Abstract
Age determination of the jumbo squid (Dosidicus gigas) has been successfully carried out over its geographic range based on the use of the rings present in the statoliths. In this study, we propose an additional method to identify ages of D. gigas off the Ecuadorian and Chilean Exclusive Economic Zones (EEZs) waters by using upper beak rostrum sagittal section (RSS) analysis. Our results show periodic growth increments and occasional checks located in the RSS surface. Growth increments meet at the internal rostral axis in the shape of chevron, and the marks in the hood are more discernable and clearer than in the crest. As has been suggested for octopi, checks are thought to represent temperature oscillation and reproductive events. Growth increments are laid down in the RSS daily, and numbers were similar to the numbers of increments used to determine age in statoliths. There was a significant regional difference in age but not in increment width when mantle length effects were considered, where squid off the warm Ecuadorian EEZ waters had a shorter lifespan and higher somatic growth in mantle length than those in the temperate Chilean EEZ waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several direct and indirect methods used to define cephalopod age and growth (Šifner, 2008). Indirect methods, i.e. length-frequency analysis, have proved inappropriate for ageing short-lived, fast-growing and highly migratory species (Jackson, 2004). Direct methods of tag-recapture and laboratory are usually unfeasible because of low recapture rate and high mortality (Šifner, 2008). However, the study of the microstructure of hard tissues is considered the most appropriate method for the estimation of cephalopod age and growth (Šifner, 2008).
Periodic growth increments are commonly found in cephalopod hard structures including statolith (Jackson, 1994), gladius (Perez et al., 1996), stylet (Leporati et al., 2008; Regueira et al., 2015) and eye lenses (Cárdenas et al., 2011) as well as beaks (Perales-Raya & Hernández-González, 1998), and these have been widely used for age determination. The statolith has been thought to be the most reliable material to estimate cephalopod, especially squid age (Arkhipkin & Scherbich, 2012).The gladius has proved to be unsuitable to define squid age over a whole lifespan (Perez et al., 1996, 2006), although the daily deposition of increments during the adult stage has proved to be suitable and adequate for individual growth studies (Arkhipkin & Bizikov, 1991). Following the successful utilisation of the beak in age studies of octopi (Hernández-López et al., 2001; Perales-Raya et al., 2010; Castanhari & Tomás, 2012), we carry out the first attempt to determine adult squid age and growth by using the growth increment in beaks.
The growth increments within beaks were first observed in squid Moroteuthis ingens (Clarke, 1965), but the periodicity of increments was not estimated until Perales-Raya & Hernández-González (1998) suggested that regular increment deposition should be related to an individual’s age. Subsequently, the daily deposition of the growth increment was validated in Octopus vulgaris (Oosthuizen, 2003; Canali et al., 2011; Perales-Raya et al., 2014a) and O. maya (Rodríguez-Domínguez et al., 2013; Bárcenas et al., 2014). Moreover, the periodic deposition of growth increments in beaks was validated in five paralarval squid Dosidicus gigas, Ommastrephes bartramii, Sthenoteuthis oualaniensis, Illex argentinus and Todarodes pacificus (Sakai et al., 2007). Hu et al. (2015) reported the microstructure of beak rostrum of D. gigas off Peruvian Exclusive Economic Zone (EEZ) and attempted to compare it with growth increments in the statolith.
Over the D. gigas geographic range, age and growth have been widely studied, mainly based on statolith microstructure analysis (Argüelles et al., 2001; Markaida et al., 2004; Chen et al., 2011, 2013; Liu et al., 2013a, b). The main objectives of the present paper are to analyse periodic growth increments in beaks and evaluate the possibility of using these as a potential material for estimating jumbo squid age, similar to what has been done for octopi. We also investigate possible regional differences in estimated age and growth rates. This study might provide additional or alternative data for determining jumbo squid age and growth, especially in cases where a statolith is unavailable.
Materials and methods
Sampling
A total of 382 beaks of D. gigas were analysed. These were obtained from the waters off the Chile and Ecuador EEZs by Chinese jigging vessels (Table 1). After removal, the beaks were soaked in soapy water, washed and then preserved in 75% ethanol for further analyses. Dorsal mantle length (ML) was measured to the nearest 1 mm and body weight (BW) to 1 g. Upper beak rostrum length (URL) was measured with a vernier caliper to the nearest 0.1 mm. Sex was identified, and maturity stages were assigned according to five stages following a modified version of Lipinski and Underhill (1995) scale: immature (stages I and II), maturing (stage III) and mature (stage IV and V).
Beak processing and ageing
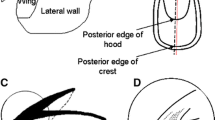
Upper beaks were selected for sectioning. The upper beak was first cut into two pieces along the post-edge of the hood and crest to the rostral tip (Fig. 1). Then, the rostral area of one piece was cut off and embedded with the section plane face down in a small mould of epoxy mixed with a hardener (Fig. 2). The embedded mould was left for 24 h to harden and then glued to a microscope slide after being cut into small blocks (~2–3 mm thickness). Each sample was ground gradually on the cutting plane to near the medial plane surface with 240, 600 and 1200 grit waterproof sandpaper, and then grinding continued with 2000 grit waterproof sandpaper until the medial plane was exposed (Fig. 2). The block was then turned over, attached to the glass slide and ground again to the medial plane. During the grinding process, the blocks needed to be constantly checked with a microscope to avoid over grinding into the medial plane. Finally, they were fine-ground with 2500 grit waterproof sandpaper and polished with 0.05 μm aluminium oxide powder.
Increments within the rostrum sagittal section (RSS) were observed at ×100 and ×400 magnifications using an Olympus light microscope, and digital images of the whole sections were captured with a Charged Coupled Device (CCD) and then processed with PhotoShop7.0 software. The number of increments in each beak was counted along the axis a little further away from the internal rostral axis (IRA) with a large increment width (Liu et al., 2015). The RSS for each beak was counted three times independently by the same skilled person, and the mean value of the three independent counts was accepted when they differed by less than 10%.
Increments periodicity validation
The statolith-determined age of each squid from Ecuador was obtained from recently unpublished data (Liu [unpubl. data]), while the statolith-determined age of squid from Chile was obtained from previously published data (Liu et al., 2013b). The number of increments in the RSS was related to the statolith-determined age by a linear regression analysis to validate its periodicity.
Data analysis
Two-group independent t test was used to compare regional differences in age and the mean increment width of each readable sample between Ecuador and Chile. ANCOVA was used to remove the size-dependent influence on regional differences with ML used as a covariate. Linear, power, exponential, logarithmic, von Bertalanffy, logistic and Gompertz models were fitted to age-URL and age-ML. The model with the least Akaike Information Criterion (AIC) value was selected to describe growth (Arkhipkin et al., 2000).
Results
RSS microstructure
After the upper beaks of D. gigas were uniformly cut and ground, a pattern of bands could be seen on the medial surfaces of the RRS across the rostrum tip to the joint of the hood (dorsal part) and crest (ventral part) (Fig. 1). The anterior portion of the bands in both parts met at the IRA in the shape of chevron, and the posterior portions were parallel to the rostrum edges (Fig. 1c). These bandings consist of a wider light increment and a thinner dark increment (Fig. 3a, b), in which the hood is more discernable and clearer than the crest (Fig. 3c, d). The increments at the tip of rostrum are narrowest (Fig. 3a), gradually becoming wider at the anterior joint (Fig. 3b), reaching to their widest at the medial part of the rostrum (Fig. 3c) and then becoming slightly narrower again to the posterior joint (Fig. 3d). In most specimens, there were prominent checks in the anterior (Fig. 4a), medium (Fig. 4b) and posterior region (Fig. 4c) of the RSS. An aberrant structure was found in the peripheral region of the hood for one specimen (Fig. 4d).
Increment width
Two-group independent t-test showed that there was a significant difference in the mean widths of increments (P = 0.000 < 0.01), with 16.1 ± 2.2 μm (11.5–22.3 mm) for squid off Ecuador and 14.1 ± 1.8 μm (9.9–18.3 mm) for squid off Chile. However, ANCOVA showed that there was no significantly regional difference in the widths of increments when ML was considered as a covariate (P = 0.629 > 0.01).
Validation of increment periodicity
The number of growth increments in the RSS for D. gigas off Ecuador and Chile both showed significant linear relationships with statolith-determined age, where the values of the regression slopes were close to 1 and the intercepts were close to 0 (Fig. 5).
Age structure
Two-group independent t-test and ANCOVA both showed that the ages of samples of D. gigas off Ecuador were significantly lower than the ages of D. gigas off Chile (P = 0.000 < 0.01). The ages of D. gigas off Ecuador were from 106 to 240 days with dominant ages from 151 to 180 days consisting of 45.9% of all the samples, whereas the ages of squid off Chile ranged from 222 to 432 days with dominant ages from 301 to 360 days accounting for 69.2% of the total sample (Fig. 6).
Rostrum growth
The least AIC value was obtained when the URL-age data for both Ecuador and Chile were described by linear curves (F test: Ecuador, F 1,213 = 228, P < 0.01; Chile, F 1,126 = 85, P < 0.01, Fig. 7) and functions as follows:
Somatic growth in ML
The least AIC value was obtained when the ML-age data for both Ecuador and Chile were described by linear curves (F test: Ecuador, F 1,220 = 1484, P < 0.01; Chile, F 1,129 = 251, P < 0.01, Fig. 8) and functions as follows:
Discussion
As demonstrated in octopi (Perales-Raya & Hernández-González, 1998; Perales-Raya et al., 2010; Bárcenas et al., 2014; Perales-Raya et al., 2014a), the RSS surface of the upper beak in D. gigas always reveals a clearly visible pattern of growth increments, where those in the hood are more discernable and clearer than those in the crest (Fig. 4c, d). Similar to that observed in M. ingens (Clarke, 1965) and O. vulgaris (Hernández-López et al., 2001), we found different increment widths along the beak (Fig. 4). Such variation is in agreement with the suggestion proposed by Perales-Raya et al. (2010) of different ontogenetic growth rates in the beak rostrum of D. gigas, with a rapid increase in the early life stages and a slight but continuous decrease in later life. The increments near the RRS tip (representing the very early stage) are usually indiscernible due to their narrower widths, while the marginal increments near the joint of the hood and crest are very clear. This contrasts with statoliths, in which early stage increments are distinct but marginal increments are ambiguous due to their narrower widths (González et al., 1996).
Checks are prominent growth increments caused by a growth delay or interruption (Canali et al., 2011). In this study, checks were clearly located in different parts of the RSS in D. gigas (Fig. 4a–c). This had previously been reported for the same species from the waters off Peru (Hu et al., 2015) and in another squid O. bartramii (Fang et al., 2016). Unfortunately, the factors contributing to these formations have yet to be rigorously explained. However, Perales-Rayaet al. (2014a, b) and Franco-Sanos et al. (2015) reported that the development of checks in octopus beaks might result from physiological changes and/or environmental variation. Based on their assumption, checks in the central part of the RSS (Fig. 4b) may relate to temperature variation, while checks in the posterior region in mature individuals (Fig. 4c) may be associated with reproductive events. Interestingly, similar to previous reports on D. gigas beaks off Peruvian waters (Hu et al., 2015), one of the specimens from our study area was found to have an aberrant structure in the hood peripheral region (Fig. 4d), but what caused the formation of this structure is unclear.
We found the mean width of the increments along the beak in tropical Ecuadorian waters (16.1 ± 2.2 μm) to be larger than in temperate Chilean waters (14.1 ± 1.8 μm). This is contrary to what was revealed by Hernández-López et al. (2001), but in agreement with findings reported by Canali et al. (2011) in O. vulgaris, where individuals collected during the summer months have thicker increments than during winter. As reported for statolith in Lolliguncula brevis (Durholtz and Lipinski, 2000), such potential temperature-dependent increment widths seem to indicate that D. gigas living in warm waters have a faster growth rate in the beak rostrum than those living in cold waters, which is confirmed by a steeper linear regression slope between the URL and the number of increments in samples from Ecuador than in those from Chile (Fig. 7). However, ANCOVA showed that there was no significantly regional difference in the widths of increments when ML was considered as a covariate, which indicates that ML is responsible for the regional difference.
Previous studies using chemical or environmental markers suggested the formation of one increment per day in the RSS and LWL of octopi (Oosthuizen, 2003; Canali et al., 2011; Rodríguez-Domínguez et al., 2013; Bárcenas et al., 2014; Perales-Raya et al., 2014b). The periodicity of increment in squid beaks was first validated for five paralarvae of five oceanic squid species by correlating it with daily deposited statolith increments or days elapsed since rearing (Sakai et al., 2007). However, the only available method to validate the periodicity of increment formation in squid beaks over their whole life is by comparison with the statolith-determined ages (Liu et al., 2015), since squid, especially adults, are difficult to rear in the laboratory (Iglesias et al., 2014). In this study, the number of increments in the RSS is similar to the statolith-determined age (Fig. 6) supporting the hypothesis of “one day, one increment” in beaks of D. gigas from Ecuador and Chile. However, age underestimation caused by erosion of the rostral tip has to be taken into account (Liu et al. 2015).
If growth increments in beaks are laid down daily as discussed above, our results are consistent with a lifespan for D. gigas of 8 months in Ecuador (Liu [unpubl. data]) and 1.5 years in Chile (Liu et al., 2013b) which is supported by statolith microstructure analysis. The somatic growth in mantle length of D. gigas showed significant linear relationship with age, and the growth rate in waters of Ecuador was higher than in waters off Chile (Fig. 8). The spatial difference in age and somatic growth could be due to a discrepancy in temperature or food availability and quality. Indeed, in warm waters, squid commonly have a shorter lifespan than the same species inhabiting temperate or cold waters (Arkhipkin, 2004) For example, S. oualaniensis living with D. gigas in tropical Ecuadorian waters have a lifespan of no more than 6 months and high growth rate in ML (Liu et al., 2016a in press), while those from the northwestern Indian Ocean have a lifespan of about 1 year and low growth rate in ML (Liu et al., 2009). As Chavez and Messié (2009) indicated, Ecuadorian waters have higher SST than Chilean waters. This might be the reason why the maximum determined age of D. gigas from Ecuadorian waters (240 days) was less than those from Chilean waters (432 days), on the contrary growth rate in ML was higher in Ecuadorian waters.
In summary, despite statolith having been systematically used to define squid age, growth increments in D. gigas RSS provide an alternative method of ageing that can be applied to assess regional differences in the growth pattern. Beaks were found to have advantages over statoliths during sampling, extracting, preserving and manipulating, and could provide additional data in those cases where a statolith was unavailable. However, bias in the determination of age due to the erosion of the rostrum tip caused by feeding cannot be ignored (Liu et al., 2015). In addition, beaks have another important advantage over statoliths in that the growth increments in the RSS in the marginal regions are always distinct, whereas this is often not the case in the corresponding area in statoliths. This distribution pattern of growth increments in the beak’s marginal regions can allow the matching of environmental factors (such as SST and chlorophyll a) at the stage of life just before capture much more accurately than when using statoliths. As a consequence, the use of such potentially accurate methods to analyse squid life history, especially when reconstructing migratory routes, have the potential to be more effective than methods which use statoliths (Liu et al., 2016b).
References
Argüelles, J., P. G. Rodhouse, P. Villegas & G. Castillo, 2001. Age, growth and population structure of the jumbo flying squid Dosidicus gigas in Peruvian waters. Fisheries Research 54: 51–61.
Arkhipkin, A. I., 2004. Diversity in growth and longevity in short-lived animals: squid of the suborder Oegopsina. Marine and Freshwater Research 55: 341–355.
Arkhipkin, A. I. & V. A. Bizikov, 1991. A comparative analysis of age and growth estimation using statoliths and Gladius in squids. In Jereb, P., S. Ragonese & S. von Boletzky (eds), Squids Age Determinations Using Statoliths. N.T.R.-I.T.T.P. Special Publication, Mazara del Vallo: 19–33.
Arkhipkin, A. I. & Z. N. Shcherbich, 2012. Thirty years’ progress in age determination of squid using statoliths. Journal of the Marine Biological Association of the UK 92(6): 1389–1398.
Arkhipkin, A. I., P. Jereb & S. Ragonese, 2000. Growth and maturation in two successive groups of the short-fi nned squid, Illex coindetii from the Strait of Sicily (Central Mediterranean). ICES Journal of Marine Science 57: 31–41.
Bárcenas, G. V., C. Perales-Raya, A. Bartolomé, E. Almansa & C. Rosas, 2014. Age validation in Octopus maya (Voss and Solís, 1966) by counting increments in the beak rostrum sagittal sections of known age individuals. Fisheries Research 152: 93–97.
Cárdenas, E. B., S. M. Correa, R. C. Guzman, N. Barahona, F. Briceño, M. J. Villegas & R. Paredes, 2011. Eye lens structure of the octopus Enteroctopus megalocyathus: evidence of growth. Journal of shellfish Research 30(2): 199–204.
Canali, E., G. Ponte, P. Belcari, F. Rocha & G. Fiorito, 2011. Evaluating age in Octopus vulgaris: estimation, validation and seasonal differences. Marine Ecology Progress Series 441: 141–149.
Castanhari, G. & A. R. G. Tomás, 2012. Beak increment counts as a tool for growth studies of the common octopus Octopus vulgaris in Southern Brazil. Boletim do Instituto de Pesca São Paulo 38(4): 323–331.
Chavez, F. P. & M. Messié, 2009. A comparison of eastern boundary upwelling ecosystems. Progress in Oceanography 83: 80–96.
Chen, X. J., H. J. Lu, B. L. Liu & C. Yong, 2011. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. Journal of the Marine Biological Association of the UK 91(1): 229–235.
Chen, X. J., J. H. Li, B. L. Liu, Y. Chen, G. Li, Z. Fang & S. Q. Tian, 2013. Age, growth and population structure of Jumbo flying squid, Dosidicus gigas, off the Costa Rica Dome. Journal of the Marine Biological Association of the UK 93(2): 567–573.
Clarke, M. R., 1965. “Growth Rings” in the beaks of the squid Moroteuthis ingens (Oegopsida: Onychoteuthidae). Malacologia 3(2): 287–307.
Durholtz, M. D. & M. R. Lipinski, 2000. Influence of temperature on the microstructure of statoliths of the thumbstall squid Lolliguncula brevis. Marine Biology 136: 1029–1037.
Franco-Santos, R. M., C. Perales-Raya, E. Almansa, M. D. Troch & D. Garrido, 2015. Beak microstructure analysis as a tool to identify potential rearing stress for Octopus vulgaris paralarvae. Aquaculture Research 1: 15.
Fang, Z., J. H. Li, K. Thompson, F. F. Hu, X. J. Chen, B. L. Liu & Y. Chen, 2016. Age, growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak microstructure. Fishery Bulletin 114(1): 34–44.
González, A. F., B. G. Castro & A. Guerra, 1996. Age and growth of the short-finned squid Illex coindetii in Galician waters (NW Spain) based on statolith analysis. Journal of Marine Science 53(5): 802–810.
Hernández-López, J. L., J. L. Castro-Hernández & V. Hernández-garica, 2001. Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fishery Bulletin 99(4): 679–684.
Hu, G. Y., X. J. Chen, B. L. Liu & Z. Fang, 2015. Microstructure of statolith and beak for Dosidicus gigas and its determination of growth increments. Journal of Fisheries of China 39(3): 361–370.
Iglesias, J., L. Fuentes & R. Villanueva, 2014. Cephalopod Culture. Springer Press, New York: 493.
Jackson, G. D., 1994. Application and future potential of statoliths increment analysis in squids and sepioids. Canadian Journal of Fisheries Aquatic and Sciences 51: 2612–2625.
Jackson, G. D., 2004. Advances in defining the life histories of myopsid squid. Marine and Freshwater Research 55: 357–365.
Leporati, S. C., J. M. Semmens & G. T. Pecl, 2008. Determining the age and growth of wild octopus using stylet increment analysis. Marine Ecology Progress Series 367: 213–222.
Lipinski, M. R. & L. G. Underhill, 1995. Sexual maturation in squid: quantum or continuum. South African Journal of Marine Science 15: 207–223.
Liu, B. L., X. J. Chen & J. S. Zhong, 2009. Age, growth and population structure of squid Sthenoteuthis oualaniensis in northwest Indian Ocean by statolith microstructure. Chinese Journal of Dalian Fisheries University 24: 206–212. (In Chinese with English abstract).
Liu, B. L., X. J. Chen, Y. Chen, S. Q. Tian, J. H. Li, Z. Fang & M. X. Yang, 2013a. Age, maturation, and population structure of the Humboldt squid Dosidicus gigas off Peruvian Exclusive Economic Zones. Chinese Journal of Oceanology and Limnology 31(1): 81–91.
Liu, B. L., X. J. Chen & Q. Yi, 2013b. A comparison of fishery biology of jumbo flying squid, Dosidicus gigas outside three Exclusive Economic Zones in the Eastern Pacific Ocean. Chinese Journal of Oceanology and Limnology 31(3): 523–533.
Liu, B. L., X. J. Chen, Y. Chen & G. Y. Hu, 2015. Determination of squid age using upper beak rostrum sections: technique improvement and comparison with the statolith. Marine Biology 162: 1685–1693.
Liu, B. L., X. J. Chen, J. H. Li & Y. Chen, 2016a. Age, growth and maturation of Sthenoteuthis oualaniensis in the Eastern Tropical Pacific Ocean by statolith analysis. Marine and Freshwater Research. doi:10.1071/MF14427.
Liu, B. L., J. Cao, S. B. Truesdell, Y. Chen, X. J. Chen & S. Q. Tian, 2016b. Reconstructing cephalopod migration with statolith elemental signatures: a case study using Dosidicus gigas. Fisheries Science 82: 425–433.
Markaida, U., C. Quiñónez-Velázquez & O. Sosa-Nishizaki, 2004. Age, growth and maturation of jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. Fisheries Research 66: 31–47.
Oosthuizen, A., 2003. A development and management framework for a new Octopus vulgaris Fishery in South Africa. PhD Thesis, Rhodes University: 183.
Perales-Raya, C. & C. L. Hernández-González, 1998. Growth lines within the beak microstructure of the Octopus vulgaris Cuvier, 1797. South African Journal of Marine Science 20: 135–142.
Perales-Raya, C., A. Bartolomé, M. T. García-Santamaría, P. Pascual-Alayón & E. Almansa, 2010. Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: Improvements and comparisons. Fisheries Research 106: 171–176.
Perales-Raya, C., A. Jurado-Ruzafa, A. Bartolomé, V. Duque, M. N. Carrasco & E. Fraile-Nuez, 2014a. Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydrobiologia 725: 105–114.
Perales-Raya, C., E. Almansa, A. Bartolomé, B. C. Felipe, J. Iglesias, F. J. Sánchez, J. F. Carrasco & C. Rodríguez, 2014b. Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of live events in octopuses. Journal of Shellfish Research 33(2): 1–13.
Perez, J. A. A., R. K. O’Dor, P. Beck & E. G. Dawe, 1996. Evaluation of gladius dorsal surface structure for age and growth studies of the short-finned squid, Illex illecebrosus (Teuthoidea: Ommastrephidae). Canadian Journal of Fisheries Aquatic and Sciences 53: 2837–2846.
Perez, J. A. A., D. C. de Aguiar & J. A. T. dos Santos, 2006. Gladius and statolith as tools for age and growth studies of the Squid Loligo plei (Teuthida Loliginidae) off Southern Brazil. Brazilian Archives of Biology and Technology 49(5): 747–755.
Regueira, M., Á. González & Á. Guerra, 2015. Determination of age and growth of the horned octopus Eledone cirrhosa (Cephalopoda: Octopoda) using stylet increment analysis. Scientia Marina 79(1): 71–78.
Rodríguez-Domínguez, A., C. Rosas, I. Méndez-Loeza & U. Markaida, 2013. Validation of growth increments in stylet, beaks and lenses as aging tools in Octopus maya. Journal of Experimental Marine Biology and Ecology 449: 194–199.
Sakai, M., N. Brunetti, J. Bower, B. Elena, T. Ichii, M. Ivanovic, Y. Sakurai, T. Wakabayashi, T. Wakabayashi & A. Yatsu, 2007. Daily growth increments in upper beak of five ommastrephid paralarvae, Illex argentinus, Ommastrephes bartramii, Dosidicus gigas, Sthenoteuthis oualaniensis, Todarodes pacificus. Squids Resources Research Conference 9: 1–7.
Šifner, S. K., 2008. Method for age and growth determination in cephalopods. Ribarstvo 66(1): 25–34.
Acknowledgments
We thanks Dr. Pauline Hilary Lovell for the English improvement. This work was funded by National Nature Science Foundation of China (NSFC41306127 and NSFC41276156). Programs Foundation of Ministry of Education of China (No. 20133104120001) and Shanghai Universities First-class Disciplines Project (Fisheries A). Y. Chen’s involvement was supported by Shanghai 1000 Talent Plan Program and SHOU International Center for Marine Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Begoña Santos
Rights and permissions
About this article
Cite this article
Liu, B.L., Chen, X.J., Chen, Y. et al. Periodic increments in the jumbo squid (Dosidicus gigas) beak: a potential tool for determining age and investigating regional difference in growth rates. Hydrobiologia 790, 83–92 (2017). https://doi.org/10.1007/s10750-016-3020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-3020-3