Abstract
There are few studies of the ecology of waterfalls despite their being prominent landscape features and of ecological interest because of their physical characteristics. We compared invertebrate assemblages of 5 waterfalls in the Australian Wet Tropics over 12–18 mo. Waterfall assemblages were distinct from those of riffles and bedrock, with some taxa particularly abundant on waterfalls (e.g. Simuliidae, Hydropsychidae) and others restricted to them (e.g. Blephariceridae, Pyralidae), and supported more species than bedrock but fewer than riffles. Differences among waterfalls related to differences in discharge, shade and habitat complexity. Waterfalls comprised a complex of microhabitat patches, with high-flow smooth, high-flow rough, vertical and spray zones most prevalent and distinguishable by gradient, roughness, water velocity, depth and invertebrate assemblages. In high-flow microhabitats rheophilic taxa (e.g. Simuliidae) were prevalent, while in the spray zone a range of madicolous taxa (e.g. various Coleoptera) occurred. Within microhabitats, temporal change was moderate, with stable composition over 12 months. Flood disturbance had limited effect on assemblages, as large flows overshot steep surfaces; drought may be more of a threat because recovery is hindered by isolation and lack of hyporheic refugia. Given their isolation, limited extent and distinctive fauna, waterfalls merit special conservation attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Descriptions of stream biota are available for a wide diversity of systems globally, but little ecological attention has been directed at ‘harsh’ or ‘extreme’ habitats that have severe physical attributes (e.g. high flow) and are difficult to access, such as rapids and waterfalls. Waterfalls typically represent only a minor part of a stream’s length, but their physical characteristics and specialised biota make them of ecological interest and conservation concern (Clayton, 1995; Rackemann et al., 2012). Waterfalls are conspicuous but isolated landscape features, characterised by an abrupt drop in flowing water, and very strong flows. They provide diverse habitats that differ in gradient, roughness, current velocity and growth of bryophytes, with a bedrock substratum but no hyporheic zone or habitable water column beyond the boundary layer (Hawkins et al., 1993). Waterfalls are important as barriers to fish passage (Northcote, 1981) and as habitats for species adapted (i) to torrential flow, including some fish (Hora, 1930), amphibians (Hora, 1930; Khan & Malik, 1987; Richards, 1992) and invertebrates such as Hydropsychidae (Boon, 1988), Blephariceridae (Zwick, 1981) and Simuliidae (Schroder, 1988); and (ii) to madicolous or hygropetric zones (thin films of water over rock surfaces), such as larvae of some Trichoptera (Kjærandsen 2005) and Tipulidae (Sinclair, 1988). Despite many records of animals associated with waterfalls, there are few reports on the spatial distribution or dynamics of their invertebrate assemblages. Exceptions include Yule (1996), who found distinct and aseasonal assemblages on an equatorial waterfall on Bougainville Island, Papua New Guinea; and Rackemann et al. (2012), who showed that the insect assemblages of 12 waterfalls in temperate Victoria, Australia, differed from those in adjacent riffles, with several taxa dependent on waterfall habitats.
As stream faunas of different sites can be spatially isolated from each other even within the same catchment (Hughes et al., 1996; Bunn & Hughes 1997; Thuesen et al. 2008), and as waterfalls typically comprise only a small proportion of stream habitat, they may represent a high level of isolation for the fauna, especially for those elements that have weak powers of dispersal. Therefore, given that waterfalls are apparently harsh environments that vary in biophysical characteristics and isolation, we predicted that their invertebrate assemblage composition would (i) be dictated by the physical environment, especially flow, (ii) not be diverse, (iii) differ among waterfall habitats, but (iv) be similar among waterfalls, (v) differ from assemblages in other stream habitats, and (vi) vary seasonally, especially with extremes of flow. We investigated these predictions for the assemblages of 5 small to moderate waterfalls in the Queensland Wet Tropics bioregion/World Heritage Area of north-eastern Australia (QWT), sampled over 12 months, and assemblages of the main habitats at a single waterfall, sampled over 18 months.
Methods
QWT streams have seasonal perennial flow, unpredictable floods and high biotic diversity, and are heterotrophic to autotrophic, depending on degree of riparian shade (Pearson et al., 2015). Stream hydrographs reflect rainfall patterns, with maxima in the summer wet season (December–March) and minima towards the end of the dry season (October–November), and exhibit rapid increases in discharge in response to rainfall events (Soulsby et al., 1997). Waterfalls occur on most upland streams, but are sparsely distributed along streams, typically with several kilometres between waterfalls.
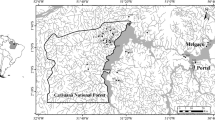
The study sites were Birthday Falls, ‘Benson Falls’, ‘No-name Falls’, ‘Alma Falls’ and Walsh Falls (Fig. 1) (Birthday Falls and Walsh Falls are gazetted names but the others are not). They were located in densely vegetated (rainforest) headwater catchments, situated on volcanic rocks, mainly rhyolite and granite. The streams at these sites are largely undisturbed, seasonally cool to warm and slightly acidic to moderately alkaline, with low solute and nutrient concentrations, and low conductivity (Table 1; Pearson & Connolly, 2000). Water clarity is generally high, except during spates. The waterfalls had variable relief, including rough and smooth rock faces with slopes from 0 to 90°, chutes, small ledges and crevices. They had laminar or turbulent flow, with low-velocity sheeting flow in vertical and peripheral (spray) zones. They differed in size, discharge, overall gradient, shade and other physical features (Table 1). They were separated from other waterfalls within the same streams by several kilometres (e.g. Birthday and Benson Falls were 4 km apart and represented about 0.25% of the ~13 km length of Birthday Ck.).
Map showing location of the Wet Tropics bioregion and World Heritage Area, its main rivers and towns, stream sites where riffle samples were taken (open circles) and the waterfall sites of this study—from north to south, WF (Walsh Falls) near Atherton, AF (Alma Falls) near the Murray River, and NF, BC and BF (No-name Falls, Birthday Falls and Benson Falls) near the southern limit of the region
Four main waterfall microhabitats (‘zones’), representing at least 90% of the surface area of the waterfalls, were identified during a pilot study and were the focus of this study. They comprised high-flow ‘rough’, high-flow ‘smooth’, ‘vertical’ and ‘spray’ zones, all of which occurred in patches across and/or down each waterfall, depending on waterfall morphology. Their extent varied temporally with discharge, as spray zones became submerged by high flows. High-flow habitats had current velocities greater than 1.0 ms−1; smooth habitats had a roughness index <1.10 (see below); rough habitats had a roughness index >1.20; vertical habitats had slopes >85°; and spray zones had a thin film of water with negligible current, and growth of bryophytes and algae.
We collected samples each month through 1991 at each waterfall, except that Birthday, Benson and No-name Falls were inaccessible in February. Additionally, seven monthly samples were taken prior to this at Birthday Falls. We used rock-climbing equipment to scale the waterfalls. Standard sampling equipment was modified to be compact and to hang from a harness. One climber (PDC) undertook all sampling, while a second climber recorded notes and received samples periodically. At the top of each waterfall, we measured, each month, water temperature, conductivity, pH, dissolved oxygen (YSI meters, Yellow Springs, Ohio, USA), and discharge (mean velocity × depth × width – Speedwatch meter, JDC Electronic SA, Switzerland) and proportion of riparian shade (from vertical photographs). At Birthday Falls, measurements were taken of slope, current velocity, water depth and substratum roughness for each sample position. Roughness was measured using a profile gauge (25 cm long, 14 pins/cm) to provide a trace of the rock profile, which was later digitised in Sigma-Scan© (Systat Software, San Jose, California) and used to calculate a roughness index (distance along the profile divided by the associated straight-line distance). The streams were not gauged, so, as stream flow in QWT headwater streams correlates closely with local rainfall (Soulsby et al., 1997), we used cumulative rainfall for periods between 1 and 80 days prior to each sample as a surrogate for antecedent flow. Cumulative rainfall was calculated from daily data recorded at Australian Bureau of Meteorology stations close to the waterfalls (Table 1).
Four samples of invertebrates were collected monthly from each zone for 18 months at Birthday Falls and for 11 or 12 months at the other waterfalls. Sample positions, stratified by habitat, were determined randomly across the face of each waterfall using measuring tapes down and across the waterfall. At each location, samples of the biota were scrubbed from the rock face with a brush into a short-handled, triangular dip net of 250-µm mesh size. A 20 × 20 cm copper-wire quadrat was attached to the front of the dip net to delineate the area to be sampled. The leading edge of the net frame was covered by a strip of neoprene (20 mm thick) to provide a good seal. Samples from the four zones were pooled for each waterfall except Birthday Falls. Samples were preserved in 70% ethanol and returned to the laboratory, where invertebrates were sorted, identified to the lowest possible taxon, and counted.
For comparisons of invertebrate assemblages from waterfalls and other habitats, we sampled invertebrates on bedrock in the stream upstream and downstream of Birthday Falls using similar methods to the above; and we used data from Cheshire et al. (2005) for riffle fauna, which was obtained from invertebrate samples from several Wet Tropics streams, including Birthday Ck., by scouring a 25 × 25 cm area of substrate upstream of a dip net with 250-µm mesh. For these comparisons we used November samples from each habitat.
Comparisons of physical characteristics among waterfalls were undertaken, where possible, using analysis of covariance (ANCOVA, in Statistix 7) of mean monthly data with date as the covariate. Comparisons between waterfall microhabitats were made using analysis of variance (ANOVA, in Statistix 7) followed by Tukey post hoc tests.
Counts of invertebrates were log transformed for analysis. Counts of taxa (‘species richness’, S) were made on raw data and were not transformed. Evenness (J) was calculated in PCORD (version 6.2—MjM Software, Gleneden Beach, Oregon, USA.; McCune & Mefford, 2011). ANCOVA and Tukey post hoc tests were used to compare log abundance, species richness and evenness between waterfalls. Descriptive statistics for these variables and correlations between them, selected species’ abundances and physical variables were calculated in Microsoft Excel©. Comparisons among 4 habitats of abundance, species richness and evenness of invertebrates were undertaken using ANCOVAs with date as the covariate. For comparisons between waterfall, riffle and bedrock samples, taxa were lumped where necessary so that levels of taxonomic resolution matched.
For multivariate analyses of quantitative data, taxa that contributed less than 0.05% of the invertebrate count were removed from data matrices. In comparisons between waterfall, riffle and bedrock samples, proportional data were used (arcsine square-root transformed) because the sampling methods differed slightly among sample types. Monthly samples were pooled for each waterfall for comparisons among waterfalls.
Descriptive statistics and correlations between physical variables and invertebrate metrics were calculated in Microsoft Excel©. PCORD was used to ordinate samples using non-metric multidimensional scaling (NMDS), followed by multi-response permutation procedures (MRPP) to test for differences between a priori groups (defined in Results section). Distance-based linear models were used to explain invertebrate assemblages in terms of physical variables using the DistLM procedure in the PERMANOVA+ add-on (Anderson et al., 2008) in PRIMER-E (version 6, PRIMER-E Ltd, Plymouth, UK; Clarke & Gorley, 2006). Seasonal cyclicity of invertebrate samples was investigated using the RELATE procedure in PRIMER-E.
Results
The waterfalls differed variously in altitude, aspect, slope, height, stream order, discharge, conductivity, shade and local rainfall, but not in mean temperature, pH or dissolved oxygen (Table 1). Wetted area was proportional to discharge, and declined substantially during the dry season on the smaller waterfalls (Alma Falls, Benson Falls, No-name Falls). The different altitudes of the waterfalls led to differences in water temperature ranges but not in overall means. Conductivity differed consistently among waterfalls, but was always low. Degree of shade differed substantially, with Birthday Falls almost entirely open, Benson Falls and No-name Falls with almost complete canopy cover, and Alma and Walsh Falls intermediate. The northerly facing waterfalls would capture greater insolation for all but about 6 weeks of the year when the sun is more southerly at QWT latitudes. High flows occurred at all waterfalls in the January to March period, but were mostly not captured by the instantaneous discharge data estimated on each sampling occasion. High flows coincided with the February sampling trip at Birthday, Benson and No-name Falls, precluding access and sample collections.
Seventy-two taxa of invertebrates and Anura were recorded from the monthly waterfall samples (Supplementary Information, Table S1). Only 13% of taxa were recorded at every waterfall but these taxa accounted for 92% of all individuals collected, of which the Simuliidae accounted for 70%. Other taxa, such as species of Ephemeroptera, Plecoptera and Hemiptera, were less common.
NMDS of monthly invertebrate samples showed distinct separation of Birthday Falls from other waterfalls on axis 1 and varying separation of the waterfalls from each other, with MRPP indicating that all waterfalls, apart from Benson and No-name Falls, differed from each other (Fig. 2). The spread of samples indicated that the assemblages at Birthday Falls and Walsh Falls were less variable over time than those of the smaller waterfalls. Total abundance was highest at Birthday Falls and lowest at No-name Falls; species richness was highest at Birthday Falls but did not differ among the other waterfalls; evenness was low at Birthday Falls compared with the other waterfalls; and values of CV indicate that abundance varied moderately over the 12-month sampling period, while richness and evenness were more consistent (Table 2; Fig. 3). Across waterfalls, there were correlations between discharge and abundance (r = 0.544, P < 0.001), species richness (r = 0.639, P < 0.001) and evenness (r = 0.625, P < 0.001), reflecting between-waterfall contrasts because, within waterfalls, only a single correlation was significant (Table 2). DistLM analysis indicated that differentiation among waterfall assemblages was related to shade and discharge. Within waterfalls, there were few relationships detected between assemblages and temperature, discharge and antecedent rainfall (Table 3): at Birthday Falls, temperature, discharge and 10- and 80-day antecedent rainfall were significant in the model; at No-name Falls, discharge and 80-day antecedent rainfall were significant; but no other model showed significant relationships. Nevertheless, abundance of several taxa showed distinct, though variable, relationships with these measures (Supplementary Information, Table S2). For example, the abundances of Chironomidae and Simuliidae positively correlated with 1-, 5- and 10-day antecedent rainfall at Benson Falls, but these variables were the only ones that these abundances did not correlate with at Birthday Falls. Further, correlations between particular taxa and individual physical variables were both positive and negative. The RELATE procedure showed that the assemblages followed a seasonal cyclical pattern: for Birthday, Benson, No-name, Alma and Walsh Falls, respectively, values of the the test statistic, ρ, were 0.254, 0.363, 0.247, 0.270 and 0.357 and of P were 0.004, 0.002, 0.005, 0.004 and 0.003.
NMDS plot (3D solution, first 2 axes) for invertebrate assemblages on 5 waterfalls (A Alma Falls; B Benson Falls; BC Birthday Falls; N No-name Falls; W Walsh Falls). Proportion of variance explained by each axis (%) and stress are shown. MRPP showed strong pairwise differences between all falls (A > 0.09, P < 0.0001) except Benson and No-name Falls (A = 0.0009, P = 0.4216)
NMDS of November samples showed distinct separation of riffle and bedrock habitats from composite waterfall samples and the waterfall microhabitat samples, confirmed by MRPP (Fig. 4). Species richness was similar among riffle samples, and much greater than in waterfalls (23.1 ± 1.31 vs. 9.9 ± 1.15 SE, respectively; F 1,44 = 49.85, P < 0.0001), while richness was greater on Birthday Falls than in nearby bedrock samples (13.33 ± 1.76 vs. 6.25 ± 1.44 SE, respectively; F 1,5 = 9.91, P = 0.0255).
NMDS plot (2D solution) of QWT riffle samples (open circles), bedrock samples from Birthday Ck. (R), composite samples from 5 waterfalls (A Alma Falls; B Benson Falls; BC Birthday Falls; N No-name Falls; W Walsh Falls) and individual microhabitats on Birthday Falls (sm smooth; r rough; v vertical; and sp spray). Proportion of variance explained by each axis (%) and stress are shown. MRPP indicated significant differences between waterfalls and riffles (A = 0.106, P < 0.0001), between waterfalls and bedrock (A = 0.209, P = 0.0032), between individual habitats and riffles (for smooth, rough, vertical and spray micro-habitats, respectively A = 0.153, 0.175, 0.097, and 0.103; P < 0.001 for each), and between individual microhabitats and stream bedrock samples (for smooth, rough, vertical and spray micro-habitats, respectively A = 0.427, 0.491, 0.306 and 0.278; P = 0.019, 0.018, 0.024 and 0.021)
At Birthday Falls, 54 taxa were collected during the 18-month sampling period (Supplementary Information, Table S1), 18 of which were recorded in all 4 habitats and accounted for 97% of all of individuals collected. The rough and smooth zones were the most similar biologically, with the Simuliidae accounting for 71% of all individuals collected. Apistomyia collini Bezzi, 1913 (Blephariceridae) and Octhebius sp. (Coleoptera) were also abundant. The different habitats were clearly differentiated by physical variables (Table 4): depth was greatest in smooth and rough zones; slope was greatest in the vertical zone, least in the smooth zone and intermediate in the rough and spray zones; roughness was greatest in the rough zone; and velocity was greatest in the smooth and rough zones and virtually absent in the spray zone. NMDS showed separation of monthly samples by habitat, confirmed by MRPP (Fig. 5). MRPP on samples grouped by quarter of the year showed a difference between samples from the first and third quarters (January–March vs. July–September), representing wet and dry season months (A = 0.029, P = 0.016); no other between-quarter analyses were significant. Invertebrate abundance was highest in the smooth and rough zones, richness highest in the spray zone and evenness higher in the vertical and spray zones. CV values across monthly samples indicate that abundance varied substantially from month to month, richness was more consistent, and evenness was intermediate (Table 5; Fig. 6). DistLM analysis indicated relationships between assemblage composition and physical variables: temperature was important in 3 habitats, reflecting the seasonal cyclicity recorded across falls; velocity was important in the smooth and rough zones; and longer-term antecedent rainfall was significant for all but the spray zone (Table 6). Correlations between physical variables and abundance of individual taxa showed that temperature was a strong influence for Chironomidae in all but the spray zone, and for a few other taxa; velocity was important for several taxa in smooth and rough zones but not in the vertical or spray zones, with a positive relationship for some (e.g. Chironomidae, Dinotoperla sp.) and negative for others (Simuliidae, Blephariceridae); roughness had a negative influence on 2 taxa in the smooth zone and 2 in the vertical zone; and antecedent rainfall had the greatest influence overall with a positive relationship with a variety of taxa across zones, but a negative relationship for Blephariceridae and Simuliidae (Supplementary Information, Table S3).
NMDS plot (2D solution) for samples from 4 habitats on Birthday Falls (sm smooth; r rough; v vertical; and sp spray zones). Proportion of variance explained by each axis (%) and stress are shown. MRPP showed significant differences between habitats for all pairwise comparisons (A > 0.046, P < 0.001, except smooth vs. rough, where A = 0.019, P = 0.023)
Discussion
The waterfalls differed most obviously in discharge and shade: for example, there was strong contrast between the small, shaded Benson Falls and the much larger, open Birthday Falls, located about 4 km apart on the same stream. Discharge varied seasonally, with regular rapid increases due to the short response times to heavy wet-season rainfall (Soulsby et al., 1997), and with low flows reducing habitat extent in the smaller waterfalls in the dry season. Stream hydraulics are important in determining the spatial and temporal patterns of invertebrate assemblages (e.g. Statzner & Higler, 1986; Davis & Barmuta, 1989; Hildrew & Giller, 1992; Gore, 1994; Quinn & Hickey, 1994), and it is clear that hydraulics and the nature of the bedrock substratum are major determinants of invertebrate assemblages on waterfalls. Microhabitats differed according to slope, roughness of the substratum and current velocity, which may range from the extreme of almost terminal velocity (~600 cm/s) during floods to moderate velocity and low depth in the vertical zone and negligible velocity and depth in the spray zones. Therefore, the apparent force of the flow in waterfalls belies the occurrence of substantial areas of wet habitat in which current velocity has less influence on invertebrates.
The 4 main microhabitats at Birthday Falls had distinct assemblages that related to their physical differences. The rough and smooth zones were the most extensive and had the most similar assemblages, typified by taxa with strong attachment capabilities. The vertical zone was unusual because much of the discharge overshoots the underlying vertical rock surfaces and their associated fauna, and therefore has some physical conditions more like those of the spray zone. Unlike the spray zone, however, the vertical zone was less likely to dry up during the dry season and did not support plant growth. The spray zone was unlike the other microhabitats, and may more closely resemble seeps, being peripheral to major flow and characterised by a thin film of water and species complement quite different from mainstream habitats (Collier & Smith, 2006).
It might be expected that waterfalls, with their dominant physical attributes, would have very similar faunas with consistent responses to environmental variables. However, while the QWT waterfalls had many taxa in common, especially rheophilic species of Simuliidae, Chironomidae, Blephariceridae and Hydropsychidae (Williams, 1980), there were distinct differences in assemblages among falls and among microhabitats. These results indicate complex responses of the fauna to biophysical variables across waterfalls, possibly relating to species’ traits (e.g. grazers such as Blepharicidae and Pyralidae being common on the less-shaded falls) and different waterfall characteristics (e.g. larger falls having greater habitat diversity, and smaller falls greater impacts of low flow), and suggest that site-specific characteristics override any effects of upstream–downstream colonisation. Assemblages of waterfalls and other stream habitats were very distinct, although they shared some taxa (e.g. Rosser & Pearson, 1995; Cheshire et al., 2005), as is the case for waterfalls on Bougainville Island (Yule, 1996) and in Victoria (Rackemann et al., 2012). Assemblages of individual waterfalls were separated from bedrock assemblages, possibly because of greater microhabitat diversity on the waterfalls. Species richness was lower in waterfalls than in stream riffles in the QWT. Richness of insects was comparable on QWT waterfalls (23–45 insect taxa per waterfall) with that on waterfalls in Victoria (14–42 taxa), from fewer waterfalls (5 vs. 12) but a greater number of samples (63 vs. 21) (Rackemann et al., 2012), possibly reflecting the greater species richness in QWT than Victorian streams (Lake et al., 1994).
Assemblages generally were stable in that they retained their main characteristics through the year, although they showed seasonal cyclicity, like other non-equatorial tropical streams, in contrast to the aseasonality of those close to the equator (Wolda, 1988; Dudgeon, 1992; Yule, 1996; Yule & Pearson, 1996; Pearson, 2014). However, despite distinct seasonality in discharge and temperature during the study period, including high flows in the wet season, the changes in the waterfall assemblages were not consistently linked to those variables. The fauna at the larger falls (Birthday and Walsh) was less variable over time than those of the other falls. In waterfalls, the adverse flood conditions typically faced by stream invertebrates that can significantly reduce abundance and richness (e.g. Wolda, 1988, 1989; Pearson, 2014) may be partly averted as high flows overshoot steep sections. Thus, while physical characteristics determined microhabitats and correlated with their fauna spatially, these characteristics had less effect temporally.
Disturbance in streams is an important structuring agent for invertebrate assemblages (e.g. Resh et al., 1988; Reice et al., 1990), including in Birthday Ck. (Rosser & Pearson, 1995), and may result from flood or drought (Lake, 2003; Pearson, 2014). However, in waterfalls the effects of spate-induced disturbance on invertebrate assemblages may be far less than expected for other stream habitats because the fauna can handle high flows or avoid them. Parts of waterfalls, particularly those that are vertical, are less physically challenging than fast-flow stream microhabitats (e.g. rocky rapids and chutes), allowing substantial faunal avoidance of putative disturbance. Absence of any major differences in patterns of response to seasonal physical change simply indicates that the fauna copes with apparent exigencies of its microhabitat. Drought is likely to affect falls by reduction of habitat overall, and especially fast-flow microhabitats favoured by Simuliidae and other taxa. Thus, it is notable that the smaller QWT waterfalls had much lower diversity than the larger ones, due probably to limited area and diversity of microhabitats, and susceptibility to habitat contraction in the dry season.
Resilience of stream assemblages to disturbance depends on habitat refugia, which include the hyporheic zone, lateral extensions of channels into floodplains, and the heterogeneity of the flow (Hildrew & Giller, 1992). QWT stream assemblages recover rapidly from moderate disturbance, recolonising from undisturbed patches or the hyporheic zone, although drought and large floods greatly increase recovery times (Rosser & Pearson, 1995; Pearson, 2014). However, it is probable that waterfall assemblages are more disturbed by very low flows than high flows because waterfalls lack suitable habitat refugia for many species (Palmer et al., 1992). On waterfalls, the hyporheic zone and lateral channels are unavailable to species that might otherwise use them, but the patchiness of flow (e.g. vertical surfaces) and possibly rock crevices, may provide disturbance refugia for the fauna, as might riparian habitat for adult insects. Colonisation from other waterfalls by drifting invertebrates is unlikely (a) during floods, as the water velocity and turbulence can be high and waterfalls upstream would also suffer the disturbance, and (b) at other times because of the typical distances involved, given that most instream movement tends to be constrained within reaches (Benson & Pearson, 1987; Bunn & Hughes, 1997). Therefore, the physical characteristics of waterfalls restrict the options for colonisation to within-waterfall movement, aerial dispersal or dormancy in situ. However, in an environment where spates are a common occurrence, particularly during the summer breeding period of many taxa, immunity from spate-induced disturbance entails obvious advantages for the waterfall assemblage and, paradoxically, may confer opportunity for recolonisation of nearby habitats from waterfalls by some species.
Although physical factors are important assemblage-structuring agents in waterfalls, as in other high-energy habitats (e.g. rocky shores—Sousa, 1984), strong competitive interactions may occur, as elsewhere in streams (Hearnden & Pearson, 1991; Pearson et al., 2015), especially given the high densities of some taxa, such as Simuliidae, which are typically evenly spaced in their preferred habitat (e.g. Harding & Colbo, 1981), and which may also compete for position with other invertebrates (e.g. Hydropsychidae—Hemphill, 1988). Interactions between physical conditions, inter- and intra-specific processes and stochastic events in structuring assemblages warrant further investigation.
Conclusion
Our results support the predictions that waterfall assemblages are largely dictated by the physical environment, are specialised but not diverse, differ from assemblages in other stream habitats in composition and diversity, differ among waterfall microhabitats, and vary seasonally. However, despite many taxa in common, waterfall faunas differed substantially from each other in overall invertebrate abundance, diversity and evenness. Waterfalls supported a distinct assemblage of species including those that were well-adapted to torrential flows and for which conditions were therefore suitable and not harsh; they also provided vertical and spray-zone microhabitats that largely avoid strong flows for many other species. For this reason, they provide a more diverse and perhaps less physically challenging environment overall than, say, rapids running over low-gradient bedrock and large boulders.
Freshwater systems demand special conservation globally because of their limited extent, high biodiversity, vulnerability and importance as a human resource (Dudgeon et al., 2006). Like streams generally, waterfalls have cultural, aesthetic and ecotourism values, as well as intrinsic ecological importance as biologically distinct habitats, and are vulnerable to the same anthropogenic influences as other stream habitats, including climate change; in this regard, the QWT is regarded as something of a refuge on the Australian continent (James et al., 2013). Many waterfalls are incidentally protected in reserves (e.g. the QWT World Heritage Area) and many are partly protected from human disturbance by being located in headwaters. However, because of their special faunas, small extent and isolation, waterfalls are also worthy of conservation in their own right (Rackemann et al., 2012). It is surprising, therefore, that only 5 waterfalls, 4 of which are located in or close to the QWT, are named in the directory of important Australian wetlands (Australian Government, 2005).
References
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth.
Australian Government, 2005. A Directory of Important Wetlands in Australia. Accessed online 2 June 2015 at http://www.environment.gov.au/topics/water/water-our-environment/wetlands/australian-wetlands-database/directory-important#inland.
Benson, L. J. & R. G. Pearson, 1987. Drift and upstream movements of macro-invertebrates in a tropical Australian stream. Hydrobiologia 153: 225–239.
Boon, P. J., 1988. Notes on the distribution and biology of Smicridea (Trichoptera: Hydropsychidae) in Jamaica. Archiv für Hydrobiologie 111: 423–433.
Bunn, S. E. & J. M. Hughes, 1997. Dispersal and recruitment in streams: evidence from genetic studies. Journal of the North American Benthological Society 16: 338–346.
Cheshire, K., L. Boyero & R. G. Pearson, 2005. Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biology 50: 748–769.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER Version 6. User Manual/Tutorial. PRIMER-E Ltd., Plymouth.
Clayton, P.D., 1995. The ecology of waterfalls in the Australian wet tropics. PhD thesis, James Cook University, Australia.
Collier, K. J. & B. J. Smith, 2006. Distinctive invertebrate assemblages in rockface seepages enhance lotic biodiversity in northern New Zealand. Biodiversity and Conservation 15: 3591–3616.
Davis, J. A. & L. A. Barmuta, 1989. An ecologically useful classification of mean and near-bed flows in streams and rivers. Freshwater Biology 21: 271–282.
Dudgeon, D., 1992. Patterns and Processes in Stream Ecology: A Synoptic Review of Hong Kong Running Waters. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z.-I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A.-H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Gore, J. A., 1994. Hydrological change. In Calow, P. & G. E. Petts (eds), The Rivers Handbook: Hydrological and Ecological Principles. Blackwell Scientific Publications, London: 33–54.
Harding, J. & M. H. Colbo, 1981. Competition for attachment sites between larvae of Simuliidae (Diptera). The Canadian Entomologist 113: 761–763.
Hawkins, C. P., J. L. Kershner, P. A. Bisson, M. D. Bryant, L. M. Decker, S. V. Gregory, D. D. McCullough, C. K. Overton, G. H. Reeves, R. J. Steedman & M. K. Young, 1993. A hierarchical approach to classifying stream habitat features. Fisheries 18: 3–12.
Hearnden, M. R. & R. G. Pearson, 1991. Habitat partitioning among mayflies (Insecta: Ephemeroptera) in an Australian tropical stream. Oecologia 87: 91–101.
Hemphill, N., 1988. Competition between two stream dwelling filter-feeders, Hydropsyche oslari and Simulium virgatum. Oecologia 77: 73–80.
Hildrew, A. G. & P. S. Giller, 1992. Patchiness, species interactions and disturbance in the stream benthos. In Giller, P. S., A. G. Hildrew & D. G. Raffaelli (eds), Aquatic Ecology: Scale, Pattern and Process. Blackwell Scientific Publications, London: 21–62.
Hora, S. L., 1930. Ecology, bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Philosophical Transactions of the Royal Society of London B 218: 171–282.
Hughes, J. M., S. E. Bunn, D. A. Hurwood, S. Choy & R. G. Pearson, 1996. Genetic differentiation among populations of Caridina zebra (Decapoda: Atyidae) in tropical rainforest streams, northern Australia. Freshwater Biology 36: 289–296.
James, C. S., J. VanDerWal, S. J. Capon, L. Hodgson, N. Waltham, D. P. Ward, B. J. Anderson & R. G. Pearson, 2013. Identifying climate refuges for freshwater biodiversity across Australia. National Climate Change Adaptation Research Facility, Gold Coast, 150 pp. ISBN: 978-1-925039-56-6.
Khan, M. S. & S. A. Malik, 1987. Buccopharyngeal morphology of tadpole larva of Rana hazarensis Dubois and Khan 1979, and its torrenticole adaptions. Biologia Gabonica 33: 45–60.
Kjærandsen, J., 2005. Species assemblages and community structure of adult caddisflies along a headwater stream in southeastern Ghana (Insecta: Trichoptera). Biodiversity and Conservation 14: 1–43.
Lake, P. S., 2003. Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48: 1161–1172.
Lake, P. S., E. S. G. Schreiber, B. J. Milne & R. G. Pearson, 1994. Species richness in streams: patterns over time, with stream size and with latitude. Verhandlungen der Internationalen Vereinigung fur Theoretische und Angewandte Limnologie 25: 1822–1826.
McCune, B. & M.J. Mefford, 2011. PC-ORD. Multivariate Analysis of Ecological Data. Version 6.20. MjM Software: Gleneden Beach, Oregon, USA.
Northcote, T. G., 1981. Juvenile current response, growth and maturity of above and below waterfall stocks of rainbow trout, Salmo gairdneri. Journal of Fisheries Biology 18: 741–751.
Palmer, M. A., A. E. Bely & K. E. Berg, 1992. Response of invertebrates to lotic disturbance: a test of the hyporheic refuge hypothesis. Oecologia 89: 182–194.
Pearson, R. G., 2014. Dynamics of invertebrate diversity in a tropical stream. Diversity 6: 771–791.
Pearson, R. G. & N. M. Connolly, 2000. Nutrient enhancement, food quality and community dynamics in a tropical rainforest stream. Freshwater Biology 43: 31–42.
Pearson, R. G., N. M. Connolly & L. Boyero, 2015. Ecology of streams in a biogeographic isolate – the Queensland Wet Tropics, Australia. Freshwater Science 34: 797–819.
Quinn, J. M. & C. W. Hickey, 1994. Hydraulic parameters and benthic invertebrate distributions in two gravel-bed New Zealand rivers. Freshwater Biology 32: 489–500.
Rackemann, S. L., B. J. Robson & T. G. Matthews, 2012. Conservation value of waterfalls as habitat for lotic insects of western Victoria, Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 23: 171–178.
Reice, S. R., R. C. Wissmar & R. J. Naiman, 1990. Disturbance regimes, resilience and recovery of animal communities and habitats in lotic ecosystems. Environmental Management 14: 647–659.
Resh, V. H., A. V. Brown, A. P. Covich, M. E. Gurtz, H. W. Li, W. Minshall, S. R. Reice, A. L. Sheldon, J. B. Wallace & R. C. Wissmar, 1988. The role of disturbance in stream ecology. Journal of the North American Benthological Society 7: 433–455.
Richards, S. J., 1992. The tadpole of the Australian frog Litoria nyakalensis (Anura, Hylidae), and a key to the torrent tadpoles of northern Queensland. Alytes 10: 99–103.
Rosser, Z. & R. G. Pearson, 1995. Responses of rock fauna to physical disturbance in two Australian tropical rainforest streams. Journal of the North American Benthological Society 14: 183–196.
Schroder, P., 1988. Gut-passage, particle selection and ingestion of filter feeding blackfly (Diptera; Simuliidae) larvae inhabiting a waterfall in Tahiti (French Polynesia). Aquatic Insects 10: 1–16.
Sinclair, B. J., 1988. The madicolous Tipulidae (Diptera) of eastern North America, with descriptions of the biology and immature stages of Dactylolabis montana (Osten Sacken) and D. hudsonica Alexander (Diptera: Tipulidae). The Canadian Entomologist 120: 569–573.
Soulsby, C., A. Pomeroy & C. Gibbins, 1997. Hydrology and hydrochemistry of a montane rainforest catchment in Queensland, Australia. Hydrochemistry: Proceedings of the Rabat Symposium, April 1997. IAHS Publication No. 244: 299–307.
Sousa, W. P., 1984. The role of disturbance in natural communities. Annual Review of Ecology and Systematics 15: 353–391.
Statzner, B. & B. Higler, 1986. Stream hydraulics as a major determinant of benthic invertebrate zonation patterns. Freshwater Biology 16: 127–139.
Thuesen, P. A., B. J. Pusey, D. R. Peck, R. G. Pearson & B. C. Congdon, 2008. Genetic differentiation over small spatial scales in the absence of physical barriers in an Australian rainforest stream fish. Journal of Fish Biology 72: 1174–1187.
Williams, W. D., 1980. Australian Freshwater Life, 2nd ed. The MacMillan Company of Australia, Melbourne.
Wolda, H., 1988. Insect seasonality: why? Annual Review of Ecology and Systematics 19: 1–18.
Wolda, H., 1989. Seasonal cues in tropical organisms. Rainfall? Not necessarily! Oecologia 80: 437–442.
Yule, C. M., 1996. Spatial distribution of the invertebrate fauna of an aseasonal tropical stream on Bougainville Island, Papua New Guinea. Archiv fur Hydrobiologie 137: 227–249.
Yule, C. M. & R. G. Pearson, 1996. Aseasonality of benthic invertebrates in a tropical stream on Bougainville Island, Papua New Guinea. Archiv fur Hydrobiologie 137: 95–117.
Zwick, P., 1981. Blephariceridae. Monographiae Biologicae 41: 1183–1193.
Acknowledgments
We thank Scott Cuthbertson, Jane Orr, Geoff Power and Lance Wilkie for assistance in sampling the waterfalls; Ros St Clair, Ian Campbell, John Hawking and Peter Zwick for taxonomic advice; and Dr. C. Colón-Gaud and two anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Checo Colón-Gaud
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clayton, P.D., Pearson, R.G. Harsh habitats? Waterfalls and their faunal dynamics in tropical Australia. Hydrobiologia 775, 123–137 (2016). https://doi.org/10.1007/s10750-016-2719-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2719-5