Abstract
We test the suitability of microbial parameters to assess environmental pressures, and investigate the abundance and phenotypic traits of prokaryotic cells in an Antarctic area. During two oceanographic cruises, carried out in 2001 and 2005, seawater samples were collected in two mooring stations of the Ross Sea. As ancillary parameters, determinations of lipopolysaccharides, chlorophyll a, and/or fractionated adenosine-tri-phosphate were done. Furthermore, in the 2005 survey, six more stations were sampled. Results show that prokaryotic cells—in terms of abundance, shape, and size—change in relation with environmental changes. Prokaryotic abundance was high, where a strong condition of eutrophication was detected. Sizes of cells showed the prevalence of very small forms (>0.1 μm3), but larger morphotypes were observed in high trophic conditions (till 0.4 μm3). In Antarctic marine systems, such prokaryotic parameters can be used to evaluate environment status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cell count and single-cell volume estimation represent primary steps in the comprehension of the role played by prokaryotes (bacteria and archaea) in ecological studies, mainly contributing to an updated quantification in terms of carbon biomass. The development of analytical protocols for studying the shapes and sizes of microorganisms in aquatic environments is in progress (Posch et al., 2009; Zeder et al., 2011). Cell morphology has biological relevance since the individual cell shapes are adaptive (Young, 2007) and can modify according to the cell lineages (Cottrel & Kirchman, 2004). Cell morphology is a sensitive biomarker in response to environmental cues in aquatic ecosystems (Żmuda, 2005; Straza et al., 2009). As a matter of fact, cell sizes and shapes quickly change conferring advantage in natural selection, helping cells cope with, and adapting to external conditions. Therefore, the main selective forces invoked in morphological and size regulation are the top down and bottom up pressures together with cell division (Young, 2007).
When studying prokaryotic biomass in aquatic systems, the most currently used method is the direct count of cell abundance—by epifluorescence microscopy or flow cytometry—and the following application of standard carbon conversion factors among which the most used is 20 fg C cell−1 (Lee & Fuhrman, 1987). Since the morphological variability implies different cell carbon contents (Bölter et al., 2002), the application of a conversion factor ad hoc—i.e., directly derived from cell volume determinations—seems to be suitable for a more reliable estimation of prokaryotic biomass in ecological studies (Posch et al., 2001; La Ferla et al., 2014).
The quantification of the prokaryotic biomass by a combination of cell counting, cell sizing, and the use of conversion factors based on cell volume has occasionally been performed in temperate marine environments (i.e., La Ferla et al., 2012 and references herein) and even less in the Southern Ocean (Delille, 2004; Stewart & Fritsen, 2004; Celussi et al., 2009). In Antarctic spring/summer periods, the Southern Ocean is a high nutrient low chlorophyll (HNLC) environment where constant sunlight, temperature, and melting ice phenomena rule the biota variability. The Ross Sea is one of the most productive areas of the Southern Ocean with primary production values about four-fold higher than those of other areas (Nelson et al., 1996). Its numerous polynyas, extensive ice shelf (covering half of the continental Antarctic shelf), and vertical and horizontal water exchanges provide a dynamic environment (Catalano et al., 2006; Smith et al., 2007; Vichi et al., 2009).
In summer 2001 and 2005, two cruises were carried out in the Ross Sea for studying the prokaryotic cell abundance (PA), biovolume (VOL) and morphotype distribution along the water column at two mooring stations (max depth of 820 m). Basic hydrologic parameters and indirect estimates of trophic assessment were done (lipopolysaccharides, LPS, and adenosine-tri-phosphate, ATP, in the 2001 cruise; LPS and chlorophyll a, Chla, in the 2005 one). In addition to the aforementioned stations, other six sites were investigated in the cruise of 2005.
The hypothesis tested in this paper is that cell abundance and phenotypic trait of prokaryotic cells are constrained by environmental pressures. The specific objectives of this study are (i) to insight a general explanation for prokaryotic cell variation—in term of abundance, shape and size—in pelagic areas of the Ross Sea; (ii) to assess whether the prokaryotic parameters are useful to describe the environmental status.
Materials and methods
Study area
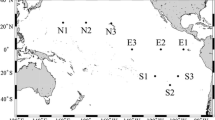
The XVI and XX PNRA Antarctic expeditions were carried out in the Ross Sea from January 20th to February 7th, 2001 and January 4th to February 14th, 2005, respectively, aboard the Italian R/V Italica. In the 2001 survey, station A was sampled one time (st A01), whereas station B was sampled twice with a time lag of 18 days (st B01 and st B101, respectively). In the 2005 survey, the same sites were reinvestigated (st A05 and st B05) together with other six stations (Fig. 1). In Table 1 the stations sampled, their coordinates and sampling depths are reported.
Sample collection
Seawater samplings were performed by using a Rosette sampler (Sea-Bird Electronics SBE32 with 24 Niskin bottles, 12 l) mounted on a CTD equipped with a Sea-Bird 9/11 plus multiparametric probe (SeaBird Electronics) with temperature- (T), conductivity- (salinity, S, and density, D), dissolved oxygen- (DO; SBE 43) -sensors.
LPS, ATP, and Chla
Measurements of LPS, a good proxy of the bacterioplankton biomass in extreme environments (Karl et al., 1999; Barnett et al., 2012), were performed in the 2001 and 2005 surveys. A total of 97 subsamples for total LPS quantification (dissolved plus particulate) were collected and preserved in pyrogen-free vials and stored at –20°C until processing. LPS was analyzed by the Quantitative Chromogenic LAL test (QCL-1000, Bio Whittaker, Inc.), using a microtiter ELX-808 spectrophotometer (Bio Whittaker, Inc.) equipped with an Automatic Microplate Reader (for 96 microtiters) and the specific software WIN-KQCL (Bio Whittaker, Inc.).
In the 2001 cruise, measurements of ATP were used as a proxy for total living biomass (Holm-Hansen & Paerl, 1972; Karl, 1980). A total of 35 subsamples (1 l) for ATP quantification were collected in sterile polycarbonate bottles. In the samples collected above 100 m depth, the pico-, nano-, and microsized ATP—18 samples for each size—were fractionated by subsequent filtrations on membranes with a 0.22, 2, and 10 µm pore sizes, respectively, after a pre-filtration on 250 µm net. The filters were immediately plunged into 3 ml boiling TRIS–EDTA phosphate buffer (pH 7.75), and the ATP was extracted at 10°C for 3 min and kept frozen (−20°C) until laboratory analysis in Italy. Extracts for ATP determination were tested by measuring the peak height of the firefly bioluminescence by a Lumat LB9507 EG&G Berthold.
In the 2005 cruise, CHLa, as an index of phytoplankton biomass, was determined in 4 l samples collected in the upper water column (max 160 m depth). The water samples (1 l) were filtered on Whatman GF/F glass-fiber filters, according to Lazzara et al. (1990). After filtration, the filters were immediately stored at −20°C. Chla was extracted in 90% acetone and read before and after acidification. Determinations were carried out with a Varian Eclipse spectrofluorometer. Maximum excitation and emission wavelengths (431 and 667 nm, respectively) were selected on a prescan with a solution of Chla from Anacystis nidulans (by Sigma Co).
Prokaryotic cell abundance, volume, and morphology
A total of 121 samples for prokaryotic abundance (PA), size (VOL), and morphology were collected in sterile polyethylene falcon tubes (50 ml), immediately fixed with pre-filtered formaldehyde (0.2 µm porosity; final concentration 2%), and stored in the dark at 4°C to prevent contamination. Within three months, two replicates of samples were filtered on polycarbonate black membranes (porosity 0.2 µm; GE Water & Process Technologies) and stained for 10 min with 4′,6-diamidino-2-phenylindole (DAPI, Sigma, final concentration 10 µg ml−1) according to Porter & Feig (1980). Stained cells were counted by a Zeiss AXIOPLAN 2 Imaging microscope (magnification: Plan-Neofluar 100× objective and 10× ocular; HBO 100 W lamp; filter sets: G365 exciter filter, FT395 chromatic beam splitter, LP420 barrier filter) equipped with the digital camera AXIOCAM-HR. Images were captured and digitized on a personal computer using the AXIOVISION 3.1 software. The standard resolution of 1300 × 1030 pixels was used for the image acquisition. Pixel size in the resulting image was 0.106 µm by automatic calibration. The system was further calibrated with fluorescent latex beads by measuring a FITC-dyed suspension of monosized latex beads (diameter 2.13 µm). According to Lee & Fuhrman (1987), pixels that constituted the fluorescent “halo” around the bacterial cells were not measured. The recognition of the cells was done by discharging the misclassified objects from count and measurement. Cell counts were performed on a minimum of 20 randomly selected fields in two replicate slides, to avoid the analytical error and to minimize the heterogeneous distribution of prokaryotic cells. When cell abundance was very low, 30–40 random microscope fields were counted. The abundances are reported as averaged cell value from two slides (coefficient of variability 27.7%). VOL (expressed in µm3) of individual cell was derived from two linear dimensions (width, W, and length, L) manually obtained. The detailed methodological procedure for the single-cell volume calculation, the volume-to-biomass conversion factor, and the cell carbon content (CCC) is reported in La Ferla et al. (2012).
The prokaryotic biomass (PB) was quantified by multiplying PA to CCC.
Phenotypic traits allowed us to classify cocci, coccobacilli, rods, vibrios, and spirillae. Cells were operationally defined as cocci if their length and width differed by less than 0.10 µm, coccobacilli if their length and width differed by more than 0.10 µm, and rods if their length was at least double their width; C-shaped and S-shaped cells were defined vibrios and spirillae, respectively (La Ferla et al., 2014).
Statistical analysis
Descriptive statistical analyses were performed with Sigma-Stat statistical package version 3.0. The normal distribution of the entire data set and of the two stations (A and B) repeated in the 2001 and 2005 cruises were assessed by normality test; data that did not meet this assumption were logarithmically transformed. The statistical significance of the differences among data was checked by two-ways ANOVA, considering stations and depths as the main factors. Post hoc multiple comparisons were performed using the Holm-Sidak’s method. Spearman–Rank Order Correlation was applied to test the correlation coefficients and P values for pairs of variables. The coefficient of variation (CV), i.e., the ratio of the standard deviation to the mean, was calculated to assess the variability among samples.
Results
Environmental parameters
The mean and range values of T, S, and DO for each station are reported in Table 2. T and S ranged between −1.996 and 2.370°C and between 33.82 and 37.73, respectively. DO range was 4.72–7.28 ml O2 l−1.
LPS, ATP and Chla
LPS values were generally higher at the stations sampled in the cruise of 2005 than at those sampled in the cruise of 2001. In the 2001 cruise, no significant differences between photic and aphotic layers in LPS values were detected, while there were in the 2005 cruise with lower values in the deep layers (Supplementary Fig. 1).
Total ATP concentration—determined only in the three stations sampled in the 2001 cruise—resulted in a wide range (3.2–1751.8 ng l−1) with high values in the photic layer of st A01. Anyhow total ATP in the aphotic layers at st A01, B01, and B101 resulted about 26, 17, and 6 times lower than in the photic layers, respectively (Supplementary Fig. 2). With respect to the size-fractioned ATP, performed in the photic layers only, different size compositions characterized the different stations. At st A01 and st B01, the microplanktonic fraction prevailed on the whole population showing the highest incidence (95 and 88% of total ATP, respectively). Conversely, the nano- and picoplanktonic fractions were almost negligible (from 2 to 7% of total ATP). A very different result was obtained for the st B101 where micro-, nano-, and picoplanktonic fractions amounted for 63, 24, and 12% of total ATP, respectively (data not shown).
In the cruise of 2005, Chla concentrations were generally low in all the stations with a mean value of 0.104 ± 0.12 mg m−3. The minimum value (0.011 mg m−3) was detected at st B05 (160 m depth), while the maximum (0.54 mg m−3) at st D (5 m depth). Chla distribution in the quadrangle bounded by the st A, H1, B, and D is reported in supplementary Fig. 3.
Prokaryotic cell abundance, volume, and morphology
In the total data set, PA ranged from 0.03 (st A01, at 500 m) to 14.43 × 105 cells ml−1 (st D, at 25 m). A higher variability with depth was observed in the 2001 cruise (CV higher than 100) than in 2005 one, with the only exception of st D (CV = 127). The range of VOL in the entire data set was 0.030 (st B101, at 150 m) −0.160 µm3 (st D, at 800 m), and the largest sizes were detected at st A01 (mean VOL 0.093 µm3). CCC varied in an overall range of 10.8–45.0 fg C cell−1 and PB widely ranged from 0.06 to 45.11 µg C l−1 at st A01, at 500 and 5 m depth, respectively.
In the cruise of 2001, PA distribution with depth showed a clear stratification along the water column with high values in the photic layer and very low values below 100 m depth (Fig. 2). At st A01, PA showed the highest abundances at surface and a peak at 40 m depth. In the cruise of 2005, the water column showed more homogeneous distributions with respect to the 2001 cruise. Only at st D, and in a lesser extent at st 5, PA displayed a stratified distribution with depth. At st A05, high PA till 100 m depth was recorded, and then they declined and increased again at 600 m depth.
In the cruise of 2001, high VOL at st A01 between 75 and 200 m depth were detected (Fig. 2); at st B01, tight increasing VOL at 50 and 75 m depths as well as at st B101, peaks at 100, 200, and 528 m depths were recorded. In the cruise of 2005, with the only exception of st H and st B05, VOL were larger in the aphotic layer than in the upper one, with significant increases at st D and 6. At st A05, VOL increased around 100 m depth as in the 2001 cruise. The class frequency of the dimensional sizes, as percentages for each station is reported in Fig. 3. The size class distribution varied within the different stations and much more between the two study years. On the whole the most frequent size, including the 46% of all the measured volumes, fell in the range 0.020–0.049 µm3 and it reached the highest percentages of frequency in the cruise of 2005. Nevertheless, in the cruise of 2001, at st A01 only the 31% of total cells fell in this dimensional range.
PB widely varied along the water column between the study years (Fig. 3). In the cruise of 2001, a strongly stratified distribution occurred in all the stations with the lowest values in the aphotic layer. In the cruise of 2005, PB resulted in narrow profiles. The distribution with depth of PB resulted similar to PA distribution. However, increasing PB values in st B101 at 528 m, st A05 at 600 m, and st 6 below 390 m were detected according to VOL profiles.
Concerning morphotype composition, the prokaryotic cells were grouped into five classes: cocci, coccobacilli, vibrios, rods, and spirillae. Filamentous forms (i.e., cells exceeding 4 µm in length) were lacking. In terms of abundance, cocci dominated the entire data set, being about the 50% of the total cells in both study periods, followed by rod (representing the 27 and 17% of total cells in the 2001 and 2005 cruises, respectively) and coccobacilli (17% in both study periods). Vibrio accounted for 7 and 13% in the 2001 and 2005 cruises, respectively. At last, spirillae were negligible in both studied periods (data not shown). Cocci, notwithstanding the greatest abundances, showed small sizes while spirillae were characterized by great sizes.
With regard to the different stations, at st A01 all the morphotypes showed the greatest sizes, in particular rods and coccobacilli in the photic layer and vibrios and cocci in the aphotic layer. In the other stations in the cruise of 2001, the mayor sizes were due to rods and vibrios while cocci, albeit numerous, generally showed lesser sizes than other morphotypes (Fig. 4). In the 2005 cruise, coccobacilli also showed relatively high sizes in aphotic layers.
Box plots showing the distribution of the morphotype sizes in the examined stations. Box widths are proportional to the square root of the number of cells. Box plots range between the 25th and the 75th percentiles of the data set and the whiskers indicate the minimum and maximum values. The black lines and squares in the box plots represent the median and mean, respectively, while the black dots the outliner
Statistical analysis
Spearman–Rank correlations among microbial and environmental parameters were computed on the entire data set and on the stations A and B. In Table 3, solely the significant correlations were reported. On the whole data, PA and PB significantly correlated with several environmental and microbial variables. VOL, instead, correlated only with total and fractionated ATP. Considering only stations A and B, correlations with hydrological parameters were lacking with the only exception of DO. Moreover, no correlations between PA, VOL, and PB with CHLa were detected, while they were differently correlated with fractionated ATP and LPS. Indirect trophic determinations resulted well correlated each other (LPS vs. Chla: r = 0.375, P = 0.0347, n = 32; LPS vs. total ATP: r = 0.695, P = 0.0012, n = 18; LPS vs. microATP: r = 0.684, P = 0.0016, n = 18; LPS vs. picoATP: r = 0.692, P = 0.0013, n = 18) (data not shown).
Applying the Kruskal–Wallis one way analysis of variance on Ranks on the entire data set, the differences in the median values among the microbial parameters (PA, VOL, and PB) were not a statistically significant. Conversely, considering only the replicated stations (A and B), PA showed significant differences among the stations and depths (Two-way ANOVA, P < 0.001), while significant differences in VOL were only recorded among the stations (P < 0.001). Post hoc multiple comparisons (by Holm-Sidak method) showed that the st A05 and st A01 were the main responsible for the spatial differences found in PA and VOL, respectively. In fact, they resulted statistically different from the others stations (Two-way ANOVA, P < 0.05). Moreover, significant differences were determined with time between st A01 and st A05 and VOL (Two-way ANOVA, P < 0.05).
Discussion
Environmental assessment
We focus our attention on the two mooring stations replicated in the years. The site A is an area of maximum primary production in the Ross sea; the site B is located in a basin characterized by high rates of accumulation and affected by seasonal intrusion of Circumpolar deep Water (CDW) (Langone et al., 2000).
During the 2001 cruise, drifting pack ice occurred over large areas of the Ross Sea with old pack ice being trapped within the new ice formations in the latter half of February (Mangoni et al., 2004). As a consequence, an extensive algal biomass occurred suggesting that the phytoplankton was a result of seeding from ice algal communities, with a major impact on the trophic structure of the entire ecosystem. ATP estimates showed station-to-station variation, corresponding to different trophic status. In particular at the st A01 marked peaks, achieved in the upper 75 m depth, revealed a strong condition of eutrophy according to Karl (1980). These values, among the highest even recorded, were of the same order of magnitude of those observed in the Gerlache Strait by Karl et al. (1991). Such feature coincided with a considerable increase in phytoplankton biomass (143 ± 20 mg Chla m−2) in the same area (Mangoni et al., 2004) with a strongly colored ice layer in the broken ice floes and a dark brown melting ice. The relatively thin ice floes and the permanence of pack ice would have provided optimal conditions for algal growth within the ice in the absence of grazing, as suggested by pheophorbide pigments. Conversely, at st B, Mangoni et al. (2004) findings (57 ± 20 mg Chla m−2) furnished a strongly different situation corroborated by our ATP estimates. This station was initially characterized by a moderate trophism (st B01) and the occurrence of a monospecific bloom of diatoms (Corethron spp.). After 18 days (st B101), the algal depletion was accompanied by an oligotrophic status (Fonda Umani et al., 2002). A wide and quick variability in total and fractionated ATP was similarly observed at Terra Nova Bay (La Ferla et al., 1995).
During the 2005 cruise, Chla evidenced a general picture of low trophism on spatial scale. Such finding could be due to the early stratification caused by the melting ice in the Ross Sea (La Ferla et al., 2008).
The LPS assay highlighted the variability among the two studied years as well as stations. In the cruise of 2001, the LPS distribution was homogeneous along the water column whereas, in the cruise of 2005, LPS showed high values at surface layers, in disagreement with PA distribution. Such incongruence was presumably due to the selective quantification of gram negative bacteria and the exclusion of archaea by the LPS determination. Low abundance or absence of archaea was reported in other Antarctic waters. In a study carried out in Summer 2005 in Terra Nova Bay, archaea were poorly represented (Lo Giudice et al., 2012). The different LPS distributions between the two study years confirm the hypothesis of Church et al. (2003) that bacteria contribute mostly to prokaryote abundance during phytoplanktonic seasonal peak i.e., in the samples collected in the 2001 cruise. However, the few studies existing on LPS distribution in Antarctic sea evidenced wider LPS values in the Gerlache Strait (summer 1989–1990, Karl & Tien, 1991) and Terra Nova Bay (spring 1990, La Ferla et al., 1995) than our estimates.
Prokaryotic cell abundance, volume, and morphology
The mean PA value obtained was in a range roughly comparable to those obtained in several Antarctic marine environments, but it resulted higher than those reported in the Indian–Australian sector of the Antarctic Ocean and in the Weddell Sea, Adelie Land, and Antarctic Peninsula (supplementary Table 1). If compared to previous studies in the Ross Sea, our determinations were similar to the findings of Monticelli et al. (2003) and Stewart & Fritsen (2004), but lower than those found by Crisafi et al. (2000), Ducklow et al. (2001) in January 1997 and Celussi et al. (2009) at surface. In the 2001 cruise, our PA spatial variability resulted in a vertical well stratified cell distribution along the water column, with very low values in the aphotic layers. It was comparable to Celussi et al. (2009) findings in Victoria Land in January–February 2006. Conversely, in the 2005 cruise PA was quite uniform with depth at st B05, while at st A05 a decreasing trend with depth was observed.
On the whole, the very small cell sizes (>0.1 μm3) prevailed, also if VOL showed to be flexible along the water column. When compared to previous research in Antarctic areas, our range was equal to that determined in January–February 1997 in the Ross Sea or encompassed that quantified in the Antarctic Peninsula (see supplementary Table 1). Nevertheless, VOL resulted smaller than those observed in the Indian-Australian Antarctic Ocean, Kerguelen Islands and, sometimes, Adelie Land (again supplementary Table 1). However, Delille used acridine orange as dye which proved to show greater cell volumes than DAPI does. Conversely, our mean VOL resulted greater than that referred by Stewart & Fritsen (2004) in Summer 1999. Finally, when compared to Mediterranean Sea (La Ferla et al., 2010, see herein Table 5; Azzaro et al., 2012), our Antarctic VOL estimates showed the occurrence of a smaller prokaryotic community. In the Western Antarctic Peninsula sea ice, the occurrence of small bacterial volumes was associated to substrate limitation in terms of both quality and quantity (Stewart & Fritsen, 2004), corroborating our findings that showed enlarged morphotypes in high trophic conditions (till 0.4 μm3).
In our study, no relation seems to link cell size with phytoplanktonic population—in terms of Chla—suggesting that no potential limitation by labile organic matter of new production constrains the cell size.
Unfortunately, the evaluation of heterotrophic nanoflagellates and protistan grazing were not taken into account in this study. However, both PA and VOL covaried with nanoplankton and microplankton biomass (in terms of ATP), respectively, conveying some kind of top down control on prokaryotic populations. Nevertheless, according to Pernthaler & Amann (2005), the length frequency classes reported in Fig. 5 suggested that predation was lacking. Reduction of the cell size and morphological transition to a cyst-like resting cell have previously been described as strategies to maintain cell integrity and function in extremely cold and salty environments (Kuhn et al., 2014). However, it must be stressed that viral abundance and its interaction with prokaryotic biomass unfortunately were not considered in this study, notwithstanding the viral infection has been recognized to be important to constrain both prokaryotic cell abundance and size (Weinbauer, 2004).
In general, VOL showed increasing sizes in the depth horizon within 100–200 or 500–800 m. These findings partially agreed with those obtained in temperate pelagic areas, where an increase in cell size with increasing depth often occurred. Nevertheless, the significant negative correlation between cell size and abundance, found in Mediterranean Sea, did not exist in the Ross Sea (La Ferla et al., 2012).
PB resulted to be higher in the photic layers than in the aphotic ones (20 times in the 2001 samples and 2 times in the 2005 ones, respectively). Moreover, in both study years, PB generally was higher than in other references in Antarctic waters (Supplementary Table 1). Quantifying the biomass by a combination of cell counts and volume determinations resulted in a more reliable estimate since the use of a standard conversion factor would deflect the biomass data. In fact, in the whole data set, our conversion factors varied in a broad range between 10 and 42 fg C cell−1, roughly corresponding to the half and double of the most used abundance-biomass conversion factor (Lee & Fuhrman, 1987). When calculating the biomass by applying 20 fg C cell−1, PB would have resulted in the range 0.068–27.4 µg C l−1; our experimental study the PB resulted in the wider range varying between 0.063 and 45.1 µg C l−1. A recent study on the bacterial biomass distribution in the global ocean assumed a conversion factor from abundance to carbon biomass of 9.1 fg C cell−1 (Buitenhuis et al., 2012). Such conservative estimate might reduced about two times our PB values. In fact considering the photic zone only, Buitenhuis et al. (2012) measured 3.2 µg C l−1 in Antarctica versus 5.35 µg C l−1 reached in our study.
As regard the morphotype analysis, cocci were the dominant group throughout this study as already reported for subtropical and temperate pelagic environments (La Ferla et al., 2014). The high contribution of cocci to total counts (almost 50%) was comparable with findings in Arctic Sea ice (Gradinger & Zhang, 1997). Nevertheless, the relatively small size of cocci did not allow them to account for the highest biomass. However, where high trophic conditions occurred (st A01), cocci were characterized by larger sizes than elsewhere. In a study on VOL and ecosystem characterization, the growth and number of coccal forms seemed to have a more efficient reproductive strategy in eutrophic environments than in oligotrophic ones (La Ferla et al., 2014). In the same context, the increases of rods shaped cells (st B01) could have been favored by reduced trophic conditions enhancing the elongated forms growth.
Consistency concerning phylotypes and morphotypes has been highlighted in some aquatic systems (Straza et al., 2009). In a study performed in coastal areas of Terra Nova Bay, the FISH analysis revealed a bacterioplankton composition that was typical of Antarctic marine environments with the Actinobacteria and Gammaproteobacteria that were equally dominant with the Cytophaga/Flavobacter group (Lo Giudice et al., 2012). The obtained results highlighted the ability of the small Actinobacteria to survive and proliferate in the Terra Nova Bay seawater as they generally showed a wide range of salt tolerance and appeared to be particularly competitive with strictly marine bacteria by better utilizing supplied carbon sources. Although freshwater and marine Actinobacteria are different groups (Newton et al., 2011), phylogenetic lineage analysis in Alpine lake showed that at least two bacterial groups were constituted by small morphotypes of Actinobacteria (Posch et al., 2009). In a different context, assemblages of ultrasmall microbial cells within the ice cover of Lake Vida (Antarctica) were dominated by the Proteobacteria (Kuhn et al., 2014). Cultivation efforts of the 0.1–0.2 μm size fraction led to the isolation of Actinobacteria-affiliated genera Microbacterium and Kocuria. Based on phylogenetic relatedness and microscopic observations, it has been hypothesized that these ultramicrocells are likely in a reduced size state as a result of environmental stress or life cycle-related conditions (Duda et al., 2012).
Environmental constrains
Hydrology did not affect PA and PB variability if considering solely the repeated stations A and B. In extreme cold habitats, temperature seems to have a limited influence on prokaryotes in terms of both abundance and metabolic activities (Margesin & Miteva, 2011). On the entire data set, also VOL was not dependent on hydrology differently to other studies carried out in temperate systems (La Ferla et al., 2012). However, in Canadian Fjord, the influence of water temperature appeared to act simultaneously with other factors (organic nutrient quality and quantity, phytoplankton activity, flagellate grazing) in controlling bacterial dynamics (Albright & McCrae, 1987). Several researches in marine and brackish environments assessed that cell sizes varied in relation to environmental trophic status (Vrede et al., 2002; La Ferla et al., 2014). In our study case, among the trophic parameters, LPS, and the different size fractions of ATP resulted directly linked to PA, PB, and VOL, and the largest cell size were detected in the photic layer of st A01. These results allowed us to affirm that the eutrophic state of such site could have affected the cell size, perhaps indirectly by a trophodynamic link with other microbial populations (see the correlation with micro- and pico-sized ATP). VOL and PA were never correlated with each other, conversely to what happens in other temperate seas (La Ferla et al., 2012).
Conclusion
The starting hypothesis that environmental characteristics modulate prokaryotic cell abundance, size, and shape in the Ross Sea resulted to be partially supported by our results. The actual level of understanding can be summarized as follows: cell abundance and volume are proportional to the richness of the environment and reflects directly the trophic status of the system; morphotypes are constrained by environment trophodynamics. The studied parameters appear to be suitable to assess the environmental status. However, the relationship between phylotypes and morphotypes need to be deepened, as well as the effects of viral lysis and predation, to get a more comprehensive ecological significance to prokaryotic shapes in Antarctic ocean.
References
Albright, L. J. & S. K. McCrae, 1987. Annual cycle of bacterial specific biovolumes in howe sound, a canadian west coast fjord sound. Applied Environmental Microbiology 53: 2739–2744.
Azzaro, M., R. La Ferla, G. Maimone, L. S. Monticelli, R. Zaccone & G. Civitarese, 2012. Prokaryotic dynamics and heterotrophic metabolism in a deep convection site of Eastern Mediterranean Sea (the Southern Adriatic Pit). Continental Shelf Research 44: 106–118.
Barnett, M. J., J. L. Wadham, M. Jackson & D. C. Cullen, 2012. In-field implementation of a recombinant factor C assay for the detection of lipopolysaccharide as a biomarker of extant life within glacial environments. Biosensors 2: 83–100.
Bölter, M., J. Bloem, K. Meiners & R. Möller, 2002. Enumeration and biovolume determination of microbial cells—a methodological review and recommendations for applications in ecological research. Biology and Fertility of Soils 36: 249–259.
Buitenhuis, E. T., W. K. W. Li, M. W. Lomas, D. M. Karl, M. R. Landry & S. Jacquet, 2012. Bacterial biomass distribution in the global ocean. Earth System Science Data Discussion 5: 301–315.
Catalano, G., G. Budillon, R. La Ferla, P. Povero, M. Ravaioli, V. Saggiomo, A. Accornero, M. Azzaro, G. C. Carrada, F. Giglio, L. Langone, O. Mangoni, C. Misic & M. Modigh, 2006. A global budget of carbon and nitrogen in the Ross Sea (Southern Ocean). In Liu, K. K., L. Atkinson, R. Quiñones & L. Talaue-McManus (eds), Carbon and Nutrient Fluxes in Continental Margins: A Global Synthesis, Global Change, The IGBP Series. Springer, Berlin.
Celussi, M., B. Cataletto, S. Fonda Umani & P. Del Negro, 2009. Depth profiles of bacterioplankton assemblages and their activities in the Ross Sea. Deep-Sea Research I 56: 2193–2205.
Church, M. J., E. F. De Long, H. W. Ducklow, M. B. Karner, C. M. Preston & D. M. Karl, 2003. Abundance and distribution of planktonic archaea and bacteria in the waters west of the Antarctic Peninsula. Limnology Oceanography 48: 1893–1902.
Cottrel, M. T. & D. L. Kirchman, 2004. Single-cell analysis of bacterial growth, cell size, and community structure in the Delaware estuary. Aquatic Microbial Ecology 34: 139–149.
Crisafi, E., F. Azzaro, R. La Ferla & L. S. Monticelli, 2000. Microbial biomass and respiratory activity related to the ice-melting upper layers in the Ross Sea (Antarctica). In Faranda, F. M., L. Guglielmo & A. Ianora (eds.), The Ross Sea Ecology. Springer, New York.
Delille, D., 2004. Abundance and function of bacteria in the Southern Ocean. Cellular and Molecular Biology 50: 543–551.
Ducklow, H., C. Carlson, M. Church, D. Kirickman, D. Smith & G. Steward, 2001. The seasonal development of the bacterioplankton bloom in the Ross Sea, Antarctica, 1994–1997. Deep-Sea Research II 48: 4199–4221.
Duda, V. I., N. E. Suzina, V. N. Polivtseva & A. M. Boronin, 2012. Ultramicrobacteria: formation of the concept and contribution of ultramicrobacteria to biology. Microbiology 81: 379–390.
Fonda Umani, S., A. Accornero, G. Budillon, M. Capello, S. Tucci, M. Cabrini, P. Del Negro, M. Monti & C. De Vittor, 2002. Particulate matter and plankton dynamics in the Ross Sea polynya of Terra Nova Bay during the austral summer 1997/98. Journal Marine Systems 36: 29–49.
Gradinger, R. & Q. Zhang, 1997. Vertical distribution of bacteria in Arctic sea ice from the Barents and Laptev seas. Polar Biology 17: 448–454.
Holm-Hansen, O. & H. W. Paerl, 1972. The applicability of ATP determination fro estimation of microbial biomass and metabolic activity. Memorie dell’Istituto Italiano di Idrobiologia 29: 149–168.
Karl, D. M., 1980. Cellular nucleotide measurements and applications in microbial ecology. Microbiological Review 44(4): 739–796.
Karl, D. M. & G. Tien, 1991. Bacterial abundances during the 1989–90 austral summer phytoplankton bloom in the Gerlache Strait. Antarctic Journal of the United States 26: 147–149.
Karl, D. M., O. Holm-Hansen, G. T. Taylor, G. Tien & D. F. Bird, 1991. Microbial biomass and productivity in the western Bransfield Strait, Antarctica during the 1986–87 austral summer. Deep-Sea Research 38: 1029–1055.
Karl, D. M., D. F. Bird, K. Björkman, T. Houlikan, R. Shackelford & L. Tupas, 1999. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science 286: 2144–2147.
Kuhn, E., A. S. Ichimura, V. Peng, C. H. Fritsen, G. Trubl, P. T. Doran & A. E. Murray, 2014. Brine assemblages of ultrasmall microbial cells within the ice cover of Lake Vida, Antarctica. Applied Environmental Microbiology 80: 3687–3698.
La Ferla, R., A. Allegra, F. Azzaro, S. Greco & E. Crisafi, 1995. Observations on the microbial biomass in two stations of Terra Nova Bay (Antarctica) by ATP and LPS measurements. Marine Ecology 16: 307–315.
La Ferla, R, F. Azzaro, M. Azzaro, G. Maimone & L. S. Monticelli, 2008. Variability of the microbial biomass and activity in the Ross Sea (Antarctica) and its implication on ecosystem carbon cycle. Geophysical Research Abstract 10, EGU2008-A-0000-EGU General Assembly 2008.
La Ferla, R., M. Azzaro, G. Budillon, C. Caroppo, F. Decembrini & G. Maimone, 2010. Distribution of the prokaryotic biomass and community respiration in the main water masses of the Southern Tyrrhenian Sea (June and December 2005). Advances in Oceanography and Limnology 2: 235–257.
La Ferla, R., G. Maimone, M. Azzaro, F. Conversano, C. Brunet, A. S. Cabral & R. Paranhos, 2012. Vertical distribution of the prokaryotic cell size in the Mediterranean Sea. Helgoland Marine Research 66: 635–650.
La Ferla, R., G. Maimone, G. Caruso, F. Azzaro, M. Azzaro, F. Decembrini, A. Cosenza, M. Leonardi & R. Paranhos, 2014. Are prokaryotic cell shape and size suitable to ecosystem characterization? Hydrobiologia 726: 65–80.
Langone, L., M. Frignani, M. Ravaioli & C. Bianchi, 2000. Particle fluxes and biogeochemical processes in an area influenced by seasonal retreat of the ice margin (northwestern Ross Sea, Antarctica). Journal Marine Systems 27: 221–234.
Lazzara, L., F. Bianchi, M. Falcucci, V. Hull, M. Modigh & M. Ribera D’Alcalà, 1990. Pigmenti clorofilliani. Nova Thalassia 11: 207–223.
Lee, S. & A. Fuhrman, 1987. Relationship between biovolume and biomass of naturally derived bacterioplankton. Applied Environmental Microbiology 53: 1298–1303.
Lo Giudice, A., C. Caruso, S. Mangano, V. Bruni, M. De Domenico & L. Michaud, 2012. Marine bacterioplankton diversity and community composition in an Antarctic coastal environment. Microbial Ecology 63: 210–223.
Mangoni, O., M. Modigh, F. Conversano, G. C. Carrada & V. Saggiomo, 2004. Effects of summer ice coverage on phytoplankton assemblages in the Ross Sea, Antarctica. Deep-Sea Research I 51: 1601–1617.
Margesin, R. & V. Miteva, 2011. Diversity and ecology of psychrophilic microorganisms. Research in Microbiology 162: 346–361.
Monticelli, L. S., R. La Ferla & G. Maimone, 2003. Dynamics of bacterioplankton activities after a summer phytoplankton bloom period in Terra Nova Bay. Antarctic Science 15(1): 85–93.
Nelson, D. M., D. J. De Master, R. B. Dunbar & W. O. Jr Smith, 1996. Cycling of organic carbon and biogenic silica in the Southern Ocean: estimates of water column and sedimentary fluxes on the Ross Sea continental shelf. Journal Geophysical Research 101: 519–532.
Newton, R. J., S. E. Jones, A. Eiler, K. D. McMahon & S. Bertilsson, 2011. A guide to the natural history of freshwater lake bacteria. Microbiology and Molecular Biology Reviews 1: 14–49.
Pernthaler, J. & R. Amann, 2005. Fate of heterotrophic microbes in pelagic habitats: focus on populations. Microbiology and Molecular Biology Review 6: 440–461.
Posch, T., M. Loferer-Krößbacher, G. Gao, A. Alfreider, J. Pernthaler & R. Psenner, 2001. Precision of bacterioplankton biomass determination: a comparison of two fluorescent dyes, and of allometric and linear volume-to-carbon conversion factors. Aquatic Microbial Ecology 25: 55–63.
Posch, T., J. Franzoi, M. Prader & M. M. Salcher, 2009. New image analysis tool to study biomass and morphotypes of three major bacterioplankton groups in an alpine lake. Aquatic Microbial Ecology 54: 113–126.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography 25: 943–948.
Smith Jr., W. O., D. J. Ainley & R. Cattaneo-Vietti, 2007. Trophic interactions within the Ross Sea continental shelf ecosystem. Philosophical Transactions of the Royal Society B 362: 95–111.
Stewart, F. J. & C. H. Fritsen, 2004. Bacteria-algae relationships in Antarctic sea ice. Antarctic Science 16: 143–156.
Straza, T. R. A., M. T. Cottrell, H. W. Ducklow & D. L. Kirchman, 2009. Geographic and phylogenetic variation in bacterial biovolume as revealed by protein and nucleic acid staining. Applied Environmental Microbiology 75: 4028–4034.
Vichi, M., A. Coluccelli, M. Ravaioli, F. Giglio, L. Langone, M. Azzaro, F. Azzaro, R. La Ferla, G. Catalano & S. Cozzi, 2009. Modelling approach to the assessment of biogenic fluxes at a selected Ross Sea site, Antarctica. Ocean Science Discussions 6: 1477–1512.
Vrede, K., M. Heldal, S. Norland & G. Bratbak, 2002. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Applied Environmental Microbiology 68: 2965–2971.
Weinbauer, M. G., 2004. Ecology of prokaryotic viruses. FEMS Microbiology Reviews 28: 127–181.
Young, K. D., 2007. Bacterial morphology: why have different shapes? Current Opinion in Microbiology 10: 596–600.
Zeder, M., E. Kohler, L. Zeder & J. Pernthaler, 2011. A novel algorithm for the determination of bacterial cell volumes that is unbiased by cell morphology. Microscopy and Microanalysis 17: 799–809.
Żmuda, M., 2005. Abundance and morphotype diversity of surface bacterioplankton along the Gdynia-Brest transect. Oceanological and Hydrobiological Studies 34: 3–17.
Acknowledgments
The research was done in the frame of BIOSESO II and ABIOCLEAR projects funded by the Italian PNRA (National Programme of Antarctic Research). The authors wish to thank two anonymous reviewers and editors for their useful comments as well as the CEFA project of PNRA for the technical support. They also thank the colleagues of IAMC, Dr. Gabriella Caruso and Dr. Giuseppe Frisone, for statistics elaboration and data typing, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Diego Fontaneto & Stefano Schiaparelli / Biology of the Ross Sea and Surrounding Areas in Antarctica
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2015_2426_MOESM1_ESM.tif
Fig. 1 Distributions of the total LPS concentrations in the photic and aphotic layers in the stations sampled in the 2001 and 2005 cruises
Rights and permissions
About this article
Cite this article
La Ferla, R., Maimone, G., Lo Giudice, A. et al. Cell size and other phenotypic traits of prokaryotic cells in pelagic areas of the Ross Sea (Antarctica). Hydrobiologia 761, 181–194 (2015). https://doi.org/10.1007/s10750-015-2426-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2426-7