Analytical Services Laboratory where they were analyzed for Permethrin (method detection limit of 0.25 µg/l). We also measured water temperature with a hand-held thermometer on each study date. We measured basic water quality using a Professional Plus sonde made by Yellow Spring Instrument that was calibrated before use and discharge using a flow meter and top-setting rod at the beginning of the study.
We collected drifting and benthic invertebrate samples to estimate the degree to which the composition, abundance, and biomass of invertebrates changed after Permethrin application. The first insecticide treatment at LaPrele Park occurred on the night of June 13th and into the morning of June 14th, 2013. We collected our first drift samples at sunset on June 13th (pre-application), and at sunrise the following two mornings (immediately after and 1 day following application) at both sites. We sampled invertebrate drift at the 9th Street Bridge during the first period to measure the invertebrate response to Permethrin 2.25 km downstream from treatment. We collected a second series of drift samples at 9th Street Bridge at sunset on June 18th prior to initial Permethrin application and at sunrise the following two mornings.
To measure invertebrate drift, we deployed three drift nets (Wildlife Supply Company, 363 µm mesh) across the stream and collected samples for 20 min at each site on each date. We calculated flow rate and volume for each drift net using the floating ball method and adjusted surface water velocity by a factor of 0.85 (Gore, 1996). On June 13th we inadvertently captured a brown trout in a drift net at LaPrele Park while collecting samples; we omitted that replicate as the fish likely altered invertebrate densities in the sample.
In addition to drift samples, we collected five benthic invertebrate samples at both our study sites (LaPrele Park and 9th Street Bridge) on four dates: two pre-application (May 29th and June 10th, 2013), one 5–6 days after spraying began (June 18th, 2013, LaPrele and June 24th, 2013, 9th Street Bridge), and one at the end of the mosquito spraying season (August 12th, 2013). We placed a Hess sampler (0.086 m2, 243 μm mesh) in the stream channel and we agitated the sediments, and scrubbed rocks and plants to capture invertebrates. We preserved all samples in ~ 75% ethanol.
In the laboratory, we separated samples into large fractions (> 2 mm) and small fractions (250 μm to 2 mm), and sorted the invertebrates from the debris. Small fractions with high densities of invertebrates were subsampled using a record player (Waters, 1969). A bucket with a sieve and wedge (1/2,1/4, or 1/8 of the area of the sieve) sat on top of a record player that rotated while the sample slowly poured out evenly. The sample retained in the wedge was sorted and identified. We identified, measured, and counted invertebrates under a dissecting microscope using Merritt et al. (2008) to identify aquatic insects and Thorp & Covich (2010) to identify non-insect invertebrates. We classified insects into adult, pupal and larval stages. For each site and date, we calculated total invertebrate density (ind./m3 (drift) and ind./m2 (benthos)), biomass (mg/m3(drift) and mg/m2(benthos)), and taxa richness. We calculated aquatic invertebrate biomass using length-mass regression (Downing, 1984; Ganihar, 1997; Benke et al., 1999). We measured the degree to which the mosquito control program altered the invertebrate assemblages using the statistical program R (R Core Development Team, 2017), including the vegan (Oksanen et al., 2013), Matrix (Bates & Maechler, 2013), and plyr (Wickham, 2011) packages. We investigated if the density and biomass of drifting invertebrates differed by site (LaPrele Park, 9th Street Bridge after treating LaPrele Park and 9th Street Bridge’s initial treatment) or treatment (pre-treatment, immediately after treatment, and ~ 24 h after treatment) using a 2-way analysis of variance (ANOVA). If treatment was significant (P < 0.05), we used Tukey’s Honest Significant Difference (Tukey’s HSD) to calculate differences. Similarly, we used ANOVA to investigate if benthic density, biomass and richness varied among sampling dates and Tukey’s Honest Significant Difference (Tukey’s HSD) to calculate differences among dates.
Results
Abiotic conditions were similar in Spring Creek across the sites and dates sampled. Water temperature was between 10 and 12°C during the study. Discharge was ~ 76 l/s. Basic water quality was sufficient to support aquatic life (Table 1). Insecticide concentrations were below the detection limit (< 0.25 µg/l) in the stream during the duration of the project.
Drift
The drifting invertebrate assemblage of Spring Creek included 37 taxa in 5 phyla and 17 orders. We collected 5 insect orders, the most abundant of which were Diptera, Ephemeroptera, and Coleoptera, respectively (Table 2). We collected 12 non-insect invertebrate taxa of which Oligochaeta, Ostracoda, and Cyclopoida were the most abundant. Taxa richness increased in the drift following treatments. Mollusks, Cnidarians, Hymenopterans, and Cladocerans appeared in drift samples after treatment, but these taxa were not detected in the drift prior to application. Interestingly, no Culicidae were detected at any site or date.
The total density of drifting invertebrates was highest immediately after application (F = 9.0, df = 2, P = 0.0015; Tukey’s HSD, P < 0.014), and the effect did not vary by site (F = 1.0, df = 2, P = 0.39). The density of all drifting invertebrates was 2.4 × higher at sunrise immediately following Permethrin application at LaPrele Park and 2.8 × higher 2.25 km downstream at 9th Street Bridge. Similarly, we observed a 2.5 × increase in invertebrate density when 9th Street Bridge received the initial treatment. A day after treatment, invertebrate drift decreased to near pre-treatment densities at all sites and dates (Fig. 2). Total invertebrate richness increased immediately after Permethrin application at all sites and dates. Richness was usually lower 24 h after treatment compared to before treatment (Table 2).
Total density (ind./m3) of drifting invertebrates increased immediately after insecticide treatment at LaPrele Park (a), and 9th Street Bridge (b, c) and decreased to near ambient drift density 1 day after treatment. LaPrele Park was initially treated on June 14th and Permethrin drifted to 9th Street Bridge 2.25 km downstream. 9th Street Bridge was initially treated on June 19th
Drifting aquatic insects increased the most immediately after Permethrin treatment (F = 9.2, df = 2, P = 0.0014; Tukey’s HSD, P < 0.016) and densities did not vary among sites (F = 1.1, df = 2, P = 0.15). The density of drifting aquatic insects was 11 × higher at LaPrele Park immediately after treatment (Fig. 3a) and decreased to < 30% of pre-treatment drift a day after treatment (pre vs. 24 h later; Tukey’s HSD, P = 0.65). Despite not fogging downstream, we observed the same response at 9th Street Bridge on these dates. Aquatic insect densities increased 3 times immediately after treatment and declined to 85% of pre-treatment drift densities a day later (Fig. 3b). When 9th Street Bridge received the initial Permethrin application, post-treatment drift densities increased 2.2 times and returned to 100% of pre-treatment densities ~ 24 h after treatment (Fig. 3c).
Density (ind./m3; a–c) and biomass (mg/m3; d–f) of drifting aquatic insects increased immediately after insecticide treatment at LaPrele Park (a, d), and 9th Street Bridge (b, c, e, f) and decreased to near ambient drift density 1 day after treatment. LaPrele Park was initially treated on June 14th and Permethrin drifted to 9th Street Bridge 2.25 km downstream. 9th Street Bridge was initially treated on June 19th
Similar to density, the biomass of drifting aquatic insects increased following treatment (F = 15.5, df = 2, P < 0.0001; Tukey’s HSD, P < 0.002) and returned to pre-treatment levels a day later (Tukey’s HSD, P = 0.57); however, aquatic invertebrate biomass did not vary among sites (F = 2.9, df = 2, P = 0.08). At LaPrele Park, biomass was 1.6 times higher after treatment and decreased to 4% of pre-treatment biomass 24 h after treatment (Fig. 3d). The biomass of drifting insects at 9th Street Bridge also increased 1.6 times immediately after fogging upstream of LaPrele and decreased to 60% of pre-treatment biomass 24 h later (Fig. 3e). When Permethrin was initially applied near 9th Street Bridge, we observed a 4 × increase in biomass immediately after treatment and biomass returned to pre-treatment levels a day later (Fig. 3f).
The density (F = 7.7, df = 2, P = 0.003, Tukey’s HSD, P < 0.05) and biomass (F = 7.1, df = 2, P = 0.004, Tukey’s HSD, P < 0.05) of aquatic non-insect invertebrates increased after Permethrin treatment. At LaPrele Park, density (1.5 ×) and biomass (1.7 ×) increased immediately after treatment, and density (4% of pre-treatment) and biomass (10% of pre-treatment) decreased a day later (pre vs. 24 h after treatment; Tukey’s HSD, P = 0.97; Fig. 4a, d). The density of non-insect invertebrates increased 3.5 × and biomass increased 2 × at 9th Street Bridge during initial treatment 2.25 km upstream, and density and biomass decreased below pre-treatment values 24 h later (Fig. 4b, e). Finally, the density (4 ×) and biomass (4.5 ×) of non-insect invertebrates in the drift increased after initial spraying at 9th Street Bridge and density and biomass decreased below pre-treatment values 24 h later (Fig. 4c, f). The density of aquatic non-insect invertebrates varied among sites (F = 4.1, df = 2, P = 0.003), but biomass did not (F = 2.1, df = 2, P = 0.15). The density of drifting non-insect invertebrates was higher at LaPrele Park compared to 2.25 km downstream at 9th Street Bridge that was not treated on that day (Tukey’s HSD, P = 0.026).
Density (ind./m3; a–c) and biomass (mg/m3; d–f) of drifting aquatic non-insect invertebrates increased immediately after insecticide treatment at LaPrele Park (a, d), and 9th Street Bridge (b, c, e, f) and decreased to near ambient drift density 1 day after treatment. LaPrele Park was initially treated on June 14th and Permethrin drifted to 9th Street Bridge 2.25 km downstream. 9th Street Bridge was initially treated on June 19th
The drift density of adult insects was similar before and after treatment (F = 0.8, df = 2, P = 0.47). The density of adult insects at LaPrele Park did not increase after treatment and densities were lowest a day after treatment (Fig. 5a). Our samples at 9th Street Bridge had high variance immediately following treatment 2.25 km upstream and we observed higher densities than pre-treatment a day later (Fig. 5b). The density of adult insects did not differ from pre-treatment values immediately after spraying began at 9th Street Bridge nor a day later (Fig. 5c). The most abundant drifting adult insects at both sites on all dates were members of the family Chironomidae followed by adults in the dipteran suborder Brachycera.
Adult insect density (ind./m3) in the drift did not increase immediately after insecticide treatment at LaPrele Park (a), and 9th Street Bridge (b, c) or a day after treatment. LaPrele Park was initially treated on June 14th and Permethrin drifted to 9th Street Bridge 2.25 km downstream. 9th Street Bridge was initially treated on June 19th
Benthos
The benthic invertebrate assemblage included 30 taxa. We collected five insect orders and 10 non-insect invertebrate groups. Twelve of these taxa were collected in drift and benthic samples. Non-insect invertebrates were more abundant (128,289 ind./m2) than insects (8320 ind./m2). Oligochaeta (73,681 ind./m2), Crustacea (42,577 ind./m2), Arachnida (7065 ind./m2), Mollusca (2284 ind./m2), Nematoda (1079 ind./m2), Mermithida (845 ind./m2), and Hirudinea (558 ind./m2) were the non-insect invertebrates present in the stream in decreasing order of abundance. Ephemeroptera (4394 ind./m2) and Diptera (3465 ind./m2) were the densest orders of insects followed by Coleoptera (174 ind./m2), Trichoptera (163 ind./m2), and Hemiptera (124 ind./m2).
Density and biomass of invertebrates varied during the summer (Table 3). The highest densities of invertebrates were collected in May before any spraying occurred (59,768 ind./m2). Total invertebrate biomass decreased over the summer but did not differ among dates (ANOVA, F = 1.12, df = 3, P = 0.36; Fig. 6). The individual biomass of invertebrates varied little during early summer, but the mean individual biomass decreased in August (ANOVA, F = 4.4, df = 3, P = 0.014, Tukey HSD, P ≤ 0.05; Fig. 6). Insect biomass was lowest in August, but biomass did not differ among dates (ANOVA, F = 1.7, df = 3, P = 0.20; Fig. 6). Tipulidae made up < 0.01% of the Diptera in benthic samples, but their large body size translated to high biomass that declined throughout the summer driving the decrease Diptera biomass in August (ANOVA, F = 2.3, df = 3, P = 0.08; Fig. 6). Non-Tanypodinae Chironomidae composed 83.6% of the Diptera in benthic samples and these insects had their highest biomass in August (ANOVA, F = 1.7, df = 3, P = 0.18; Fig. 6). Simulidae and Muscidae biomass were also highest in August. The biomass of non-insect invertebrates did not vary among dates (ANOVA, F = 0.89, df = 3, P = 0.46; Fig. 6). Crustaceans had the lowest biomass in August, but the decrease was not significant (ANOVA, F = 1.6, df = 3, P = 0.22; Fig. 6). Taxa richness was highest in August (ANOVA, F = 3.1, df = 3, P = 0.06; Fig. 6; Tukey HSD, P < 0.05).
Discussion
Incidental exposure to Permethrin increased the drifting invertebrates and reduced the biomass of benthic invertebrates in Spring Creek, Wyoming USA. The higher number of taxa in drift samples after treatment suggested that many invertebrates responded to the insecticide within the stream, not simply the most intolerant taxa. The appearance of hymenopterans, an order of terrestrial insects, indicated that Permethrin affected non-target terrestrial insects near the stream. Studies have repeatedly shown that insecticides can increase invertebrates entering the drift in streams (Wallace & Hynes, 1981). For example, a mass invertebrate drift response was observed in a small Alaskan stream when Permethrin was applied adjacent to the stream to control spruce beetles (Werner & Hilgert, 1992). Similarly, Everts et al. (1983) found that incidental exposure to Permethrin caused mortality in a variety of insects in a riverine forest habitat. Invertebrates in our study responded to Permethrin application even when an ultra-low volume fogger was used. Wallace and Hynes (1981) explained that aerial applications of pesticide require less pesticide, but can disperse the chemicals better, possibly affecting more organisms. High concentrations of Permethrin in a small Canadian headwater stream not only increased drift but also reduced the densities of invertebrates in the benthos, including Ephemereoptera, Plecoptera, and Trichoptera (Kreutzweiser & Sibley, 1991).
Although Permethrin concentrations in Spring Creek remained below detection levels (≤ 0.25 μg/l) throughout the experiment, lab experiments found a LC50 for C. dilutus with Permethrin concentrations of ~ 0.159 μg/l (Hasenbein et al., 2015). The current detection level may not be sensitive enough to detect lethal concentrations of Permethrin, let alone sub-lethal concentrations. Permethrin detection in water is difficult as it is hydrophobic, lipophilic, and disperses quickly to plants and sediment when it lands on water. The plants and sediment in Spring Creek could be absorbing and retaining Permethrin as observed in other studies, especially in LaPrele Park’s pond (Sundaram, 1991; Moore et al., 2014). Organic matter retaining chemicals could lead to prolonged exposure for aquatic invertebrate herbivores and may affect the stream throughout the year.
Incidentally introducing Permethrin to Spring Creek may affect vertebrates in the stream because Permethrin is lethal to many gilled aquatic organisms including fish (Coats et al., 1989). Permethrin can bioaccumulate as observed in the blood of crocodiles in Costa Rica (Grant et al., 2013). Wallace and Hynes (1981) surmised that the chemicals measured in fish tissue came from the insects they ate. Pesticides consumed can alter the physiology of vertebrates. For example, brown trout showed an increase in brain acetylcholinesterase (AChE) activity, an enzyme that breaks down neurotransmitters, and decreased muscle AChE activity for 3 weeks after a pyrethroid spraying event in an Australian stream (Davies & Cook, 1993). Davies and Cook (1993) inferred that the brown trout’s ingestion of affected invertebrates caused the changes in the trout’s physiology because the concentration of the pyrethroid in the stream was much lower than the concentration necessary to induce physiological changes in a laboratory study. Brown trout are present in Spring Creek and may receive higher doses of pyrethroids from invertebrates if the pesticide is accumulating in the stream. Decades of annual insecticide treatments could lead to higher concentrations if the chemical remains in aquatic organisms or the stream itself between the summer spraying seasons.
Decreased biomass of aquatic invertebrates in streams that receive overspray from mosquito control programs may translate into less food for resident fish, amphibians, and riparian birds. The diet of brown trout mirrored the invertebrates available in the drift of an Australian stream treated with a pyrethroid insecticide (Davies & Cook, 1993). Fewer insects in the benthos may also decrease the adult insects emerging from the stream and make less food available to riparian insectivores. For example, introduced fish reduced the invertebrates in California lakes; fewer adult insects emerged and subsequently lowered the density of Rosy finches (Leucosticte tephrocotis dawsoni Grinnell, 1913) around the lake (Epanchin et al., 2010). Conversely, a decline in stream invertebrates may lead to an overabundance of primary producers such as filamentous algae. Davies and Cook (1993) hypothesized that a decrease in invertebrate scrapers caused an algal bloom several weeks after a pyrethroid spraying event in their study stream. Pesticide treatments in Spring Creek may reduce the number of scrapers, but the Spring Creek watershed is entirely within the city of Laramie and many disturbances may be present in an urban environment. For example, runoff from fertilized lawns could cause blooms of algae. We did not measure the concentration of other pollutants in the stream, which are likely altering Spring Creek in unknown ways. Our data suggest that invertebrates are drifting downstream after pesticide treatments; however, runoff of other pollutants may also be changing the invertebrate assemblage in Spring Creek.
Decades of insecticide treatments may have changed the invertebrate assemblage of Spring Creek. Non-insect invertebrates (94% by density) dominated the invertebrate assemblage in Spring Creek. Similarly, non-insect invertebrates with a life span of less than 1 year dominated an insecticide-treated headwater stream in the southeastern United States (Whiles & Wallace, 1992). In Spring Creek, non-insect invertebrates composed 58% of the assemblage by biomass and that increased to 86% after spraying initially. Chung et al. (1993) observed that a stream switched from being dominated by insects to mostly non-insect invertebrates after insecticide treatments. The stream took 2 years to recover to an insect dominated assemblage after spraying ceased. Recovery times are taxa specific (Wallace & Hynes, 1981); while some taxa may be able to recover within weeks, others can take years (Wallace & Hynes, 1981; Chung et al., 1993).
Benthic taxa richness increased at the end of the mosquito spraying season. The increase in taxa richness indicated that new taxa likely colonized the stream. Wallace and Hynes (1981) thought recolonization was the first step in a stream’s recovery after applying insecticide. Recolonization can come from eggs hatching, upstream drift, and movement within the benthos (Wallace & Hynes, 1981; Whiles & Wallace, 1992). Hatching eggs can either resist the chemical or were laid in the stream after the disturbance (Whiles & Wallace, 1992). The small individual body size in August benthic samples supported the idea that eggs hatched and Spring Creek was being colonized. By ceasing pesticide treatment, invertebrates may be allowed to recolonize the stream; however, chemical residuals may remain in the stream. LaPrele Park’s pond could have increased particulate matter in the water column as observed in a pond in the study site of Sundaram (1991), allowing Permethrin to persist in organic matter, sediments, or in the tissue of organisms after the last spraying event. Permethrin could flow downstream of the pond after the summer (Sundaram, 1991), and persistence likely depends on many stream factors and the life cycles of the stream’s organisms.
Our study did not measure the survival, behavior, or fitness of invertebrates after Permethrin treatment, but insecticides can have lethal or sub-lethal effects on invertebrates. Sub-lethal effects of insecticides can include decreased growth, changes in behavior or increased mobility. Invertebrate bioassays during insecticide treatments showed that individuals initially survived treatment but died shortly after (Wallace & Hynes, 1981). Some individuals even exhibited increased activity for a short period before perishing. Invertebrates in Spring Creek may have been alive or dead in the drift; however, we did not observe their status after retrieving samples. Invertebrates strongly responded to treatment by drift rates increasing up to 11 × the initial rates; however, we do not know if the concentrations in Spring Creek were lethal or not.
Permethrin spraying events during the summer in Spring Creek caused increases in the drift of invertebrates and reduced benthic invertebrate biomass. The U.S. Environmental Protection Agency previously required a buffer distance when spraying Permethrin near streams; however, these rules were lifted because of the threat of West Nile Virus to human health. Reestablishing a minimum distance from the stream or reducing the frequency of treatment may reduce the volume of insecticide entering the stream. Our study demonstrates insecticide treatments affect the biota 2.25 km downstream. The distance that insecticides travel in streams likely depends on many factors such as water velocity, transient storage, and the amount of vegetation. The invertebrates we captured at the downstream site originated an unknown distance upstream. The magnitude of the response did not differ between the upstream sprayed site and the downstream location, so we assume that downstream invertebrates were affected by insecticide. Re-evaluating the frequency and routes of insecticide treatment may reduce the effects on non-target animals while managing mosquito populations.
References
Anziska, Y. M. D. & A. Rahman, 2016. Pediatric myasthenia gravis exacerbation associated with permethrin cream. Muscle and Nerve 55: E11–E12.
Baser, S., F. Erkoc, M. Selvi & O. Kocak, 2003. Investigation of acute toxicity of permethrin on guppies Poecilia reticulata. Chemosphere 51: 469–474.
Bates, D. & M. Maechler, 2013. Matrix: sparse and dense matrix classes and methods. In. R package version 1.0-12.
Benke, A. C., A. D. Huryn, L. A. Smock & J. B. Wallace, 1999. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 18: 308–343.
Brittain, J. E. & T. J. Eikeland, 1988. Invertebrate drift: a review. Hydrobiologia 166: 77–93.
Chang, X., D. Zhong, Q. Fang, J. Hartsel, G. Zhou, L. Shi, F. Fang, C. Zhu & G. Yan, 2014. Multiple resistance and complex mechanisms Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. PLoS Neglected Tropical Diseases 8: e2889.
Chung, K., J. B. Wallace & J. W. Grubaugh, 1993. The impact of insecticide treatment on abundance, biomass, and production of litterbag fauna in a headwater stream: a study of pretreatment, treatment, and recovery. Limnologica 28: 93–106.
Coats, J. R., D. M. Symonik, S. P. Bradbury, S. D. Dyer, L. K. Timson & G. J. Atchison, 1989. Toxicology of synthetic pyrethroids in aquatic organisms: an overview. Environmental Toxicology and Chemistry 8: 671–679.
Cox, R. L. & W. T. Wilson, 1984. Effects of permethrin on behavior of individually tagged honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environmental Entomology 13: 375–378.
Currie, B. J. & J. S. McCarthy, 2010. Permethrin and ivermectin for scabies. The New England Journal of Medicine 362: 717–725.
Davies, P. E. & L. S. J. Cook, 1993. Catastrophic macroinvertebrate drift and sublethal effects on brown trout, Salmo trutta, caused by cypermethrin spraying on a Tasmanian stream. Aquatic Toxicology 27: 201–224.
Downing, J. A. & F. H. Rigler, 1984. A manual on methods for the assessment of secondary productivity. Fresh Waters 18: 566–592.
Epanchin, P. N., R. A. Knapp & S. P. Lawler, 2010. Nonnative trout impact an alpine-nesting bird by altering aquatic-insect subsidies. Ecology 91: 2406–2415.
Everts, J. W., K. Van Frankenhuyzen, B. Roman & J. H. Koeman, 1983. Side-effects of experimental pyrethroid applications for the control of tsetse flies in a riverine forest habitat (Africa). Archives of Environmental Contamination and Toxicology 12: 91–97.
Ganihar, S. R., 1997. Biomass estimates of terrestrial arthropods based on body length. Journal of Biosciences 22: 219–224.
Gore, J. A., 1996. Discharge measurements and streamflow analysis. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology. Academic Press, San Diego, CA: 53–74.
Grant, P. B. C., M. B. Woudneh & P. S. Ross, 2013. Pesticides in blood form spectacled caiman (Caiman crocodilus) downstream of banana plantations in Costa Rica. Environmental Toxicology and Chemistry 32: 2576.
Hasenbein, S., S. P. Lawler, J. Geist & R. E. Connon, 2015. The use of growth and behavioral endpoints to assess the effects of pesticide mixtures upon aquatic organisms. Ecotoxicology 24: 746–759.
Impoinvil, D. E., S. Ahmad, A. Troyo, J. Keating, A. K. Githeko, C. M. Mbogo, L. Kibe, J. I. Githure, A. M. Gad, A. N. Hassan, L. Orshan, A. Warburg, O. Calderón-Arguedas, V. M. Sánchez-Loría, R. Velit-Suarez, D. D. Chadee, R. J. Novak & J. C. Beier, 2007. Comparison of mosquito control programs in seven urban sites in Africa, the Middle East, and the Americas. Health Policy 83: 196–212.
Johnson, K. R., P. C. Jepson & J. J. Jenkins, 2008. Esfenvalerate-induced case-abandonment in the larvae of the caddisfly (Brachycentrus americanus). Environmental Ecotoxicology and Chemisty 27: 397–403.
Kells, S. A. & S. N. Hymel, 2016. The influence of time and distance traveled by bed bugs, Cimex lectularius, on permethrin uptake from treated mattress liners. Pest Management Science 73: 113–117.
Kreutzweiser, D. P. & P. K. Sibley, 1991. Invertebrate drift in a headwater stream treated with permethrin. Archives of Environmental Contamination and Toxicology 20: 330–336.
Kyong, S., S. B. Symington, S. H. Lee, D. M. Soderlund & J. M. Clark, 2008. Three mutations identified in the voltage-sensitive sodium channel α-subunit gene of permethrin-resistant human head lice reduce permethrin sensitivity of house fly Vssc1 sodium channels expressed in Xenopus oocytes. Insect Biochemistry and Molecular Biology 38: 296–306.
Merritt, R. W., K. W. Cummins & M. B. Berg (eds), 2008. An Introduction to the Aquatic insects of North America, 4th ed. Kendall Hunt Publishing, Dubuque, IA.
Moore, M. T., R. Kroger, M. A. Locke, R. E. Lizotte, S. Testa & C. M. Cooper, 2014. Diazinon and permethrin mitigation across a grass-wetland buffer. Bulletin of Environmental Contamination and Toxicology 93: 574–579.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens & H. Wagner, 2013. Vegan: community ecology package. R package version 1.15-4.
Peterson, R. K. D., C. J. Preftakes, J. L. Bodin, C. R. Brown, A. M. Piccolomini & J. J. Schleier, 2016. Determinants of acute mortality of Hippodamia convergens (Coleoptera: Coccinellidae) to ultra-low volume permethrin used for mosquito management. PeerJ 4: 1–17.
Sparkes, A., C. Bessant & N. Bates, 2016. Permethrin risk to cats. The Veterinary Record 178: 480.
Sundaram, Kanth M. S., 1991. Fate and short-term persistence of permethrin insecticide injected in a northern Ontario (Canada) headwater stream. Pesticide Science 31: 281–294.
R Core Development Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Reactions Weekly, 2011. Permethrin: erythema multiforme-like irritant contact dermatitis: case report. Reactions Weekly 1372: 28.
Reactions Weekly, 2015. Permethrin overdose: various toxicities in an infant following extensive dermal exposure and possible ingestion: case report. Reactions Weekly 1539: 236.
Thorp, J. H. & A. P. Covich (eds), 2010. Ecology and Classification of North American Freshwater Invertebrates, 3rd ed. Elsevier, New York.
Toynton, K., B. Luukinen, K. Buhl & D. Stone, 2009. Permethrin general fact sheet. National Pesticide Information Center, Oregon State University Extension Services.
U.S. Environmental Protection Agency, 2009. Reregistration eligibility decision (RED) for permethrin. EPA 738/R-09/06. Washington, DC.
Waters, T. F., 1969. Subsampler for dividing large samples of stream invertebrate drift. Limnology and Oceanography 14: 813–815.
Waters, T. F., 1972. The drift of stream insects. Annual Review of Entomology 17: 253–272.
Wallace, R. R. & B. N. Hynes, 1981. The effect of chemical treatments against blackfly larvae on the fauna of running waters. In Laird, M. (ed.), Blackflies. Academic Press, London: 237–258.
Whiles, M. R. & J. B. Wallace, 1992. First-year benthic recovery of a headwater stream following a 3-year insecticide-induced disturbance. Freshwater Biology 28: 81–91.
Wickham, H., 2011. The split-apply_combine strategy for data analysis. Journal of Statistical Software 40: 1–29.
Werner, R. A. & J. W. Hilgert, 1992. Effects of permethrin on aquatic organisms in a freshwater stream in south-central Alaska. Journal of Economic Entomology 85: 860–864.
Zika Virus, 2014. Center for Disease Control.
Acknowledgements
We thank the Wyoming Natural Diversity Database staff, Alexis Lester and Katrina Cook for their help with benthic samples, Oliver Wilmot for his help collecting field samples, and Keith Wardlaw and the Mosquito and Integrated Pest Management of Laramie for logistical support. The Wyoming Research Scholars Program, the University of Wyoming Biodiversity Institute, Laramie Rivers Conservation District, and Wyoming IDeA Networks for Biomedical Research Excellence supported this project. Conversations with Mark Andersen sparked this project’s creation. This project was Sarah Wurzel and Morgan Ford’s undergraduate research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Verónica Ferreira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wurzel, S., Ford, M.A., Dority, D. et al. Evaluating the impact of Permethrin on non-target invertebrates in an urban stream. Hydrobiologia 847, 91–104 (2020). https://doi.org/10.1007/s10750-019-04074-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04074-3