Abstract
Bloom-forming cyanobacteria have been observed with increasing frequency in Poyang Lake, China since 2000. In the present study, we examined the mechanisms responsible for the horizontal distribution of these blooms in the main body of Poyang Lake. Specifically, we addressed the hypothesis that colonies of cyanobacteria are first generated in the Eastern Bay (EB, lentic region) and then advected offshore by large-scale horizontal transport processes, with the colonies subsequently found in Northern Poyang Lake (NPL, lotic region). The cyanobacteria biomass in the EB was significantly greater than that in NPL (P < 0.0001), and negative correlations were observed between cyanobacteria biomass and nutrient concentrations in the EB. However, significant correlations were not observed between cyanobacteria biomass and nutrient concentrations in NPL. The peak abundance of cyanobacteria was first observed in the EB, with the cyanobacterial abundance peak in NPL lagging the EB peak by approximately 1 month. These results are all consistent with the cyanobacteria distribution hypothesis and show the potential for the accumulation of cyanobacteria in NPL that are normally considered unsuitable for in situ growth in EB. Variations of cyanobacteria biomass in eutrophic lakes, including Yangtze-connected (Poyang Lake and Dongting Lake) and Yangtze-isolated (Taihu) lakes, were monitored during summer (July–August), and eutrophic or even polytrophic conditions were not observed to support the development of cyanobacteria. Instead, a high rate of water flow with short retention times was the key factor preventing the accumulation of cyanobacteria in these eutrophic lakes. Therefore, the mean cyanobacteria biomass was significantly lower in the Yangtze-connected lakes (Poyang Lake, 1.01 mg l−1; Dongting Lake, 1.71 mg l−1) than in Taihu Lake at Meiliang Bay (13.54 mg l−1) or the mouth (3.45 mg l−1) (P < 0.0001); however, the biomass more closely resembled Taihu Lake at the center (0.88 mg l−1). As expected, the cyanobacteria biomass was lower in the lakes with hydraulic connections to the Yangtze River compared with those isolated from the Yangtze River. This study revealed that hydrological parameters dominated the accumulation of cyanobacterial blooms in the Yangtze-connected eutrophic lakes in eastern China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake eutrophication, especially in shallow lakes, involves nutrient enrichment (especially nitrogen and phosphorus) that is often followed by significant shifts in the phytoplankton assemblage toward cyanobacteria (Nixon, 1995). The concept that harmful algal blooms (HABs) occur in response to enhanced nutrient loads has recently been reviewed by Gowen et al. (2012), who found that the evidence relating HABs to anthropogenic nutrient enrichment was often equivocal. The presence of cyanobacteria does not necessarily indicate eutrophic conditions because several comparatively nutrient-poor lakes also support dense populations of cyanobacteria (Konopka, 1982; Raikow et al., 2004). On the other hand, when comparing different aquatic systems, even with similar nutrient contents and in the same climatic regions, inverse deductions are not valid; eutrophic or even polytrophic conditions do not necessarily support cyanobacterial development.
The four largest freshwater lakes, Poyang, Dongting, Taihu, and Chaohu lakes, are located in the floodplain of the middle and lower reaches of the Yangtze River, southeastern China. The region is controlled by the East Asian monsoon and characterized by hot, wet conditions during summer and cold, dry conditions during winter (Academia Sinica, 1985; Yi et al., 2003). Annual precipitation ranges between 1000 and 1600 mm, with more than 70% fallings between April and October (Academia Sinica, 1985). The prevailing wind direction changes seasonally, with southwest and south winds prevailing in spring and summer and northwest winds prevailing in winter. With the development of extensive agriculture, fishing, farming, and urbanization during recent decades, these four lakes have all undergone eutrophication (Cheng & Li, 2006). However, the phytoplankton assemblages in these aquatic systems show a variety of responses. The proliferation of cyanobacterial blooms in Taihu and Chaohu lakes has been linked to anthropogenic nutrient over-enrichment (Chen et al., 2003a; Deng et al., 2007; Liu et al., 2011), whereas several taxa of planktonic cyanobacteria occur in Poyang Lake that cannot outcompete eukaryotic algae despite extremely high nutrient contents (Wu et al., 2013). In another large Yangtze-connected lake, Dongting Lake, massive algal blooms have not been reported.
Even within a single water body, spatial heterogeneity of phytoplankton has been observed, and this phenomenon has received the attention of limnologists for many years. Algal distribution is usually attributed to changes in water temperature, light intensity, and nutrient availability at large temporal and spatial scales of water bodies (Chen et al., 2003a; Deng et al., 2007; Liu et al., 2011). Some studies have investigated the phytoplankton and nutrient levels of different lake areas (Chen et al., 2003a, b), because there is indeed a relationship between increasing bloom frequency and magnitude and the nutrient status of water bodies (Walker & Havens, 1995; Carstensen et al., 2007; Davidson et al., 2012). However, in other opinion (Webster, 1990; Verhagen, 1994), the spatial aggregation or patchiness of phytoplankton is not always related to nutrient gradients, and at least one determining factor is required for cyanobacterial growth to override nutrient effects, and this factor can offer an unequivocal explanation of the seemingly contradictory distribution patterns of planktonic cyanobacteria in different lakes or different areas of a lake.
Hydrological characteristics such as water currents are considered the dominant factor controlling phytoplankton distribution in some water bodies (George, 1981; Webster, 1990). Different water current features provide an explanation for why HABs occur in some enriched waters, but are less frequent or absent in others. A Microcystis bloom is not simply caused by nutrients, which tend to be associated with in situ growth rates of Microcystis but are also related to water currents in lakes (Webster, 1990; Verhagen, 1994; Wu et al., 2010). Clearly, water currents flow at considerable speed in large lakes (Hu & Pang, 2004); the transport of phytoplankton may include the advective movement of superficial bloom scums as well as the transport of algae by lake currents.
Historically, all the lakes along the Yangtze River were once connected to the main river stream. Beginning in the 1950s, dams and thousands of kilometers of dykes were built for flood control, land reclamation, and irrigation as well as for disease vector control, such as for blood flukes. More than a hundred lakes were cut off and isolated from the Yangtze River channels (WWF UK Case Study, 2011), and only Poyang and Dongting lakes retain free connections with the river. However, the Poyang Lake Water Control Project (hereinafter referred to as the Poyang Lake Dam) has been submitted to the National Development and Reform Commission (NDRC) for approval in 2012.
To better understand the mechanisms of cyanobacterial blooms in large lakes, it is essential to study the horizontal distribution of cyanobacteria and transport processes between different areas in a particular lake. By investigating the horizontal distribution of cyanobacteria and their transport by water currents in Poyang Lake, we have attempted to offer a reasonable explanation for the presence or absence of cyanobacteria in different lakes under similar climatic and eutrophic conditions. We would also like to draw attention to hydrological parameters that appear to have as great of an effect on the development of cyanobacteria in different eutrophic water bodies as chemical parameters. Finally, the Yangtze-isolated lake, Taihu is used as a demonstration that hydrological conditions changed (e.g., decreased flow velocities and increased residence times) by the planned closure of the lake’s outlet to the Yangtze River may cause algal blooms to occur more often and to be more intense.
Materials and methods
Lake description
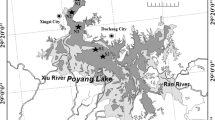
Poyang Lake (24o29′–30o04′N, 113o34′–118o28′E) is the largest freshwater lake in China (Fig. 1) and plays an important role in maintaining the unique biota of the Yangtze River floodplain ecosystem. This lake serves all the main functions of an ecosystem, including provisioning, supporting, and regulating environmental functions as well as providing cultural and recreational functions for local residents (Li et al., 2009). A section of Poyang Lake was designated by the Chinese government as a wetland of international importance under the Ramsar Convention in 1992. A small isolated lake—Junshan is located in the southernmost Poyang Lake region since 1959 (Fig. 1).
Poyang Lake is located in northern Jiangxi Province near the southern bank of the middle and lower reaches of the Yangtze River, which account for 9% of the Yangtze River basin. Poyang Lake is an overflow lake, and it increases in size with water inflows from its drainage catchment (89% of the lake’s inflow), overflow events of the Yangtze River (3% of the lake’s inflow), and other sources such as rainfall and groundwater discharge (8% of the lake’s inflow) (Li et al., 2015). It decreases in size with reduced water inflow from its catchment and the Yangtze River, reduced precipitation, deep drainage, and evaporation. In addition, the lake receives and releases water in response to seasonal variations (Shankman et al., 2006, 2012), and a 13-m difference in water height occurs between the summer rainy season and winter dry season in Poyang Lake (Zhu & Zhang, 1997). According to the report by the Yangtze River Water Resources Commission, although backflow from the Yangtze River to Poyang Lake occurs frequently (12 backflow events per year on average) during 1950–2007, the published data indicate that the frequency of backflow reduced since 2001 (Gao et al., 2014).
Taihu (30o05′–32o08′N, 119o08′–121o55′E) is a large and shallow freshwater ecosystem with a long residence time (Jin et al., 1990). The surface area is 2338 km2 and the average water depth is about 2.0 m. Water current velocity ranges from 0.1 to 0.3 m s−1, and surface currents normally follow wind direction in Taihu (Qin et al., 2007). Meiliang Bay is one of the most eutrophic bays in the northern part of the lake. For further details on morphological, hydrological, and biological properties of Lake Taihu, see Qin et al. (2007).
Sample collection and lab analysis
The many regions of Lake Poyang are completely lotic environments during the dry season (winter), when small channels flow along the lake bed. However, in the wet season (summer), the Poyang Lake area is approximately 4,000 km2, which is much larger than in the dry seasons (Zhu & Zhang, 1997; Shankman et al., 2006) and enables more extensive and comprehensive sampling. Therefore, a summer (July–August) sampling program was conducted at 80 sites that represent the entirety of Poyang Lake from 2011 to 2014. To investigate the cyanobacteria transport processes within this complex lake system, a more intensive sampling program was conducted weekly at four sites (PY5, PY9, PY10, PY15) from June 1st to November 1st (2012–2013), and these sites were selected for the following reasons: Poyang Lake receives surface runoff mainly from five major rivers (Ganjiang, Fuhe, Xinjiang, Raohe, and Xiushui Rivers) and flows into the Yangtze River through a narrow and deep channel at its northern mouth (junction of Poyang Lake and the Yangtze River; see Fig. 1); therefore, if a cyanobacterial bloom occurs upstream, after a certain amount of days without backflow events, the colonies can be traced at those sampling sites. A summer (July–August) sampling program was also performed at Dongting Lake from 2012 to 2013, with 34 sites covering the entire lake. It is noteworthy that the sampling date is on those days without backflow from the Yangtze River into Poyang Lake.
Long-term data sets (July and August 1992–2002) of total nitrogen (TN) and total phosphorous (TP) concentrations and cyanobacteria biomasses covering the three Taihu locations (mouth, Meiliang Bay and center) were supplied by the Taihu Laboratory for Lake Ecosystem Research (TLLER).
Selected environmental parameters, including water temperature, pH, and electrical conductivity, were obtained using a Hydrolab DataSonda 5 Multiprobe (Hach company, United States) in situ. Water depth was measured using a Speedtech SM-5 Portable Depth Sounder (United States). Water transparency (Secchi depth) was determined using a Secchi disk. Lake water samples were collected with a Ruttner-sampler at three depths (the surface, middle, and bottom layers of the lake) in each sampling site. The vertically integrated water samples were blended with the water samples taken from three depths of whole water column and preserved with acid-cleaned 10 L plastic buckets, and kept cool and shaded immediately prior to transport to the laboratory. Suspended solids, chemical oxygen demand (CODMn), and nutrient concentrations, including TN, TP, ammonium-N (NH4-N), nitrite-N (NO2-N), nitrate-N (NO3-N), and orthophosphate (PO4-P), were analyzed according to APHA (1998). Chlorophyll a (chl a) concentrations were obtained by filtering lake water through GF/F filters (47 mm; Whatman) and conducting a spectrophotometric assay according to Lorenzen (1967) after extraction in hot 90% ethanol. Wind speed and direction measurements were performed with a handheld anemometer at each sampling site in Poyang Lake.
Phytoplankton biomass
Phytoplankton samples were fixed with Lugol’s iodine solution (1% v/v) and allowed to settle for 48 h prior to counting with a microscope (Chen et al., 2003a), and taxonomic identification was performed according to Hu & Wei (2006). The mean cell volume was calculated using the appropriate geometric configurations (Hillebrand et al., 1999). Volume values were converted to biomass assuming that 1 mm3 of volume was equivalent to 1 mg of fresh-weight biomass (Chen et al., 2003a). In these samples, the dominant cyanobacterial species were Microcystis spp., Anabaena spp. and Planktothrix spp.
Hydrological data
The lake circulation parameters were obtained from the 2D hydrodynamic model MIKE 21 for Poyang Lake (Li et al., 2014, 2015). MIKE 21 is a finite-volume model that can be used to determine the temporal and spatial changes in both water surface elevations and depth-averaged velocities, in response to wind, river inputs, and a variety of other surface water forcing functions (Danish Hydraulic Institute (DHI), 2014), and it has been applied to coastal oceans, lagoons, and lakes (Warren & Bach, 1992; Niemann et al., 2006; Martinelli et al., 2010). Li et al. (2014, 2015) developed a 2D depth-averaged hydrodynamic model of Poyang Lake using MIKE 21 code to explore the dynamics of water levels, water surface areas, and water flow patterns, and MIKE 21 is considered appropriate for the wide and shallow characteristics of Poyang Lake (Zhang et al., 2014, 2015; Zhang & Werner, 2015). Therefore, only a brief description of the code is given here (Fig. 2).
Data analysis
Northern Poyang Lake (NPL, referred to as the lotic region) connects Poyang Lake with the Yangtze River, and it is narrow with fast water flow (0.4–0.6 m s−1, Fig. 9). The Eastern Bay (EB, referred to as the lentic region), however, is located in a relatively calm (below 0.1 m s−1, Fig. 9) and stable water column, and its hydrological conditions are much different from those in NPL. The average water depth of NPL is 8.7 m and the mean depth of EB is 3.0 m (Wu et al., 2014). To characterize the in situ growth of cyanobacteria in NPL and the EB, the cyanobacteria biomass was regressed on environmental parameters obtained from 16 and 12 sampling sites, respectively.
All statistical analyses were performed with the Statistical Program for Social Sciences (SPSS-IBM, New York, NY) 13.0 software and SigmaPlot 10.0 (Systat Software, Chicago, III). Different regression equations were derived to predict the values of the dependent variable (cyanobacteria biomass) using the independent variables (environmental parameters). The best regression equations were defined as those having the highest r 2 and those that were significant (P < 0.05) for all parameters as well as for the total model. All data were log(x + 1)-transformed and standardized to improve normality and homoscedasticity for regression and scatter-plot. The areal interpolation methods (ArcGIS 10.2 software packages, United States) are used for mapping the horizontal distribution of cyanobacteria in Fig. 5.
Results
Northern Poyang Lake and Eastern Bay
During the period from 2011 to 2014, the average cyanobacteria biomass during summer in the EB and NPL were 1.83 mg l−1 and 0.47 mg l−1, respectively (Tables 1, 2). Higher average concentrations of TN, NO3-N, and PO4-P were found in NPL, and significant differences in TN, TP, NO3-N, and PO4-P concentrations were observed between NPL and the EB (box plots in Fig. 3). The values of NH4-H were not significantly different between the two regions, and cyanobacteria biomass was typically and significantly higher in the EB than in NPL (P < 0.0001) (Fig. 3).
Separate nutrient-cyanobacteria biomass regressions were analyzed in the EB region (Fig. 4). The r 2 values of the nutrient-cyanobacteria biomass regressions were lower than 0.1, and P value for the model was higher than 0.05, indicating that the nutrient concentrations could not explain the variation of cyanobacteria biomass in the NPL region (these regression lines are not presented in Fig. 4). With regard to the EB, the coefficients of determination of the regression were highest for the TP-cyanobacteria relationship (0.50), suggesting that the cyanobacteria biomass was most affected by TP concentrations. The r 2 values were also relatively high in the regressions of TN-cyanobacteria (0.42), NH4-N-cyanobacteria (0.39) and NO3-N-cyanobacteria (0.31). These plots revealed negative and significant relationships between nutrient concentrations and cyanobacteria biomass in the EB of Poyang Lake.
Spatial and temporal distribution of cyanobacteria
The horizontal distribution pattern of cyanobacteria biomass during summer in the whole water column (the surface, middle and bottom layers of the lake) of Poyang Lake is presented in Fig. 5. The cyanobacteria biomass was generally lower in 2013 and 2014 than in 2012 across the entire lake. From 6 to 13 July and 17 to 21 July 2012, the cyanobacteria biomass was generally higher than 1 mg l−1 across the entire lake except for the northern portion. Patches of relatively high cyanobacteria biomass (more than 4 mg l−1) occurred in certain bays (including the EB) of Poyang Lake. From 6 to 19 July 2013, patches of relatively high cyanobacteria biomass (more than 1 mg l−1) occurred in certain bays (including the EB) as well as at the northern mouth of the lake (Fig. 5b). Overall, the cyanobacteria biomass in summer in 2012 and 2013 was higher in the bays than in the north and open lake. However, in the same time period for 2014 (from 11 to 23 July), the cyanobacteria biomass at the stations in the bays was lower than that from the open water and NPL (Fig. 5c). High cyanobacteria biomass was clearly observed at Junshan Lake (Fig. 5a, b). Because of the construction of the dyke, transport of cyanobacteria between Junshan and Poyang Lake is impossible.
Variations in the cyanobacteria biomass at the four monitoring sites (sites PY5, PY9, PY10, and PY15) located at the northern mouth of the lake are shown in Figs. 6 and 7. In 2012, the peak cyanobacteria biomass at sites PY5, PY9, and PY15 was 0.65, 0.33, and 0.27 mg l−1 occurred on August 10, August 26, and August 20, respectively. In 2013, the highest cyanobacteria biomass of the water column at sites PY5, PY9, PY15, and PY10 were 2.94, 4.93, 2.20, and 17.52 mg l−1, respectively, and occurred on August 18 or 28. It should be noted that the peaks of cyanobacteria biomass at those monitoring sites all appeared in the middle and late of August 2012 and late August 2013, approximately 30 days after the summer sampling programs were conducted (Fig. 5a, b). Other peaks also occurred, such as on July 30, 2012 when the cyanobacteria biomass of site PY5 was 0.54 mg l−1.
The scatter-plot of nutrient concentrations versus cyanobacteria biomass in the Yangtze-connected lakes (Poyang and Dongting) and the Yangtze-isolated lake (Taihu) is presented in Fig. 8. Regionally, the average cyanobacteria biomass was significantly higher in Meiliang Bay (13.54 mg l−1) than in the other two Taihu locations and Yangtze-connected lakes (P < 0.0001). The second-highest cyanobacteria biomass (3.45 mg l−1) was observed at the Taihu mouths with a similar level found in Dongting Lake (1.71 mg l−1). The lowest average cyanobacteria biomass (0.88 mg l−1) was found at the Taihu center, and it was not significantly different from the value in Poyang Lake (1.01 mg l−1). The mean TN and TP concentrations at the Taihu mouth (TN = 4.44 mg l−1; TP = 0.24 mg l−1) were significantly higher than those in other areas of Taihu as well as in the Yangtze-connected lakes (P < 0.0001). The average TN and TP concentrations in Poyang Lake (TN = 1.43 mg l−1; TP = 0.07 mg l−1) and Dongting Lake (TN = 1.81 mg l−1; TP = 0.08 mg l−1) were close to that at the Taihu center (TN = 1.43 mg l−1; TP = 0.06 mg l−1) and Meiliang Bay sites (TN = 1.89 mg l−1; TP = 0.10 mg l−1).
Water current structures
A snapshot of the simulated depth-averaged flow patterns over the summer (July–August 2010) for Poyang Lake obtained by the MIKE 21 hydrodynamic model is shown in Fig. 9. In general, large-scale mean circulation patterns produced by the model exhibit northward flow in the lake that represents the prevailing flow pattern of the lake (Zhu & Zhang, 1997; Wang & Dou, 1998; Li et al., 2014). A difference of approximately 0.2–0.3 m s−1 was observed between the lake’s flow channels and other areas of the lake (Fig. 9a). As expected from most river-induced flows, the water velocities near the river mouths (approximately 0.4 m s−1) were significantly higher than the velocities at any other areas of Poyang Lake (approximately 0.1 m s−1). In addition, local patterns of water circulation in the lake may be caused by shoreline irregularities and “island trapping” (Inoue & Wiseman, 2000) because sharp bathymetric features and relatively large velocity gradients, such as those found in the center and eastern portions (EB) of the lake, generally cause the formation of gyres (Fig. 9b, c).
a Map of the depth-averaged velocity averaged over the flood periods (July–August) of MIKE 21 runs and (b and c) close-ups of the velocity fields in regions of interest for July 15, 2010 and August 15, 2010, respectively. Note that the blue lines represent the streamlines, and the ‘Undefined Value’ represents dry land
Discussion
Eutrophication and cyanobacterial bloom development
According to the “Environmental Quality Standards for Surface Water” (GB3838-2002) in China, which defines five grades of water quality from I (best) to V (worst), the water quality of Poyang Lake is currently Grade III–IV, but has reduced to Grade V over the last 10 years, whereas it was Grade I before 2000 (Wang, 2014). In addition, eutrophication in Poyang Lake has also intensified. Research shows that the average TP and TN contents in the water during summer in 1988 were 0.08 mg l−1 and 0.68 mg l−1, respectively. Within 8 years, the TP content had reached 0.15 mg l−1 and the TN content had increased to 2.38 mg l−1 (Li, 1996; Zhu & Zhang, 1997). Poyang Lake has been subject to eutrophic conditions for more than half of every year (the period from autumn to early spring) since 1990, indicating that eutrophication of the lake is serious (Lu, 1996). Our large-scale investigations also indicate deteriorating water quality and eutrophication in Poyang Lake (Fig. 8). It is well known that cyanobacterial dominance in single water bodies is characteristic of highly eutrophic waters in Taihu (Chen et al., 2003a). However, previous studies of the phytoplankton in Poyang Lake in the 1980s and 1990s and from 2009 to 2011 demonstrated that diatoms were dominant, not cyanobacteria (Xie et al., 2000; Wang et al., 2004a, b; Wu et al., 2013). The presence of cyanobacteria, which is often associated with blooms, has been observed in different areas of Poyang Lake since 2000 (Table 1), and cyanobacterial blooms in this lake are patchy and ephemeral (Fig. 2).
Horizontal distribution and transport of cyanobacteria
The cyanobacteria biomass in the EB (lentic region) was significantly greater than in NPL (lotic region) (P < 0.0001) (Fig. 3f). Higher nutrient concentrations in NPL may be the results of nutrients and phytoplankton biomass from upstream Poyang Lake, but these nutrient levels were not responsible for cyanobacterial growth because a significant correlation was not observed between cyanobacteria biomass and nutrient concentrations. However, negative correlations were observed in the EB between cyanobacteria biomass and nutrient concentrations (Fig. 4). Such an inverse seasonal pattern of high nutrients and low cyanobacteria biomass (and vice versa) is not common in permanent lentic water bodies of low turbidity, although it is common in the shallow, turbid lakes of the lower and middle stretches of the Yangtze River and its delta plain (Chen et al., 2003b; Wu et al., 2006). In most freshwater ecosystems, the biomass of phytoplankton tends to increase with increasing TP concentrations (e.g., Prairie et al., 1989; Basu & Pick, 1996). They are assuming that a substantial portion of the total nutrient pools will be immediately available for algal growth and is thus allocated to phytoplankton biomass. However, in Poyang Lake where is subject to frequent flood events, the effects of nutrient enrichment on phytoplankton growth may be obscured by resetting floods that prevent biomass accrual. In addition, the negative slopes may be caused by other limiting factors, such as seasonal delay in the bulk of nutrient loads (Liu et al., 2015) and suitable physical habitat in the EB (shallow, warm water with sufficient light availability and calm currents). Significant inverse relations between TP and chl a were also reported from the turbid lake (Higginsville Lake) performed by Jones et al. (1998). According to Tockner et al. (1999), the accumulation of nutrients in a floodplain can be counterbalanced by the relocation of nutrients through their incorporation into algal biomass and drift into the main river channel. Thus, phytoplankton may plays an important role in remobilizing the nutrient pool retained in Poyang Lake. The drastic drop in inorganic nutrient concentrations (NO3-N, NH4-N and PO4-P) observed in association with high cyanobacteria biomass in the EB could therefore be explained by the high demand for those nutrients by phytoplankton in summer and subsequent transfer of those nutrients to higher trophic levels. These results may indicate in situ growth of cyanobacteria in the EB of Poyang Lake.
Our field observations of cyanobacteria distribution in Poyang Lake showed that cyanobacteria accumulated in the lentic region from July 6 to 13 and July 17 to 21, 2012 and July 6 to 19, 2013 (Fig. 5a, b). However, in the sampling from July 11 to 23, 2014, this species did not accumulate in the lentic region, but rather accumulated the lotic region of the lake (Fig. 5c). Several studies have shown that water flow rate is a key or controlling variable for the development of cyanobacterial blooms (Hötzel & Croome, 1994; Sherman et al., 1998). Low flows are considered to stabilize the water environment, increase light availability, create longer retention times and allow for the release of nutrients from sediments (MDBMC, 1994). There has also been speculation that increased flows may dilute the concentrations of cyanobacteria or transport them downstream (Sherman & Webster, 1997). As a result of the high water flow in NPL, it seems unlikely that the surface patches in this area were caused by the in situ growth of cyanobacteria.
In Poyang Lake, at least two factors might account for horizontal differences in cyanobacteria biomass. First, the main current trajectory extends from the EB to NPL (Fig. 9b). Cyanobacteria tend to be strongly affected by water current (Webster, 1990; Verhagen, 1994; Wu et al., 2010) and cyanobacteria (as well as other pollutants) that start within the EB have a tendency to move into the main flow channels of the lake, move further downstream to the narrow channel at the lake’s northern end, and eventually leave Poyang Lake. These transport trajectories are created by the predominant northward flow along with the relatively faster water velocities in the deep channels (Fig. 9a). Second, wind may be the dominant factor controlling phytoplankton patchiness in lakes (Platt & Denman, 1980; George, 1981). Wind creates a number of different water movements such as waves, seiches, and underwater currents, and the power of the movements depends on various factors, including wind speed, wind direction, and lake morphology (Imboden & Wuest, 1995). The prevailing winds throughout summer are southeasterly and southerly in Poyang Lake and have a mean velocity of 2.85 m s−1. Strong winds in summer may promote the transport of cyanobacteria colonies and produce a more general dispersion and dilution of colonies in the EB. Thus, the traveling patches of cyanobacteria in NPL, such as those observed from July 11 to 23, 2014, will be dictated by balance among processes, such as between water currents and wind.
An unexpectedly large number of cyanobacteria are retained for much longer periods at the EB because of local clockwise water recirculation (Fig. 9c), suggesting that the area may be especially heavily polluted. This type of local circulation pattern dominated most of the bays and lasted for the entire flooding period as observed from the field data (e.g., Zhu & Zhang, 1997; Zhang et al., 2014). In the present study, we observed that a high biomass of cyanobacteria was trapped within the small area of the EB (Fig. 5c), indicating a much longer residence time in this region. Because of a combination of water currents and wind, the cyanobacteria were transported from the EB to NPL; after almost 30 days, peaks of cyanobacteria biomass were observed in the north (Figs. 6, 7). This expected result coincides with evidence (MIKE 21 was combined with dye tracer simulations) that suggests the average residence times are less than 10 days along the Poyang Lake’s main flow channels; whereas approximately 30 days are estimated in the summer (Li et al., 2015).
Comparisons with other lakes
A number of studies have addressed variations in cyanobacteria occurring in different eutrophic lakes in China (Deng et al., 2007; Liu et al., 2011), and the results highlight the importance of nutrients for the growth and accumulation of bloom-forming cyanobacteria in lakes (Davidson et al., 2012). Interestingly, although the nutrients drive in situ growth of cyanobacteria in the EB of Poyang Lake, the biomass was significantly lower than that in Meiliang Bay and the mouths of Taihu Lake (P < 0.0001) (Fig. 8). It is well known that Poyang Lake and Dongting Lake are eutrophic, but there is limited information in the published literature on the occurrence of harmful cyanobacterial blooms in these Yangtze-connected lakes. However, since the mid-1980s, blooms of the toxin-producing cyanobacterium Microcystis spp. have occurred every summer in the northern part of Taihu Lake (Qin et al., 2007). Therefore, we conclude that whether the lakes are freely connected with the Yangtze River catchment is the key factor for bloom formation, and compared with Yangtze-isolated lakes, Yangtze-connected lakes have a shorter retention time and faster flow rate, which increases the likelihood that cyanobacteria biomass will be transported downstream. Another Yangtze-connected lake that is similar to Poyang Lake is Dongting Lake. It has an average residence time of approximately 30 days, whereas Taihu and Chaohu have much longer average residence times of 264 and 127 days, respectively (Jin et al., 1990; Wang & Dou, 1998). It is reasonable to assume that the growth of cyanobacteria would be adversely affected by a short retention time because of flushing or elevated flow velocity. Under existing short retention times, the likelihood of sustained cyanobacterial blooms occurring in the Yangtze-connected lakes is small. In the Ebro River, although water velocities ranging from 0.2 to 0.3 m s−1 (Sabater & Muñoz, 1990), which are lower than those in NPL (0.4 m s−1), cyanobacterial blooms are absent and have not been recorded.
Implications for Poyang Lake Dam operation
Since 1949, the middle and lower Yangtze River basins have lost one-third of their original lake area to cropland (Yin & Li, 2001), whereas many lakes that were once naturally connected with rivers have become isolated or dam controlled. The shift of lakes from river fed to dammed or isolated greatly increases the probability of eutrophication and water quality degradation and increases the frequency of cyanobacterial blooms. According to Zhang et al. (2012), the Three-Georges Dam Projects intensifies the extremes of wet and dry conditions of Poyang Lake, and now, further reduces the water level over the dry seasons. The proposed large-scale water project, the Poyang Lake Dam was designed at 2.8 km wide with sluice gates across the narrowest part of the channel that links Poyang Lake with the Yangtze River (Li, 2011). The sluice gates would hold back water in dry seasons and keep the level at a maximum depth of 12 m (Li, 2011). In this case, residence times of Poyang Lake would be prolonged, and northward flowing currents in the lake would also be disturbed. Consequently, the initiation of cyanobacterial blooms may not be transported downstream, and do not leave Poyang Lake. High nutrient concentrations and a long residence time might support the expansion of cyanobacterial blooms in the temporal duration and spatial extent of such blooms in Poyang Lake.
Conclusions
The results of this work highlight the important role of hydrological forces in structuring the horizontal distribution of cyanobacteria in Poyang Lake. In a more general context, cyanobacterial assemblages have been shown to benefit from shallow disconnected water bodies with nutrient-rich. The existence and accumulation of cyanobacteria patches in NPL could be considered the result of water currents. In addition, cyanobacteria levels are lower in lakes that are hydraulically connected with the Yangtze River compared with those that are isolated or dammed, although concentrations of both TN and TP tend to be similar to those in isolated lakes. In river-connected and eutrophic lakes, nutrient availability only weakly limits cyanobacteria growth, and the occurrence of cyanobacterial blooms appears to depend on lake water retention time and hydrology. Additionally, our study provides baseline data for future assessments of ecological change and a regional monitoring method for cyanobacterial blooms in Poyang Lake.
References
APHA (American Public Health Association), 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. American Public Health Association, Washington, DC.
Basu, B. K. & F. R. Pick, 1996. Factors regulating phytoplankton and zooplankton biomass in temperate rivers. Limnology and Oceanography 41: 1572–1577.
Carstensen, J., P. Henriksen & A. S. Heiskanen, 2007. Summer algal blooms in shallow estuaries: definition, mechanisms, and link to eutrophication. Limnology and Oceanography 52(1): 370–384.
Chen, S. L., 2012. Accumulation of cyanobacterial blooms in the littoral zone of Poyang Lake. Nanchang Evening Post. 18 October: A14 (in Chinese).
Chen, Y. W., B. Q. Qin, K. Teubner & M. T. Dokulil, 2003a. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. Journal of Plankton Research 25: 445–453.
Chen, Y., C. Fan, K. Teubner & M. Dokulil, 2003b. Changes of nutrients and phytoplankton chlorophyll a in a large shallow lake, Taihu, China: an 8-year investigation. Hydrobiologia 506–509: 273–279.
Chen, Y., Xu, C., Liu, X., 2011. Initiation and formation of cyanobacterial blooms in Poyang Lake. Chinese Society for Phytoplankton, Representative Assembly-8th Annual Conference, the 16th Academic Conference (in Chinese).
Cheng, X. & S. Li, 2006. An analysis on the evolvement processes of lake eutrophication and their characteristics of the typical lakes in the middle and lower reaches of Yangtze River. Chinese Science Bulletin 51(13): 1603–1613.
Danish Hydraulic Institute (DHI), 2014. MIKE 21 flow model: Hydrodynamic module user guide. Danish Hydraulic Institute Water and Environment, Hørsholm, Denmark: 132pp.
Davidson, K., R. J. Gowen, P. Tett, E. Bresnan, P. J. Harrison, A. Mckinney, S. Milligan, D. K. Mills, J. Silke & A. M. Crooks, 2012. Harmful algal blooms: how strong is the evidence that nutrient ratios and forms influence their occurrence? Estuarine, Coastal and Shelf Science 115: 399–413.
Deng, D. G., P. Xie, Q. Zhou, H. Yang & L. G. Guo, 2007. Studies on temporal and spatial variations of phytoplankton in Lake Chaohu. Journal of Integrative plant biology 49(4): 409–418.
Gao, J. H., J. Jia, A. J. Kettner, F. Xing, Y. P. Wang, X. N. Xu, Y. Yang, X. Q. Zou, S. Gao, S. Qi & F. Liao, 2014. Changes in water and sediment exchange between the Changjiang River and Poyang Lake under natural and anthropogenic conditions, China. Science of the Total Environment 481: 542–553.
George, D. G., 1981. Wind-induced water movements in the south basin of Windermere. Freshwater Biology 11: 37–60.
Gowen, R. J., P. Tett, E. Bresnan, K. Davidson, A. McKinney, S. Milligan, D. K. Mills, J. Silke, P. Harrsion & A. M. Crooks, 2012. Anthropogenic nutrient enrichment and blooms of harmful micro-algae. Oceanography and Marine Biology: An Annual Review 50: 65–126.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hötzel, G. & R. Croome, 1994. Long term phytoplankton monitoring of the Darling River at Burtundy, New South Wales: incidences and significance of cyanobacterial blooms. Australian Journal of Marine and Freshwater Research 45: 747–760.
Hu, W. & Y. Pang, 2004. Hydrodynamic characteristics of lakes and numerical simulation. In Qin, B., W. Hu, W. Chen, et al. (eds), Process and Mechanism of Environmental Changes of Lake Taihu. Science Press, Beijing: 119–143.
Hu, H. J. & Y. X. Wei, 2006. The Freshwater Algae of China: Systematics, Taxonomy and Ecology. Science Press, Beijing. (in Chinese).
Imboden, D. M. & A. Wuest, 1995. Mixing mechanisms in lakes. In Lerman, A., D. M. Imboden & J. Gat (eds), Lakes: Chemistry, Geology, Physics. Springer, Berlin: 83–138.
Inoue, M. & W. J. Wiseman, 2000. Transport, mixing and stirring processes in a Louisiana estuary: a model study. Estuarine, Coastal and Shelf Science 50: 449–466.
Jin, X. C., H. L. Liu, Y. Q. Tu, Z. X. Zhang & X. Zhu, 1990. Eutrophication of Lakes in China. Chinese Research Academy of Environmental Sciences, Beijing.
Jones, J. R., M. F. Knowlton & M. S. Kaiser, 1998. Effects of aggregation on chlorophyll-phosphorus relations in Missouri Reservoirs. Journal of Lake and Reservoir Management 14(1): 1–9.
Konopka, A., 1982. Buoyancy regulation and vertical migration by Oscillatoria rubescens in Crooked Lake, Indiana. British Phycological Journal 17: 427–442.
Li, B., 1996. Research on the present situation of water pollution and the forecast and planning for water quality in Poyang Lake. Resource and Environment in the Yangtze Valley 59: 60–66.
Li, J., 2011. Dam Proposal for Poyang Lake Causes Controversy, China Daily.
Li, F., L. Zhen, H. Q. Huang, P. Han, X. L. Liu, L. G. Jiang & Y. J. Wei, 2009. Impacts of land use functional change on WTA and economic compensation for core stakeholders: a case study in Poyang Lake. Resources Science 31(4): 580–589. (in Chinese).
Li, Y., Q. Zhang, J. Yao, A. D. Werner & X. Li, 2014. Hydrodynamic and hydrological modeling of Poyang Lake catchment system in China. Journal of Hydrologic Engineering 19(3): 607–616.
Li, Y., Q. Zhang & J. Yao, 2015. Investigation of residence and travel times in a large floodplain lake with complex lake-river interactions: Poyang Lake (China). Water 7: 1991–2012.
Liu, X., X. Lu & Y. Chen, 2011. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: an 11-year investigation. Harmful Algae 10: 337–343.
Liu, X., K. Qian & Y. Chen, 2015. Effects of water level fluctuations on phytoplankton in a Changjiang River floodplain lake (Poyang Lake): implications for dam operations. Journal of Great Lakes Research 41(3): 770–779.
Lorenzen, C. J., 1967. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnology and Oceanography 12: 343–346.
Lu, L., 1996. Investigation on Poyang Lake water pollution by eutrophication. Journal of Lake Science 8: 241–247.
Martinelli, L., B. Zanuttigh & C. Corbau, 2010. Assessment of coastal flooding hazard along the Emilia Romagna littoral, IT. Coastal Engineering 57(11–12): 1042–1058.
MDBMC, 1994. The Algal Management Strategy for the River Murray. Murray-Darling Basin Minesterial Council, Canberra.
Niemann, S. L., Jensen, J. H., Zyserman, J. A., Brøker, I., Baek, S., 2006. Morphological modeling of a Danish tidal inlet. Coastal Engineering: 3308–3320.
Nixon, S. W., 1995. Coastal eutrophication – a definition, social causes, and future concerns. Ophelia 41: 199–219.
Platt, T. & K. Denman, 1980. Patchiness in phytoplankton distribution. In Morris, I. (ed.), the Physiological Ecology of Phytoplankton. Blackwell, Oxford: 413–431.
Prairie, Y. T., C. M. Duarte & J. Kalff, 1989. Unifying nutrient chlorophyll relationships in lakes. Canadian Journal of Fisheries and Aquatic Sciences 46: 1176–1182.
Qin, B., P. Xu, Q. Wu, L. Luo & Y. Zhang, 2007. Environmental Issues of Lake Taihu, China. Hydrobiologia 581: 13–14.
Raikow, D. F., O. Sarnelle, A. E. Wilson & S. K. Hamilton, 2004. Dominance of the noxious cyanobacterium Microcysits aeruginosa in low nutrient lakes is associated with exotic zebra mussels. Limnology and Oceanography 49(2): 482–487.
Sabater, S. & I. Muñoz, 1990. Successional dynamics of the phytoplankton in the lower part of the river Ebro. Journal of Plankton Research 12(3): 573–592.
Shankman, D., B. D. Heim & J. Song, 2006. Flood frequency in China’s Poyang Lake region: trends and teleconnections. International Journal of Climatology 26(9): 1255–1266.
Shankman, D., B. D. Heim, T. Nakayama, R. Li, D. Wu & W. C. Remington, 2012. Hydroclimate analysis of severe floods in China’s Poyang Lake region. Earth Interactions 16(14): 1–16.
Sherman, B., Webster, I., 1997. Flow and the control of cyanobacterial growth in river weir pools. Proceedings of the AWWA 17th Federal Convention, Melbourne: 423–428.
Sherman, B. S., I. T. Webster, G. J. Jones & R. L. Oliver, 1998. Transitions between Aulacoseira and Anabaena dominance in a turbid river weir pool. Limnology and Oceanography 43(8): 1902–1915.
Sinica, Academia, 1985. Physical Geography of China: Climate. Academic Press, Beijing.
Tockner, K., D. Pennetzdorfer, N. Reiner, F. Schiemer & J. V. Ward, 1999. Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria). Freshwater Biology 41: 521–535.
Verhagen, J. H. G., 1994. Modeling phytoplankton patchiness under the influence of wind-driven currents in lakes. Limnology and Oceanography 39: 1551–1565.
Walker Jr, W. W. & K. E. Havens, 1995. Relating algal bloom frequencies to phosphorus concentrations in Lake Okeechobee. Lake and Reservoir Management 11(1): 77–83.
Wang, S., 2014. Poyang Lake: Ecological Security. Science Press, Beijing. (in Chinese).
Wang, S. & H. Dou, 1998. Chinese Lakes. Science Press, Beijing. (in Chinese).
Wang, X. H., Z. W. Fan, L. J. Cui, B. Y. Yan & H. R. Tan, 2004a. Wetland Ecosystem Assessment of Poyang Lake. Science Press, Beijing. (in Chinese).
Wang, T. Y., J. Q. Wang & J. P. Wu, 2004b. The comparison of species diversity of phytoplankton between spring and autumn in Lake Poyang. Journal of Fudan University 43: 1073–1077. (in Chinese).
Warren, I. R. & H. K. Bach, 1992. MIKE 21: a modeling system for estuaries, coastal waters and seas. Environment Software 7(4): 229–240.
Webster, L. T., 1990. Effect of wind on the distribution of phytoplankton cells in lakes. Limnology and Oceanography 35: 989–1001.
Wu, S., P. Xie, S. Wang & Q. Zhou, 2006. Changes in the patterns of inorganic nitrogen and TN/TP ratio and the associated mechanism of biological regulation in the shallow lakes of the middle and lower reaches of the Yangtze River. Science in China: Series D Earth Sciences 49(Supp.): 126–134.
Wu, X., F. Kong, Y. Chen, X. Qian, L. Zhang, Y. Yu, M. Zhang & P. Xing, 2010. Horizontal distribution and transport processes of bloom-forming Microcystis in a large shallow lake (Taihu, China). Limnologica 40: 8–15.
Wu, Z., Y. Cai, X. Liu, C. Xu, Y. Chen & L. Zhang, 2013. Temporal and spatial variability of phytoplankton in Lake Poyang: the largest freshwater lake in China. Journal of Great Lakes Research 29: 476–483.
Wu, Z., H. He, Y. Cai, L. Zhang & Y. Chen, 2014. Spatial distribution of chlorophyll a and its relationship with the environment during summer in Lake Poyang: a Yangtze-connected lake. Hydrobiologia 732: 61–70.
WWF UK Case Study, 2011. HSBC: safeguarding the Changjiang. Celebrating 10 years of conservation success.
Xie, Q. M., C. C. Li & C. L. Peng, 2000. Primary studies on community ecology of phytoplankton in Lake Poyang. Jiangxi Science 18: 162–166.
Xu, C., S. Li, W. Chai & Y. Chen, 2012. A newly recorded cyanobacterial species in water blooms occurred in Lake Poyang-Merismopedia convolute Breb, Kützing. Journal of Lake Sciences 24(4): 643–646.
Xu, H., M. Sun, H. Sui, J. Li & W. Yan, 2003. Microcystin contamination of fish on Poyang Lake in Jiangxi province. Journal of Hygiene Research 32(3): 192–194.
Yin, H. F. & C. G. Li, 2001. Human impact on floods and flood disasters on the Yangtze River. Geomorphology 41: 105–109.
Yi, S., Y. Saito, Q. Zhao & P. Wang, 2003. Vegetation and climate changes in the Changjiang (Yangtze River) Delta, China, during the past 13,000 years inferred from pollen records. Quaternary Science Reviews 24: 1501–1519.
Zhang, Q. & A. D. Werner, 2015. Hysteretic relationships in inundation dynamics for a large lake-floodplain system. Journal of Hydrology 527: 160–171.
Zhang, Q., L. Li, Y. G. Wang, A. D. Werner, P. Xin, T. Jiang & D. A. Barry, 2012. Has the Three-Gorges Dammade the Poyang Lake wetlands wetter and drier? Geophysical Research Letters 39(20): L20402.
Zhang, Q., X. C. Ye, A. D. Werner, Y. L. Li, J. Yao, X. H. Li & C. Y. Xu, 2014. An investigation of enhanced recessions in Poyang Lake: comparison of Yangtze River and local catchment impacts. Journal of Hydrology 517: 425–434.
Zhu, H. H. & B. Zhang, 1997. The Lake Poyang. Press of University of Science and Technology of China, Hefei: 1–12.
Acknowledgments
The limnological study of Poyang Lake and Dongting Lake is part of a long-term study supported by Poyang Lake Laboratory for Wetland Ecosystem Research (PLWER). We are grateful to all staff who collected and processed samples for the monitoring program. We also express our sincere thanks to the Taihu Laboratory for Lake Ecosystem Research (TLLER), Chinese Academy of Sciences, which kindly supplied us the monitoring data. This study was financially supported by the projects of National Basic Research Program (Grant 2012CB417006) and National Natural Science Foundation of China (Grant 41301088).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Rights and permissions
About this article
Cite this article
Liu, X., Li, YL., Liu, BG. et al. Cyanobacteria in the complex river-connected Poyang Lake: horizontal distribution and transport. Hydrobiologia 768, 95–110 (2016). https://doi.org/10.1007/s10750-015-2536-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2536-2