Abstract
In temperate streams, water temperature and organic matter inputs from surrounding forest vary along the altitude. We tested if the different features of streams of similar size determined by an altitudinal gradient might differentially affect the processing rate of different quality leaves (alder, oak and beech). To distinguish the relative contribution of microbial decomposition from overall decomposition, fine- and coarse-mesh bags were used. We determined decomposition rates, leaf-N and -P concentration, microbial respiration (fine bags), invertebrate colonisation (coarse bags) and density and identity of benthic invertebrates in three second-order streams. Alder decomposed faster than the other species in all three streams and regardless of mesh size due to its lower values of C:N, C:P and N:P. Unexpectedly, microbial decomposition rate did not vary among streams for any of the leaf species. The total decomposition rate of alder and oak showed a negative trend along the altitudinal gradient, the magnitude of the change in decomposition rates being similar for both species. The density and structure of the invertebrate community differed along the altitudinal gradient, related to temperature and surrounding vegetation, determining the decomposition rate. Unexpectedly, sensitivity of decomposition rate of different quality leaves to temperature does not differ along the gradient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic matter inputs (e.g. wood, leaf litter) from the surrounding vegetation are the main source of matter and energy to maintain aquatic food webs in temperate forested streams (Tank et al., 2010). This detritus is degraded primarily by microorganisms, mainly aquatic hyphomycetes (Pascoal & Cássio, 2004), and invertebrate detritivores (Graça, 2001), incorporating the dead organic matter into consumer biomass (Tank et al., 2010). Accordingly, organic matter decomposition is a key process in these ecosystems, which transfers energy and matter across trophic levels (Perkins et al., 2010), controls nutrient cycling (Cheever et al., 2012) and contributes greatly to the global carbon cycle by the release of CO2 (Battin et al., 2009).
Leaf litter decomposition is modulated by several environmental factors. Among them, temperature is clearly an important parameter since it influences chemical reactions and metabolic rates of organisms and, hence, affects biological activities (Brown et al., 2004; Davidson & Jansens, 2006; Davidson et al., 2006). In fact, it has been reported that temperature modulates leaf litter decomposition rates through its effect on the organisms driving such process (Friberg et al., 2009; Boyero et al., 2011; Pérez et al., 2011). Thus, it is expected that, within a same region, streams of similar size and order distributed in an altitudinal gradient under dissimilar thermal regimes show different litter processing capacities (Martínez et al., 2014). However, the structure and species composition of the surrounding vegetation vary along the altitudinal gradient determined by the thermal regime (Woodward, 1987), leading to a change in leaf litter inputs into streams. The intrinsic quality of the resource is one of the most important factors influencing organic matter decomposition in freshwater systems; materials with a higher nutrient concentration and a lower content of structural (lignin) and secondary compounds (tannins, phenolics) decompose faster (Ostrofsky, 1997; Casas et al., 2013). Nevertheless, when temperature and resource quality act jointly, the substrate quality might override the effects of temperature on this ecological process, as has been found in terrestrial ecosystems (Coûteaux et al., 1995; Aerts, 2006). In these environments, some authors (Fierer et al., 2005; Conant et al., 2008; Wetterstedt et al., 2010) have reported that low-quality material decomposition (structurally complex C substrates) appears to be more sensitive to temperature increase than high-quality ones, possibly because the microbial enzymatic reactions require a higher net activation energy to metabolise structurally complex C substrates (Bosatta & Ågren, 1999). This suggests that organic substrate quality would have a significant and predictable influence on the sensitivity of microbially mediated decomposition to temperature, which might also occur in freshwater ecosystems (Fernandes et al., 2012). However, whereas studies on individual effects of these two factors on litter processing are common in the literature, few studies have so far addressed the interaction between temperature and detritus quality and their implications on litter processing in freshwater ecosystems (see Ferreira & Chauvet, 2011a; Fernandes et al., 2012; Gonçalves et al., 2013). Furthermore, most of these studies were performed under controlled laboratory conditions and focused only on microbial activity or on metabolism and growth of a single detritivore species (González & Graça, 2003; Díaz-Villanueva et al., 2011) being unable to capture the complexity of natural systems. Thus, altitudinal gradients could be a useful tool for examining the influence of substrate quality and temperature on the decomposition process in the natural environment. Moreover, along these gradients, differences in stream biotic communities are expected since they are influenced by elevation-dependent variables such as water temperature and the development and composition of surrounding forest (Swan & Palmer, 2006; Friberg et al., 2009; Martínez et al., 2014; Taylor & Chauvet, 2014), determining leaf litter decomposition rates (Martínez et al., 2013a).
Thus, the goal of the present study was to assess if the features of streams located along an altitudinal gradient might differentially affect the processing rate of leaf species with different qualities. For this, leaf litter of three common and native tree species, alder (Alnus glutinosa (L.) Gaertner), oak (Quercus robur L.) and beech (Fagus sylvatica L.), was incubated in three streams that differed, due to their differences in altitude, in mean winter water temperature and surrounding forest composition. Regarding leaf species quality, alder presents higher nutrient concentration (nitrogen and phosphorous) and lower lignin content (recalcitrant carbon) than the other two species (see Ostrofsky, 1997 to compare Alnus, Quercus and Fagus). To distinguish the relative importance and contribution of microbial decomposers and invertebrate detritivores to litter processing, fine- and coarse-mesh bags were used. We hypothesise that (1) alder leaves decompose faster than oak and beech leaves because of their higher nutrient concentrations, irrespective of the stream; (2) the decomposition rates in fine bags of all leaf litter species vary among streams being positively related to temperature; (3) the decomposition rates in coarse bags of all leaf litter species vary among streams being determined by temperature and macroinvertebrate assemblages; (4) with a decrease in altitude, the magnitude of the change in decomposition rates will be higher in oak and beech litter than in alder litter due to an increase in temperature.
Materials and methods
Study sites
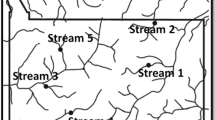
The study was conducted in three second-order headwater streams (S1, S2 and S3) with siliceous substrata, and flowing into the Atlantic Ocean (Cordillera Cantábrica, northern Spain, Table 1). The streams showed differences in their daily mean water temperature (recorded hourly) monitored with Smart Button temperature data loggers (ACR Systems Inc., Surrey, BC, Canada) from November 2011 to April 2012, which where inversely related to the elevation of each site (Table 1). The streams were selected from a pool of 25 sites monitored the previous year within an approximately 2500 km2 area. The selected streams presented oligotrophic waters (<700 µg n l−1, < 25 µg p l−1) following the trophic status classification by Dodds et al. (1998), values of conductivity lower than 100 µS cm−1, a high cover of native vegetation in the watershed and a temperature range of about 4.5°C between the coldest and the warmest one (Table 1). S1 runs through heath and F. sylvatica forest, S2 through F. sylvatica and Q. robur forests, and S3 through Q. robur forest, also featuring tree plantations of Eucalyptus globulus Labill. in upland areas of this watershed (Table 1). Nonetheless, riparian vegetation is dominated by native tree species in the three streams, with F. sylvatica at S1 and A. glutinosa, Fraxinus excelsior L. and Corylus avellana L. at S2 and S3.
Stream water characteristics
During the study period, selected water physical and chemical variables were measured in the three streams on nine occasions, when oxygen saturation, conductivity, pH (WTW multiparametric sensor), and river flow (Martin Marten Z30, Current-meter) were measured in situ. Additionally, water samples were taken from each stream with polyethylene bottles and transported to the laboratory in refrigerated chambers for alkalinity and nutrient analyses. In the laboratory, water samples were immediately filtered (preweighed 0.7 μm pore size glass fibre filters, Whatman GF/F). Subsamples of the filtered water were used to determine alkalinity by titration to an end pH of 4.5 (APHA, 2005). Nitrate concentration was determined by capillary ion electrophoresis (Agilent CE); ammonium was measured by the salicylate-hypochlorite method, nitrite by the sulphanylamide method and soluble reactive phosphorus (SRP) by the molybdate method (APHA, 2005).
Decomposition experiment
Leaves of three common tree species in the study area were used for the experiment: alder (A. glutinosa), oak (Q. robur) and beech (F. sylvatica). Alder is a typical species of riparian forest and oak and beech form natural forests in this area. The three leaf litter species differ in quality in terms of nitrogen and phosphorous concentration, with alder having the highest values of both nutrients (Table 2).
In October and November 2011, fresh fallen leaves were collected from the forest soil, were air-dried, and approximately 3 g (±0.25) of each species were placed separately into fine-mesh bags (15 × 20 cm, 0.5 mm mesh size) and 5 g (±0.25) into coarse-mesh bags (20 × 25 cm, 5 mm mesh size). The use of coarse and fine bags is recommended by Gessner & Chauvet (2002) and 5 mm and 0.5 mm mesh size bags have been broadly used in decomposition experiments and allows for distinguishing the relative importance and contribution of microbial decomposers from overall decomposition keeping the same environmental conditions (e.g. Flores et al., 2013; Ferreira & Canhoto, 2014; Mariluan et al., 2015). The bags were placed in the streams on November 21, 2011. At each site, 16 iron bars were anchored randomly in selected riffles on the streambed along 50 m, and six bags (two mesh sizes × three species of leaf litter) were tied to each bar by nylon lines. In total, 96 bags were placed in each streambed (32 bags per leaf litter species). Additionally, four bags of each species and mesh type were located in one of the streams (S3) to determine the mass-loss by leaching (24 h after incubation). The bags were retrieved (four fine bags and four coarse bags per leaf litter species from each stream) after 7 days (t7) and, thereafter, when losses roughly corresponded to 25% (t25), 50% (t50) and 75% (t75) of the initial mass (corrected for leaching) of each species. We estimated the collecting dates based on the mass loss from the previous samplings. The incubation period of fine-mesh bags was 105 days for alder and 156 days for oak and beech, whereas that of coarse-mesh bags was 50 days for alder and 125 days for oak and beech. At each collection, the bags were placed individually in plastic bags and transported in refrigerated containers to the laboratory. On each sampling date, the remaining leaf material from each bag was rinsed with filtered stream water through a 500 µm sieve to remove sediments and macroinvertebrates and was subsequently oven-dried (70°C, 72 h) and weighed to determine leaf dry mass (DM), and combusted (500°C, 4 h) to determine the remaining ash-free dry mass (AFDM). Subsamples of each bag were ground with a Culatti mill to pass through a 1.0-mm mesh and were stored at −20°C for nutrient analyses. The carbon and nitrogen concentrations were determined with a Perkin Elmer series II CHNS/O elemental analyser and phosphorus concentration was determined spectrophotometrically after mixed acid digestion (molybdenum blue method; Allen et al., 1974). Results were expressed as a percentage elemental content (C, N and P) of leaf litter dry mass.
Oxygen consumption
At the t25 sampling, the microbial respiration of the leaf litter of fine-mesh bags was measured using a dissolved oxygen measurement system (Strathkelvin 928 System). Five 12-mm discs of each leaf species were incubated in chambers with 3 mL 100% dissolved O2 saturated filtered stream water (10°C, 40 min). An extra chamber without discs was used as control. Oxygen consumption rates were determined, considering the oxygen concentration in the sample and the control over a 20-min interval and were corrected for the time and disc mass. The results were expressed as mg O2 g−1 DM h−1.
Macroinvertebrates
At the t50 sampling (36 days of incubation), macroinvertebrates were sorted from coarse-mesh bags on a 0.5-mm sieve and preserved in 70% ethanol. On the same sampling date, five benthic samples (Surber 0.09 m2, 0.5 mm mesh size) were taken from randomly chosen riffles in each stream. Macroinvertebrates from bags and the benthos were identified to genus level (some to family, Oligochaeta to order), counted and assigned to shredders or non-shredders (Tachet et al., 2002). The two groups of macroinvertebrates were oven-dried (60°C, 72 h) and weighed separately to obtain their DM per bag.
Statistical analyses
Water temperature comparisons were performed with one-way ANOVA (factor: stream) followed by the Tukey HSD test (Zar, 2010), considering daily mean temperature (n = 158) as a replicate. The same analysis was performed for other physical and chemical characteristics of the water for each stream. Breakdown rates were estimated both by a linear model (M t = M 0 − b·t; where b is the loss rate, M t is the remaining % AFDM at time t and M 0 is the initial % AFDM after correcting for leaching) and an exponential model (M t = M 0 ·e −kt; where k is the exponential loss rate). The correction of data for initial leaching provided a better fit of mass remaining data to the linear models (higher goodness of fit) in 12 of 18 rates calculated, and predicted better the initial amount of leaf mass (95–101% at t = 0) in all cases. Thus, only linear breakdown rates are presented here. In the figures, mean and standard error (SE) of rates are presented; both calculated using temporal replicas for each mesh size, stream and leaf species. The decay rates of the three species were compared by a two-way ANCOVA (factors: stream, leaf species) with days as a covariate. Initial leaf nitrogen and phosphorus concentrations were compared using one-way ANOVA (factor: leaf species), with post hoc comparisons performed by the Tukey test (Zar, 2010). Leaf-N and -P dynamics during the incubation were tested by three-way ANOVA (factors: stream, date, leaf species). Differences in leaf litter-associated macroinvertebrates (density and biomass) among the three studied streams were tested using two-way ANOVA (factors: stream, leaf species), and benthic fauna was analysed using one-way ANOVA (factor: stream). Bivariate relationships were tested by ANCOVA and linear regression analyses. To search for general differences in benthic macroinvertebrate assemblages among streams, non-metric multidimensional scaling (NMDS) was performed based in the Bray-Curtis dissimilarity matrix, followed by PERMANOVA (106 permutations). When necessary, data were transformed (log10 (x + 1)) and all mixed model parameters were estimated by means of restricted maximum likelihood. All statistical analyses were performed using the R statistical program (version 2.11.1; R Development Core Team, 2010).
Results
Stream water characteristics
During the study period (November 2011–April 2012), streams differed in water temperature (ANOVA: F 2,471 = 270.57, P < 0.001), representing a range of about 4.5°C (mean ± SE; S1: 4.29 ± 0.15°C < S2: 6.76 ± 0.15°C < S3: 8.79 ± 0.11°C). All streams showed oxygen-saturated water, with low mineralisation, neutral pH, and low dissolved nutrient concentrations (Table 1). Although all streams presented oligotrophic waters (<600 µg n l−1, <15 µg p l−1), S1 had the lowest nitrate concentration, while S3 the highest one (Table 1; ANOVA: F 2,24 = 114.47, P < 0.001). The conductivity and alkalinity differed statistically among streams (ANOVACond: F 2,24 = 96.25, P < 0.001; ANOVAAlk: F 2,24 = 15.05, P < 0.001); however, the range of these values was very narrow (Table 1). The streams did not present statistical differences in the other physical and chemical characteristics.
Leaf litter decomposition
In general, the leaf litter of all species decomposed faster in coarse-mesh bags than in fine-mesh bags (Fig. 1; ANCOVA: F 1,216 = 51.79, P < 0.001). The decomposition rate of leaf litter incubated in fine-mesh bags ranged from 0.109%AFDM d−1 in beech at S1 to 0.545% AFDM d−1 in alder at S2. The breakdown rate from litter in coarse bags ranged from 0.123%AFDM d−1 in beech at S1, to 1.417%AFDM d−1 in alder at S3. There were differences among leaf species both in fine and coarse bags (Fig. 1, ANCOVAF: F 2,109 = 134.59, P < 0.001; ANCOVAC: F 2,119 = 189.88, P < 0.001), with alder, the richest material in terms of nitrogen and phosphorus content, showing the highest rate in all streams. In fine bags there were no differences in the decomposition rate among streams regardless of leaf species. However, in coarse bags, the lowest rates were recorded for all species at S1 (lowest nitrate concentration and coolest water temperature). The breakdown rates of alder and oak showed a negative trend along the altitudinal gradient (i.e. higher decomposition rate with a decrease in altitude). During the incubation period, the differences in nitrogen and phosphorous concentration among species remained constant both in fine (Fig. 2, ANOVAN: F 2,94 = 2719.35, P < 0.01; ANOVAP: F 2,94 = 578.61, P < 0.01) and coarse bags (Fig. 2; ANOVAN: F 2,94 = 2813.37, P < 0.01; ANOVAP: F 2,94 = 599.58, P < 0.01). Only the material incubated in fine bags in S3, the stream with a higher dissolved nitrate, was more enriched in nitrogen than that submerged in the other two streams (F 2,94 = 16.81, P < 0.01). However, this trend was not observed for the %P content.
Microbial respiration and leaf litter decomposition
The oxygen consumption rate ranged from 0.13 mg O2 g−1 DM h−1, in beech leaves at S3, to 0.46 mg O2 g−1 DM h−1 in alder leaves at S1. The respiration rate differed among leaf litter species and was highest in alder leaves, the material that was most decomposed after 20 days of incubation (Fig. 3; ANOVA: F 2,26 = 26.70, P < 0.001) and that presented highest concentration of N and P. Among streams, the oxygen consumption rate tended to be higher at S1 but the difference was not statistically significant (Fig. 3; ANOVA: F 2,26 = 2.98, p = 0.068). Moreover, microbial respiration correlated positively with the decomposition rate in fine-mesh bags (r 2 = 0.85, P < 0.001).
Macroinvertebrates and leaf litter decomposition
A total of 59 macroinvertebrate taxa were identified in benthic samples (32 in S1, 43 in S2 and 47 in S3), 16 of which were shredders (9 in S1, 12 in S2 and 14 in S3). The macroinvertebrate community structure differed among streams (PERMANOVA: PseudoF 2,14 = 6.80, P < 0.001). Shredder assemblages were dominated by plecopterans (Capnioneura and genera of Nemouridae) in S1, plecopterans (Capnioneura and genera of Nemouridae) and trichopterans (Sericostoma) in S2, and crustaceans (Echinogammarus) and trichopterans (Lepidostomidae) in S3. The density of total benthic macroinvertebrates and shredders was lowest in S1 and highest in S3 (Table 3; ANOVA: F 2,15 = 9.66, P = 0.003; ANOVA: F 2,15 = 3.95, P = 0.048). Similarly, the biomass of total macroinvertebrates and shredders was lowest in S1 and highest in S3 (Table 3; ANOVA: F 2,15 = 10.40, P = 0.002; ANOVA: F 2,15 = 7.63, P = 0.007).
In total, 47 taxa were identified from macroinvertebrates colonising leaf bags. The total macroinvertebrate richness ranged from 13 in oak leaves in S1 to 26 in oak leaves in S2. Out of the total taxa, 12 were shredders, ranging from 4 in oak leaves in S3 to 10 in alder leaves in S2. The composition of shredder assemblages in colonising bags reflected the benthic macroinvertebrate composition in each stream. The total macroinvertebrate density per gram of remaining AFDM differed among leaf species (Fig. 4; ANOVA: F 2,27 = 19.77, P < 0.001): alder bags were more colonised than oak and beech bags in all streams. Among streams, bags of the three species incubated in S1 were less colonised than those in S2 and S3 (ANOVA: F 2,27 = 5.02, P = 0.014). In terms of biomass, there were also differences among leaf litter species (Fig. 4; ANOVA: F 2,27 = 11.27, P < 0.001), and streams (ANOVA: F 2,27 = 24.89, P < 0001), with the lowest macroinvertebrate biomass found in S1.
Alder, the most nutritive species among the selected ones, was most densely colonised in terms of shredder density per gram of remaining AFDM (Fig. 4; ANOVA: F 2,27 = 21.91, P < 0.001), but this did not differ significantly among streams (Fig. 4; ANOVA: F 2,27 = 0.0.44, P = 0.646). The shredder biomass per gram of remaining AFDM was higher in alder bags than that in beech bags (ANOVA: F 2,27 = 13.58, P < 0.001). Among streams, the leaf packs in S3 contained a greater biomass of shredders than those in S1 (Fig. 4; ANOVA: F 2,27 = 13.50, P < 0.0001). The variability of breakdown rate among leaf species was positively correlated with shredder density (Fig. 5; ANCOVA: F 1,7 = 33.15, P < 0.001).
Discussion
Alder leaves decomposed much faster than those of oak and beech in both mesh types, regardless of the stream considered. This variation was related to differences in microbial metabolism (estimated via oxygen consumption), and the colonisation by detritivores, showing a clear preference by the stream biota for better quality substrates (based on nutritional composition). It is known that the physical and chemical characteristics of leaves, such as toughness, roughness and toxic substances or nutrient content, determine microbial decomposer colonisation and activity (Bärlocher & Oertli, 1978; Dang et al., 2007; Kearns & Bärlocher, 2008) as well as its consumption by invertebrate detritivores (Hladyz et al., 2009; Kominoski & Pringle, 2009). Specifically, the softer texture of alder leaves and their higher nutrient concentration than oak and beech leaves might favour the colonisation of microbial decomposers and macroinvertebrate detritivores and explain their faster processing rate.
Microbial decomposition rates remained unchanged along the altitudinal gradient despite the difference in mean water temperature among streams (4.5°C). This lack of a response was unexpected since an enhancement of decomposition rate with an increase in temperature has been often reported in both field (Friberg et al., 2009; Boyero et al., 2011; Ylla et al., 2014) and under controlled laboratory conditions (Ferreira & Chauvet, 2011b, Martínez et al., 2014) as a result of the stimulation of the metabolism of microbial decomposers. Furthermore, the inter-stream nitrate difference, highest in the warmer stream (S3) where the materials present the higher nitrogen concentrations compared to those from the other streams, makes our results even more unexpected due to the positive synergetic effect between temperature and dissolved nutrient availability on the decomposition rate (Ferreira & Chauvet, 2011b; Martínez et al., 2014). The results found for terrestrial (Fierer et al., 2005; Conant et al., 2008; Wetterstedt et al., 2010) and, recently, also for freshwater ecosystems (Fernandes et al., 2012; Ylla et al., 2014) show that low-quality resources (structurally complex C substrates) are more sensitive to temperature. In addition, it has been reported that the decomposition rate of low-quality species is more sensitive to dissolved nutrient availability (Molinero et al., 1996; Pérez et al., 2014). Therefore, we expected the microbial decomposition of oak and beech litter to be more enhanced than that of alder at higher temperature and with higher contents of dissolved nitrates. However, our results do not support the previous findings, the rates being similar among streams whatever the species. Thus, the present work reinforces the idea that the relationships among water temperature, dissolved nutrients, resource quality, and leaf decomposition by microbial activity do not always follow the same pattern.
The breakdown rate in coarse-mesh bags (combined microbial and detritivore activity), in general, was higher with decreasing altitude. As the decrease in altitude does not mean an increase in stream order or in flow discharge, we discard physical abrasion as explanation of the differences. Thus, the observed response could be reflecting the direct influence of temperature on metabolism and resource processing (Gillooly et al., 2001; Brown et al., 2004) which would be consistent with previous works (Friberg et al., 2009; Dossena et al., 2012; Taylor & Chauvet, 2014). However, temperature plays also a key role determining biotic community structure (Mouthon & Daufresne, 2006), interspecific relationships (Jiang & Morin, 2007), population dynamics (Dokulil, 2013), and species distribution (Castella et al., 2001). In the present study, the macroinvertebrate community structure varied among streams with density and biomass increasing along the temperature gradient, as Friberg et al. (2009) and Dossena et al. (2012) reported. Moreover, the composition and development of surrounding vegetation defined by the gradient of altitude, determined in turn by thermal regime (Woodward, 1987), could also be influencing the structure, composition and abundance of in-stream macroinvertebrate communities (Swan & Palmer, 2006). The warmer streams (S2 and S3) are surrounded by a temperate riparian forest of A. glutinosa, F. excelsior and C. Avellana which offer greater diversity of leaf litter inputs of high quality compared to the coldest one (S1), where river banks are completely dominated by F. sylvatica. Thus, S2 and S3 are able to support a more abundant community compared to S1 (Martínez et al., 2013b). Considering the close relationship between benthic detritivores (density and biomass) and leaf litter processing rates in the same region (Martínez et al., 2013a), the density and biomass of shredders explain greatly the observed inter-site variability in breakdown rates.
As decomposition in fine-mesh bags, the breakdown rate of low-quality resources in coarse-mesh bags did not show a greater sensitivity to temperature and dissolved nutrient availability than that of high-quality leaves. It has been reported under laboratory conditions that stream shredders feeding on low-quality resources show lower assimilation efficiencies of nutrients (N and P) when the temperature increases (Díaz-Villanueva et al., 2011). As these consumers must maintain their elemental composition (C: N: P) within a narrow range (Sterner & Elser, 2002), nutrient limitations might be addressed by increasing the feeding rate (Frost et al., 2005). Therefore, an increase in feeding rates of low-quality resources was expected to satisfy their nutrient demands. However, streams are not as simple as isolated microcosms and provide a different type of resource to complex biotic communities. Thus, macroinvertebrate detritivores are not obliged to feed on the experimentally introduced poor material, since they are able to select resources in relation to their properties (Hladyz et al., 2009; Kominoski & Pringle, 2009) to fulfil their nutritional demands (Sterner & Elser, 2002; Woodward, 2009). In fact, beech breakdown rates were similar in fine- and coarse-mesh bags, pointing to an insignificant role of detritivore feeding on this resource.
Conclusion
In summary, the main results are as follows: (1) the intrinsic quality of leaf litter plays a key role in determining decomposition rates; (2) unexpectedly, microbial-mediated decomposition does not differ among streams along the altitudinal gradient with differences in temperature and dissolved nutrients; (3) the features of the streams along the altitudinal gradient determine the structure and activity of macroinvertebrate detritivores, which determine greatly the breakdown rate; and (4) the sensitivity of decomposition rates of different quality leaves to temperature does not vary along altitudinal gradient. Thus, previous findings under laboratory conditions concerning temperature effects on leaf litter processing are not so easy to extrapolate to natural systems, due to interactions with other local factors. Predictions about the effects of future temperature increases on leaf litter decomposition and stream functioning should not only focus on shifts in biotic metabolic activities, since changes in the thermal regime would also alter many environmental variables, including the riparian vegetation and the benthic community composition.
References
Aerts, R., 2006. The freezer defrosting: global warming and litter decomposition rates in cold biomes. Journal of Ecology 94: 713–724.
Allen, S. E., H. M. Grimshaw, J. A. Parkinson & C. Quarmby, 1974. Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, Oxford.
APHA (American Public Health Association), 2005. Standard Methods For the Examination of Water and Wastewater, 21st ed. American Public Health Association, American Water Works Association, and Water Environment Federation, Washington, DC.
Bärlocher, F. & J. J. Oertli, 1978. Inhibitors of aquatic hyphomycetes in dead conifer needles. Archiv für Hydrobiologie 81: 462–474.
Battin, T. J., S. Luyssaert, L. A. Kaplan, A. K. Aufdenkampe, A. Richter & L. J. Tranvik, 2009. The boundless carbon cycle. Nature Geoscience 2: 598–600.
Bosatta, E. & G. I. Ågren, 1999. Soil organic matter quality interpreted thermodynamically. Soil Biology and Biochemistry 31: 1889–1891.
Boyero, L., R. G. Pearson, M. O. Gessner, L. A. Barmuta, V. Ferreira, M. A. S. Graça, et al., 2011. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecology Letters 14: 289–294.
Brown, J. H., J. F. Gillooly, A. P. Allen, V. M. Savage & G. B. West, 2004. Toward a metabolic theory of ecology. Ecology 85: 1771–1789.
Casas, J. J., A. Larrañaga, M. Menéndez, J. Pozo, A. Basaguren, A. Martínez, J. Pérez, J. M. González, S. Mollá, C. Casado, E. Descals, N. Roblas, J. A. López-González & J. L. Valenzuela, 2013. Leaf litter decomposition of native and introduced tree species of contrasting quality in headwater streams: how does the regional setting matter? Science of the Total Environment 458–460: 197–208.
Castella, E., H. Adalsteinsson, J. E. Britain, G. M. Gislason, A. Lehmann, V. Lencioni, et al., 2001. Macrobenthic invertebrate richness and composition along a latitudinal gradient of European glacier-fed streams. Freshwater Biology 46: 1811–1831.
Cheever, B. M., E. B. Kratzer & J. R. Webster, 2012. Immobilization and mineralization of N and P by heterotrophic microbes during leaf decomposition. Freshwater Science 31: 133–147.
Conant, R. T., R. A. Drijber, M. L. Haddix, W. J. Parton, E. A. Paul, A. F. Plante, J. Six & M. Steinweg, 2008. Sensitivity of organic matter decomposition to warming varies with its quality. Global Change Biology 14: 868–877.
Coûteaux, M. M., P. Botter & B. Berg, 1995. Litter decomposition, climate and litter quality. Trends in Ecology and Evolution 10: 63–66.
Dang, C. K., M. O. Gessner & E. Chauvet, 2007. Influence of conidial traits and leaf structure on attachment success of aquatic hyphomycetes on leaf litter. Mycologia 99: 24–32.
Davidson, E. A. & I. A. Janssens, 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173.
Davidson, E. A., I. A. Janssens & Y. Q. Luo, 2006. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Global Change Biology 12: 154–164.
Díaz-Villanueva, V., R. Albariño & C. Canhoto, 2011. Detritivores feeding on poor quality food are more sensitive to increased temperatures. Hydrobiolgia 678: 155–165.
Dokulil, M. T., 2013. Impact of climate warming on European inland waters. Inland Waters 4: 27–40.
Dodds, W. K., J. R. Jones & E. B. Welch, 1998. Suggested classification of stream trophic state: distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Research 32: 1455–1462.
Dossena, M., G. Yvon-Durocher, J. Grey, J. M. Montoya, D. M. Perkins, M. Trimmer & G. Woodward, 2012. Warming alters community size structure and ecosystem functioning. Proceedings of the Royal Society B-Biological Sciences 279: 3011–3019.
Fernandes, I., C. Pascoal, H. Guimarães, R. Pinto, I. Sousa & F. Cássio, 2012. Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshwater Biology 57: 2306–2317.
Ferreira, V. & C. Canhoto, 2014. Effect of experimental and seasonal warming on litter decomposition in a temperate stream. Aquatic Sciences 76: 155–163.
Ferreira, V. & E. Chauvet, 2011a. Future increase in temperature more than decrease in litter quality can affect microbial litter decomposition in streams. Oecologia 67: 279–291.
Ferreira, V. & E. Chauvet, 2011b. Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Global Change Biology 17: 551–565.
Fierer, N., J. M. Craine, K. McLauchlan & J. P. Schimel, 2005. Litter quality and the temperature sensitivity of decomposition. Ecology 86: 320–326.
Flores, L., J. R. Díez, A. Larrañaga, C. Pacoal & A. Elosegi, 2013. Effects of retention site on breakdown of organic matter in a mountain stream. Freshwater Biology 58: 1267–1278.
Friberg, N., J. B. Dybkjær, J. S. Olafsson, G. M. Gislason, S. E. Larsen & T. L. Lauridsen, 2009. Relationships between structure and function in streams contrasting in temperature. Freshwater Biology 54: 2051–2068.
Frost, P. C., M. A. Evans-White, Z. V. Finkel, T. C. Jensen & V. Matzek, 2005. Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109: 18–25.
Gessner, M. O. & E. Chauvet, 2002. A case for using litter breakdown to assess functional stream integrity. Ecological Applications 12: 498–510.
Gillooly, J. F., J. H. Brown, G. B. West & V. M. Savage, 2001. Effect of size and temperature on metabolic rate. Science 239: 2248–2251.
Gonçalves, A. L., M. A. S. Graça & C. Canhoto, 2013. The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecology 6: 546–553.
González, J. M. & M. A. S. Graça, 2003. Conversion of leaf litter to secondary production by a shredding caddis-fly. Freshwater Biology 48: 1578–1592.
Graça, M. A. S., 2001. The role of invertebrates on leaf litter decomposition in streams – a review. International Review of Hydrobiology 86: 383–393.
Hladyz, S., M. O. Gessner, P. S. Giller, J. Pozo & G. Woodward, 2009. Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshwater Biology 54: 957–970.
Jiang, L. & P. J. Morin, 2007. Temperature fluctuation facilitates coexistence of competing species in experimental microbial communities. Journal of Animal Ecology 76: 660–668.
Kearns, S. G. & F. Bärlocher, 2008. Leaf surface roughness influences colonization success of aquatic hyphomycete conidia. Fungal Ecology 1: 13–18.
Kominoski, J. S. & C. M. Pringle, 2009. Resource–consumer diversity: testing the effects of leaf litter species diversity on stream macroinvertebrate communities. Freshwater Biology 54: 1461–1473.
Mariluan, G. D., V. Díaz-Villanueva & R. J. Albariño, 2015. Leaf litter breakdown and benthic invertebrate colonization affected by seasonal drought in headwater lotic systems of Andean Patagonia. Hydrobiologia. doi:10.1007/s10750-015-2324-z.
Martínez, A., A. Larrañaga, A. Basaguren, J. Pérez, C. Mendoza-Lera & J. Pozo, 2013a. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
Martínez, A., A. Larrañaga, J. Pérez, E. Descals, A. Basaguren & J. Pozo, 2013b. Effects of pine plantations on structural and functional attributes of forested streams. Forest Ecology and Management 310: 147–155.
Martínez, A., A. Larrañaga, J. Pérez, E. Descals & J. Pozo, 2014. Effects of temperature on leaf-litter decomposition in low-order forested streams: field and microcosm approaches. FEMS Microbiology Ecology 87: 257–267.
Molinero, J., J. Pozo & E. González, 1996. Litter breakdown in streams of the Agüera catchment: influence of dissolved nutrients and land use. Freshwater Biology 36: 745–756.
Mouthon, J. & M. Daufresne, 2006. Effects of the 2003 heatwave and climatic warming on mollusc communities of the Saône: a large lowland river and of its two main tributaries (France). Global Change Biology 12: 441–449.
Ostrofsky, M. L., 1997. Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. Journal of the North American Benthological Society 16: 750–759.
Pascoal, C. & F. Cássio, 2004. Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Applied Environmental Microbiology 70: 5266–5273.
Pérez, J., M. Menéndez, S. Larrañaga & J. Pozo, 2011. Inter– and intra–regional variability of leaf litter breakdown in reference headwater streams of northern Spain: Atlantic versus Mediterranean streams. International Review of Hydrobiology 96: 105–117.
Pérez, J., J. Galán, E. Descals & J. Pozo, 2014. Effects of fungal inocula and habitat conditions on alder and eucalyptus leaf litter decomposition in streams of northern Spain. Microbial Ecology 67: 245–255.
Perkins, D. M., J. Reiss, G. Yvon-Durocher & G. Woodward, 2010. Global changes and food webs in running waters. Hydrobiologia 657: 181–198.
R Development Core Team (2010). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org.
Sterner, R. W. & J. J. Elser, 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton.
Swan, C. M. & M. A. Palmer, 2006. Composition of speciose leaf litter alters stream detritivore growth, feeding activity and leaf breakdown. Oecologia 147: 469–478.
Tachet, H., P. Richoux, M. Bournaud & P. Usseglio-Polatera, 2002. Invertébrés d’eau douce: systématique, biologie et écologie. CNRS, Paris: 587.
Tank, J. L., E. J. Rosi-Marshall, N. A. Griffiths, S. A. Entrekin & M. L. Stephen, 2010. A review of allochthonous organic matter dynamics and metabolism. Journal of the North American Benthological Society 29: 118–146.
Taylor, B. R. & E. Chauvet, 2014. Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia 721: 239–250.
Wetterstedt, J. Å. M., T. Persson & G. I. Ågren, 2010. Temperature sensitivity and substrate quality in soil organic matter decomposition: results of an incubation study with three substrates. Global Change Biology 16: 1806–1819.
Woodward, F. I., 1987. Climate and plant distribution. Cambridge University Press, Cambridge.
Woodward, G., 2009. Biodiversity, ecosystem functioning and freshwater food webs: assembling the jigsaw puzzle. Freshwater Biology 54: 2171–2187.
Ylla, I., C. Canhoto & A. M. Romaní, 2014. Effects of warming on stream biofilm organic matter use capabilities. Microbial Ecology 68: 132–145.
Zar, J. H., 2010. Biostatistical Analysis. Prentice Hall, Upper Saddle River.
Aknowledgements
This study was funded by the Spanish Ministry of Science and Innovation (Projects CGL2010-22129-C04-01 and CGL2011-23984) and by the Basque Government (Research Grant IT-302-10). The authors thank the technicians of SGIker’s SCAB Service, of the University of The Basque Country, UPV/EHU, for the nitrate measurements. A. Martínez was granted by the Basque Government and the University of The Basque Country, UPV/EHU. S. Monroy was granted by Spanish Ministry of Economy and Competitiveness.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Checo Colón-Gaud
Aingeru Martínez and Silvia Monroy contributed equally to this work.
Rights and permissions
About this article
Cite this article
Martínez, A., Monroy, S., Pérez, J. et al. In-stream litter decomposition along an altitudinal gradient: does substrate quality matter?. Hydrobiologia 766, 17–28 (2016). https://doi.org/10.1007/s10750-015-2432-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2432-9