Abstract
Although the assembly of stream macroinvertebrates is regulated by environmental heterogeneity at multiple spatial scales, field bioassessment studies that explicitly considered such scale-dependency are rare. Here, we investigated how large scale longitudinal gradients and local microhabitat structure jointly regulate the assembly of macroinvertebrate communities along a Mediterranean river. We compared community composition, metrics and functional feeding traits among three microhabitat categories (grain-size >20 cm; grain-size <20 cm; organic substrata) along three river sectors (up-, middle-, downstream), which reflected a gradient of anthropogenic modification. Macroinvertebrate assemblages varied mostly over the large-scale longitudinal gradient, but the influence of local micro-habitat features was evident at the within-sector scale. The effects of micro-habitats appeared stronger for feeding traits compared to simple taxonomic metrics, supporting the hypothesis that feeding traits are sensitive to river substratum character. Beta-diversity among micro-habitat types was smaller in the modified downstream sector, which supported more homogeneous communities. An explicit consideration of spatial scales is recommended when interpreting results from environmental assessment studies. In the Aniene River, the influence of local-scale substratum character on macroinvertebrates depended on the longitudinal gradient in anthropogenic pressure. Also, the findings suggest that taxonomic and functional metrics reflect processes operating at different spatial scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benthic macroinvertebrates are widely used as biological indicators for assessing the integrity and functionality of lotic habitats (e.g. Lenat & Barbour, 1994; Hering et al., 2004; Larsen et al., 2012; Manfrin et al., 2013), and are frequently monitored to detect changes in hydrological and sediment regimes as well as in water quality of river in ecosystems (Boulton et al., 1992; Pace et al., 2013).
River ecosystems are hierarchically organised, where reaches (that often represent the sampling units) are nested within rivers, rivers within catchments and catchments within larger basins (Heino et al., 2004; Allan & Castillo, 2007). In this context, the assembly of river invertebrates is driven by processes operating at different spatio-temporal scales (Arscott et al., 2003; García-Roger et al., 2013).
This scale-dependent identification of the key drivers shaping macroinvertebrate assemblages is increasingly acknowledged (Mykrä et al., 2007; Larsen et al., 2015), but the explicit consideration of spatial resolution is rarely included in monitoring studies. For example, investigations at reach scale (Beisel et al., 1998; Peeters et al., 2004), clearly revealed the importance of depth, substrate and hydraulic conditions (Reice, 1980; Schmera & Eros, 2004; Barnes et al., 2013), food availability (Beisel et al., 2000) and biotic factors (Kelly et al., 2003; Nelson, 2011). Conversely, over larger scales, latitudinal gradients and variability in land-use/land-cover and bedrock geology appear to exert stronger influence and thus mask local scale effects (Allan, 2004; Mykrä et al., 2007). Also within a small microhabitat scale (~1 m2), the association of macroinvertebrate assemblages with key stream-bed features appears to be context dependent. For example, Minshall (1984) reported decreasing macroinvertebrate diversity with increasing cobble size, while Allan & Castillo (2007) observed that the diversity and abundance of macroinvertebrates increased with median grain size, likely reflecting the highly dynamic and ephemeral nature of microhabitat patches in flowing waters. Similarly, Costa & Melo (2008) found that microhabitat features were more important than stream location in determining macroinvertebrate assemblages in tropical streams. This scale and context dependency in the apparent importance of drivers shaping benthic assemblages in rivers is particularly relevant for impact-assessment programmes where the scale of measurement should coincide with the scale at which the organisms respond.
In central Italy, many rivers are characterised by marked longitudinal patterns in anthropogenic pressure, with up- and middle-stream sections draining semi-natural or extensive agricultural areas, while downstream sections being detrimentally stressed by urbanisation, impoundment and industrial sewage effluents (Solimini et al., 2001; Mancini et al., 2005; Manfrin et al., 2013). In these circumstances, the extent to which benthic assemblages reflect differences among local micro-habitat types or larger-scale longitudinal variation is not clear. Surprisingly, this issue remains largely unexplored despite its relevance for informing mandatory monitoring programmes and the increasing consideration given to microhabitats by the Water Framework Directive (European Commission, 2000) and the subsequent introduction of the multi-habitat sampling method (Star Consortium, 2003).
To address this issue, we sampled benthic macroinvertebrates in different microhabitat types along the course of the Aniene river (central Italy); this river shows a characteristic longitudinal gradient in both natural (upstream) and anthropogenic (downstream) factors, and can be categorised in three distinct sectors (Solimini et al., 2001; Manfrin et al., 2013). Specifically, we aimed to (1) quantify the relative influence of large scale longitudinal gradients and small scale microhabitat features on the structure and function of macroinvertebrate assemblages; (2) assess whether the longitudinal anthropogenic gradient caused the homogenization of assemblages among microhabitat types and (3) assess the specific effects of micro-habitat types on invertebrate assemblages at the smaller within-sector scale.
Materials and methods
Study area and sampling design
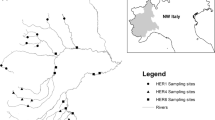
The study was conducted in October 2008 and June 2009 on the River Aniene, draining calcareous area in Central Italy from 1100 to 15 m a.s.l. (Wasson et al., 2006; Traversetti et al., 2013). This is a second-order river with a basin area of 1.453 km2 and a total length of 98 km and it is the major tributary of the River Tiber (Traversetti et al., 2014). The upper 25 km flows through semi-natural areas with limited human impact, except for a little hydro-electric dam. The downstream river sector, conversely, drains landscapes characterised by urbanisation (e.g. Subiaco and Vicovaro towns) and industrial activities, with negative impact on the river’s biotic integrity (Solimini et al., 2001; Traversetti et al., 2014). The River Aniene may, therefore, be divided into three main longitudinal sectors according to macroinvertebrate assemblage patterns and the major anthropogenic effects (Solimini et al., 2001; Manfrin et al., 2013; Traversetti & Scalici, 2014): upstream (Sector 1, 0–23 km from source; S1); midstream (Sector 2, 24–48 km from source; S2) and downstream (Sector 3, 49–99 km from source; S3) (Fig. 1). Macroinvertebrates were collected from 8 sampling sites: 3 sites in S1; 2 sites in S2 and 3 sites in S3.
Location of the investigated sites on the Aniene River within the three longitudinal river sectors: S1 = upstream; S2 = middle-stream; S3 = downstream. Common names and geographic coordinates (Latitude; Longitude): A = Filettino (33T 358488 m E; 4637280 m N); B = Trevi (33T 352780 m E; 4635426 m N); C = Jenne (33T 346117 m E; 4635426 m N); D = Subiaco (33T 340718.20 m E; 4643137 m N); E = Anticoli Corrado (33T 333595 m E; 4654173 m N); F = Vicovaro (33T 324656 m E; 4652680 m N); G = CastelMadama (33T 322936 m E; 4650229 m N); H = Tivoli (33T 315203 m E; 4647864 m N)
Macroinvertebrate collection was carried out according to the multi-habitat AQEM consortium sampling design (Star Consortium, 2003). Before collecting samples, we surveyed the microhabitat types within each site, and identified 10 different types based on both composition (inorganic and organic) and on grain size (Supplementary Material—Appendix 1). Ten benthic samples were collected for each site with a standard Surber sampler (25 × 25 cm with a mesh size of 0.5 mm) disturbing manually the substrate for 1 min. The 10 samples were distributed proportionally to each microhabitat occurrence (e.g. a microhabitat covering 60 % of the total area was sampled in 6 different points within the sampling site), following the official standards. All specimens were removed from the net, sorted (at order level) in the field, and fixed immediately in 70 % ethanol. In the laboratory, macroinvertebrates were identified to species or species-group (Ephemeroptera), genus (Plecoptera and Trichoptera) and family level (e.g. Simuliidae, Lumbricidae; see Supplementary Material—Appendix 2 for the complete taxonomic list).
Dataset organisation and statistical analyses
To increase the statistical power of the analysis and to increase the number of replicates within each microhabitat category, we defined three broad microhabitat categories as: C1, coarse inorganic substrata (>20 cm); C2, fine inorganic substrata (C2 < 20 cm) and C3, organic substrata (Supplementary Material—Appendix 3).
The process of pooling the ten micro-habitat types into three broad categories also allowed us to limit the potential biases associated with the use of proportional sampling. In other words, the employment of the official proportional multi-habitat sampling inherently gives more weight to the most common micro-habitat types. The effects of this potential bias were therefore limited by the pooling procedure.
Feeding habits of macroinvertebrates were based on six feeding categories (deposit feeders, shredders, scrapers, filterers, piercer and predators) according to Tachet et al. (1994), Usseglio-Polatera (1994), Usseglio-Polatera & Tachet (1994) and Tachet et al. (2000). We used a fuzzy coding approach to determine the affinity of each taxon to each category, thus accounting for intra-genus and intra-family variation (Chevenet et al., 1994). Affinity scores ranged between 0 and 3 or 0 and 5, and reflected the relative strength of association of a taxon for a given trait category (Dolédec et al., 2006). Affinity scores were then standardised between 0 and 1. Then, affinity values were multiplied by the relative abundance of each taxon within each microhabitat group. We obtained two traits-by-microhabitat category matrices (October 2008 and June 2009) weighted by the relative abundance of taxa in each microhabitat group (Larsen & Ormerod, 2010; Manfrin et al., 2013).
To quantify the relative importance of river sector and microhabitat categories in driving macroinvertebrate assemblages (aim 1), we used a permutational multivariate analysis of variance (perMANOVA, (Anderson, 2001). This analysis was run on log-transformed macroinvertebrate abundance (Ab), using the ‘adonis’ function in the R package “Vegan” (Oksanen et al., 2013). Taxonomic distances among the sampling sites were computed as Bray-Curtis dissimilarities. Statistical significance was tested using 999 permutations, which were constrained within river sectors in order to account for the nested sampling design (i.e. microhabitat groups nested in river sector).
Plots from the non-metric multidimensional scaling (nMDS) were applied to the log-transformed invertebrate dataset to aid the visual interpretation of perMANOVA results. An nMDS was also performed to visualise differences among microhabitat categories within each river sector. Distances among the samples were computed as Bray-Curtis dissimilarities. nMDS was performed in the R package “Vegan” (Oksanen et al., 2013).
To achieve our second aim (i.e. longitudinal homogenization of micro-habitats), we estimated taxa beta-diversity among micro-habitats within river sectors as multivariate dispersion using the “betadisper” function on the animal abundance, adjusted for unequal number of samples within sectors. Beta-diversity was then expressed as the average distance to group centroid (Anderson et al., 2006). Difference in average distance to centroid among river sectors was tested by ANOVA and Tukey’s HSD post hoc comparison (Yandell, 1997) in the R package “Vegan” (Oksanen et al., 2013).
To achieve our third aim (specific micro-habitat effects within sector), the effects of microhabitat type on invertebrate abundance (Ab) rarefied richness (rr; Sanders, 1968), Shannon diversity index (Sh; Shannon, 2001), and feeding traits were analysed separately within each river sector using linear mixed effects (LME) models (R package “lme 4”; Bates et al., 2007). To account for the effect of different sampling sites within sector, LME models considered microhabitat categories as fixed factor and the sampling sites within sectors as random factor. The significance of the fixed factor was tested by comparing the models with the respective reduced models (without the fixed factor) with likelihood ratio tests (χ 2). Residuals were tested for normality by applying the Shapiro–Wilk test (Shapiro & Wilk, 1965). To assess among which microhabitat type community metrics and feeding trait differed, we run post hoc multiple comparison analyses using the function “testInteractions” of R package “phia” (De Rosario-Martinez, 2013). To reduce Type I error or the false rejection of the null hypothesis, the sequential Bonferroni procedure (Holm, 1979) was calculated (Feeley et al., 1999). All the statistical analyses were performed separately for October 2008 and June 2009.
Results
In October 2008, a total of 26,047 individuals and in June 2009, 21,482 belonging to 80 taxa were collected (Supplementary Material—Appendix 2).
Macroinvertebrates assemblages showed a longitudinal pattern along the river system, as their grouping in the nMDS ordination followed the three sequential river sectors (Fig. 2). The perMANOVA analysis confirmed that river sector was the main driver of the macroinvertebrate structure explaining 27 and 25 % of the taxonomic variance in October and June, respectively (Table 1). Although significant, microhabitat categories explained only a small proportion of the variance in taxonomic structure (i.e. 4 and 6 % in October and June, respectively, Table 1).
Distribution plots of macroinvertebrates log(x + 1)-abundances of the eight sampling localities (see Fig. 1) obtained using non-metric multidimensional scaling (nMDS) in October 2008 and June 2009. Ellipses represent 95 % confidence interval for upstream (S1), middle-stream (S2) and downstream (S3) river sectors. Polygons delimit each microhabitat group considered within each sampling site: circles = C1; triangles = C2; crosses = C3 (for acronyms see Supplementary Material—Appendix 1). nMDS stress October = 0.19; nMDS stress June = 0.18
In June, despite the high variance, beta-diversity among micro-habitat types decreased significantly (F = 4.921; P = 0.0097) in the downstream river sector (S1–S3: P = 0.046; S2–S3: P = 0.014). These differences were not observed in October.
At the within-sector scale, the difference among organic (C3) and inorganic (C1 and C2) microhabitats was evident in the nMDS ordinations in June as shown by 95 % interval ellipses not overlapping (Fig. 3). The Linear Mixed Model analysis showed that the upstream river sector (S1) supported the highest macroinvertebrate diversity and taxonomic richness, with no significant difference among microhabitat categories in both seasons. In the middle river sector, significant differences in total abundance and diversity among microhabitat categories were recorded in June 2009, while in the downstream river sector, rarefied richness and total abundance were significantly different in October 2008 (Table 2; Fig. 4).
Distribution plots of macroinvertebrates log(x + 1)-abundances grouped by microhabitat category (i.e. C1, C2, C3;—for acronyms, see Supplementary Material—Appendix 1) within each river sectors (S1 = upstream; S2 = middle-stream; S3 = downstream) obtained by non-metric multidimensional scaling (nMDS) in October 2008 and June 2009. Ellipses represent 95 % confidence interval for each microhabitat category. nMDS stress October: S1 = 0.0.17; S2 = 0.16; S3 = 0.18. nMDS stress June: S1 = 0.20; S2 = 0.14; S3 = 0.16
Box and Whisker plots of macroinvertebrates total abundance (Ab × 1000), rarefied richness, Shannon diversity medians (squares in bars) (± standard deviations) among the three microhabitat categories(i.e. C1, C2, C3;—for acronyms, see Supplementary Material—Appendix 1) within each river sector (S1 = upstream; S2 = midstream; = S3 = downstream) in October 2008 (Oct) and June 2009 (Jun). Outliers showed as round dots. Significant (Asterisk) pairwise comparisons are shown as: A = C1–C2; B = C2–C3; C = C1–C3. For statistical significance values see Table 2
Three categories of feeding traits showed significant differences among microhabitat categories within the three river sectors (Table 3). Particularly, shredders discriminated among microhabitat categories in the upstream and middle sector in both seasons. Specifically, they were more abundant in the organic microhabitat (C3), compared to the inorganic microhabitats (C1 and C2) (Table 3, Fig. 5). Scrapers abundance differed in the middle and downstream sector in October 2008 and June 2009, respectively, with higher abundances in the finer substrata (C2) (Table 3; Fig. 5). Deposit feeders were more abundant in the inorganic microhabitats (C1 and C2) in the downstream sector in June 2009 (Table 3; Fig. 5).
Box plots showing significant linear mixed effects (see Table 3) on functional trait category medians (squares in bars) (± standard deviations) for each microhabitat grouped category (i.e. C1, C2, C3;—for acronyms, see Supplementary Material—Appendix 1) within each river sector (S1 = upstream; S2 = midstream; = S3 = downstream) in October 2008 (Oct) and June 2009 (Jun). Feeding trait categories: Dep = deposit feeder; Shr = shredder; Scr = scraper. Significant (Asterisk) pairwise comparisons are shown as: A = C1–C2; B = C2–C3; C = C1–C3. For statistical significance values see Table 3
Discussion
The main result of this study supports the hypothesis that our perception of the key abiotic drivers shaping natural assemblages is contingent upon the chosen scale of observation (Paavola et al., 2006; Larsen et al., 2009), with clear implications for environmental assessment.
Macroinvertebrate assemblages in the Aniene River appeared to be primarily regulated by processes operating at larger spatial scales (i.e. river sector), while they seemed to respond only secondarily to the structure of microhabitats. Such clear longitudinal gradient in assemblage structure likely reflects the marked longitudinal changes in both natural (gradient, temperature, shading) and anthropogenic (the progressive increase in human pressures from upstream to downstream) factors that characterise the study river, as previously shown (Solimini et al., 2001; Manfrin et al., 2013; Traversetti & Scalici, 2014).
The second aim of the study was to verify if the longitudinal gradient in anthropogenic pressure exerted a homogenising effect at the local micro-habitat scale. Anthropogenic alteration of habitats often leads to biotic homogenization of assemblages, either by reducing environmental heterogeneity or by directly affecting the structure of communities (Olden et al., 2004; Chase, 2007; Passy & Blanchet, 2007). More generally, increasing environmental harshness (i.e. strong abiotic filters) can reduce compositional heterogeneity among locations by decreasing the importance of stochastic processes in regulating assemblages (Chase, 2007). Here we hypothesised that the longitudinal increase in human pressure, namely sewage effluents, nutrient enrichment and impoundment, represented a deterministic abiotic filter that would homogenise assemblages (i.e. lower beta-diversity) among different micro-habitat types (Donohue et al., 2009). This hypothesis was partly supported by the results, where compositional heterogeneity among micro-habitats was lower in the downstream river sector, but only in June. It is possible that the effect of nutrient enrichment was stronger during summer low flows in June, compared to October, where higher flows diluted excess nutrients from treatment plants (Manfrin et al., 2013).
At the within-sectors, the relationship between macroinvertebrate community metrics and microhabitat features also appeared context dependent. In the upstream sector, abundance, rarefied richness and diversity of taxa did not show significant variation among the three microhabitat categories, which all supported relatively rich and diverse assemblages. Conversely, differences in the community metrics were evident in the middle and downstream sectors, where relatively higher invertebrate densities and diversity were observed in fine inorganic and organic substrata. Also, these differences were not due to changes in the availability of micro-habitat types along the river, which showed little longitudinal variation (Supplementary Material—Appendix 3). Overall, however, middle and downstream river sectors supported less rich and diverse assemblages, compared to the upstream sector. In contrast to structural aspects, the analysis of biological traits showed differences in feeding strategies among microhabitats along the entire river corridor and in both seasons. The higher sensitivity of feeding traits (compared to taxonomic indices) to microhabitat characteristics has been observed in numerous studies (e.g. Huamantinco & Nessimian, 1999; Tomanova & Usseglio-Polatera, 2007; Costa & Melo, 2008; Larsen & Ormerod, 2010). Specifically, the representation of feeding traits is expected to reflect the availability of key food sources, which, in turn, is strongly influenced by local substratum features (e.g. macrophytes stands, or leaf-litter patches). In the Aniene river, shredders were influenced by microhabitat type especially in the up-middle sectors where they were more abundant within fine inorganic and organic substrata, likely reflecting the local availability of coarse organic material (i.e. leaflitter) (Allan & Castillo, 2007). Scrapers and deposit feeders were more abundant within fine inorganic substrata, especially in the mid-downstream sectors, where lower water velocity and turbulence allowed the accrual of algae and the deposition of fine organic material.
Before drawing conclusions, some limitations of the study need to be discussed, including the limited sample size and taxonomic resolution. In particular, our findings are based on observations from a single river, hindering generalisations at larger spatial scales or across different eco-regions. Also, the different levels of resolution at which invertebrates were identified (species, genus, family) could have potentially limited our ability to quantify community variation, especially over small spatial gradients such as the micro-habitat scale (Li et al., 2001; Heino et al., 2004). Nonetheless, as explained in the introduction, the Aniene river is a fair representative of many Apennine rivers in central Italy that show similar longitudinal gradients. Moreover, benthic invertebrates are difficult to identify at the species level, and there is extensive evidence showing that community patterns and metrics appear consistent across different levels of taxonomic resolution (Heino & Soininen, 2007; Carneiro et al., 2010).
Conclusions
Findings from this study further highlight the importance of an explicit consideration of spatial scales in both fundamental and applied ecological research (Larsen et al., 2009; Chase, 2014; Larsen et al., 2015). Longitudinal factors were the main force driving macroinvertebrate assemblage over the study river. Nonetheless, by combining information on community composition and feeding traits, we found that macroinvertebrates were also influenced by different microhabitat types within each river sector.
The results also lend support to the simultaneous use of taxonomic and functional measures (e.g. feeding traits) in environmental assessment studies, especially where weak abiotic gradients are present (Heino et al., 2013; Gallardo et al., 2014) or where interest is given towards local-scale influences (Paller et al., 2014). In agreement with Beisel et al. (2000) and Costa & Melo (2008), our findings suggest that riverine conservation actions should preserve natural microhabitat patchiness. Stream bio-monitoring programmes should consider this scale-dependent variability in assemblage characteristics because: (i) small-scale variability in density and composition show that few replicate samples are not sufficient to estimate macroinvertebrates assemblage variability at a site and (ii) sectors from the same stream may support widely different benthic assemblages.
An inherent corollary of the present findings with practical implications is that the use of multi-habitat sampling, advocated by the Water Framework Directive, appears indeed well suited to describe benthic assemblages in streams and rivers.
References
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual review of ecology, evolution, and systematics 35: 257–284.
Allan, J. D. & M. M. Castillo, 2007. Stream Ecology: Structure and Function of Running Waters. Springer Science & Business Media, New York.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral ecology 26(1): 32–46.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9(6): 683–693.
Arscott, D. B., K. Tockner & J. Ward, 2003. Spatio-temporal patterns of benthic invertebrates along the continuum of a braided Alpine river. Archiv für Hydrobiologie 158(4): 431–460.
Barnes, J. B., I. P. Vaughan & S. J. Ormerod, 2013. Reappraising the effects of habitat structure on river macroinvertebrates. Freshwater Biology 58(10): 2154–2167.
Bates, D., D. Sarkar, M. D. Bates & L. Matrix, 2007. The lme4 package. R package version 2(1): 74.
Beisel, J.-N., P. Usseglio-Polatera, S. Thomas & J.-C. Moreteau, 1998. Stream community structure in relation to spatial variation: the influence of mesohabitat characteristics. Hydrobiologia 389(1–3): 73–88.
Beisel, J.-N., P. Usseglio-Polatera & J.-C. Moreteau, 2000. The Spatial Heterogeneity of a River Bottom: A Key Factor Determining Macroinvertebrate Communities. Springer, Berlin.
Boulton, A. J., C. G. Peterson, N. B. Grimm & S. G. Fisher, 1992. Stability of an aquatic macroinvertebrate community in a multiyear hydrologic disturbance regime. Ecology 73: 2192–2207.
Carneiro, F. M., L. M. Bini & L. C. Rodrigues, 2010. Influence of taxonomic and numerical resolution on the analysis of temporal changes in phytoplankton communities. Ecological Indicators 10(2): 249–255.
Chase, J. M., 2007. Drought mediates the importance of stochastic community assembly. Proceedings of the National Academy of Sciences 104(44): 17430–17434.
Chase, J. M., 2014. Spatial scale resolves the niche versus neutral theory debate. Journal of vegetation science 25(2): 319–322.
Chevenet, F., S. Doledec & D. Chessel, 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshwater biology 31(3): 295–309.
Costa, S. S. & A. S. Melo, 2008. Beta diversity in stream macroinvertebrate assemblages: among-site and among-microhabitat components. Hydrobiologia 598(1): 131–138.
De Rosario-Martinez, H., 2013. Phia: post-hoc interaction analysis. R package version 01-3.
Dolédec, S., N. Phillips, M. Scarsbrook, R. H. Riley & C. R. Townsend, 2006. Comparison of structural and functional approaches to determining landuse effects on grassland stream invertebrate communities. Journal of the North American Benthological Society 25(1): 44–60.
Donohue, I., L. A. Donohue, B. N. Ainín & K. Irvine, 2009. Assessment of eutrophication pressure on lakes using littoral invertebrates. Hydrobiologia 633(1): 105–122.
European Commission, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy Official Journal 22 December 2000 L 327/1. European Commission, Brussels.
Feeley, M., R. J. DeRubeis & L. A. Gelfand, 1999. The temporal relation of adherence and alliance to symptom change in cognitive therapy for depression. Journal of consulting and clinical psychology 67(4): 578.
Gallardo, B., S. Doledec, A. Paillex, D. B. Arscott, F. Sheldon, F. Zilli, S. Merigoux, E. Castella & F. A. Comín, 2014. Response of benthic macroinvertebrates to gradients in hydrological connectivity: a comparison of temperate, subtropical, Mediterranean and semiarid river floodplains. Freshwater Biology 59(3): 630–648.
García-Roger, E. M., M. D. M. Sánchez-Montoya, N. Cid, S. Erba, I. Karaouzas, I. Verkaik, M. Rieradevall, R. Gómez, M. L. Suárez & M. R. Vidal-Abarca, 2013. Spatial scale effects on taxonomic and biological trait diversity of aquatic macroinvertebrates in Mediterranean streams. Fundamental and Applied Limnology/Archiv für Hydrobiologie 183(2): 89–105.
Heino, J. & J. Soininen, 2007. Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biological Conservation 137(1): 78–89.
Heino, J., P. Louhi & T. Muotka, 2004. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49(9): 1230–1239.
Heino, J., D. Schmera & T. Erős, 2013. A macroecological perspective of trait patterns in stream communities. Freshwater Biology 58(8): 1539–1555.
Hering, D., O. Moog, L. Sandin & P. F. Verdonschot, 2004. Overview and application of the AQEM assessment system. Hydrobiologia 516(1–3): 1–20.
Holm, S., 1979. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics 6: 65–70.
Huamantinco, A. & J. Nessimian, 1999. Estrutura e distribuição espacial da comunidade de larvas de Trichoptera (Insecta) em um tributário de primeira ordem do Rio Paquequer, Teresópolis. RJ. Acta Limnologica Brasiliensia 11(2): 1–16.
Kelly, D., J. Dick, W. Montgomery & C. MacNeil, 2003. Differences in composition of macroinvertebrate communities with invasive and native Gammarus spp. (Crustacea: Amphipoda). Freshwater Biology 48(2): 306–315.
Larsen, S. & S. Ormerod, 2010. Combined effects of habitat modification on trait composition and species nestedness in river invertebrates. Biological Conservation 143(11): 2638–2646.
Larsen, S., I. Vaughan & S. Ormerod, 2009. Scale-dependent effects of fine sediments on temperate headwater invertebrates. Freshwater Biology 54(1): 203–219.
Larsen, S., L. Mancini, G. Pace, M. Scalici & L. Tancioni, 2012. Weak concordance between fish and macroinvertebrates in Mediterranean streams. PloS one 7(12): e51115.
Larsen, S., M. Scalici & L. Tancioni, 2015. Scale dependent biodiversity patterns in Mediterranean river catchments: a multi taxa approach. Aquatic Sciences. doi:10.1007/s00027-014-0390-3.
Lenat, D. R. & M. T. Barbour, 1994. Using benthic macroinvertebrate community structure for rapid, cost-effective, water quality monitoring: rapid bioassessment. Biological monitoring of aquatic systems Lewis Publishers, Boca Raton: 187–215.
Li, J., A. Herlihy, W. Gerth, P. Kaufmann, S. Gregory, S. Urquhart & D. P. Larsen, 2001. Variability in stream macroinvertebrates at multiple spatial scales. Freshwater Biology 46(1): 87–97.
Mancini, L., P. Formichetti, A. Anselmo, L. Tancioni, S. Marchini & A. Sorace, 2005. Biological quality of running waters in protected areas: the influence of size and land use. Biodiversity & Conservation 14(2): 351–364.
Manfrin, A., S. Larsen, L. Traversetti, G. Pace & M. Scalici, 2013. Longitudinal variation of macroinvertebrate communities in a Mediterranean river subjected to multiple anthropogenic stressors. International Review of Hydrobiology 98(3): 155–164.
Minshall, G., 1984. Aquatic insect-substratum relationships. In Resh, V. H. & D. M. Rosenberg (eds), The Ecology of Aquatic Insects. Praeger Publishers, New York: 358–400.
Mykrä, H., J. Heino & T. Muotka, 2007. Scale-related patterns in the spatial and environmental components of stream macroinvertebrate assemblage variation. Global Ecology and Biogeography 16(2): 149–159.
Nelson, S. M., 2011. Comparisons of macrophyte breakdown, associated plant chemistry, and macroinvertebrates in a wastewater dominated stream. International Review of Hydrobiology 96(1): 72–89.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Stevens & H. Wagner, 2013. Vegan: Community Ecology Package. R package version 2.0-7. http://www.CRANR-projectorg/package=vegan.
Olden, J. D., N. L. Poff, M. R. Douglas, M. E. Douglas & K. D. Fausch, 2004. Ecological and evolutionary consequences of biotic homogenization. Trends in Ecology & Evolution 19(1): 18–24.
Paavola, R., T. Muotka, R. Virtanen, J. Heino, D. Jackson & A. Mäki-Petäys, 2006. Spatial scale affects community concordance among fishes, benthic macroinvertebrates, and bryophytes in streams. Ecological Applications 16(1): 368–379.
Pace, G., N. Bonada & N. Prat, 2013. Long-term effects of climatic–hydrological drivers on macroinvertebrate richness and composition in two Mediterranean streams. Freshwater Biology 58(7): 1313–1328.
Paller, M. H., S. C. Sterrett, T. D. Tuberville, D. E. Fletcher & A. M. Grosse, 2014. Effects of disturbance at two spatial scales on macroinvertebrate and fish metrics of stream health. Journal of Freshwater Ecology 29(1): 83–100.
Passy, S. I. & F. G. Blanchet, 2007. Algal communities in human-impacted stream ecosystems suffer beta-diversity decline. Diversity and Distributions 13(6): 670–679.
Peeters, E. T., R. Gylstra & J. H. Vos, 2004. Benthic macroinvertebrate community structure in relation to food and environmental variables. Hydrobiologia 519(1–3): 103–115.
Reice, S. R., 1980. The role of substratum in benthic macroinvertebrate microdistribution and litter decomposition in a woodland stream. Ecology 61: 580–590.
Sanders, H. L., 1968. Marine benthic diversity: a comparative study. American naturalist 102: 243–282.
Schmera, D. & T. Eros, 2004. Effect of riverbed morphology, stream order and season on the structural and functional attributes of caddisfly assemblages (Insecta: Trichoptera) Annales de Limnologie-International Journal of Limnology. Cambridge Univ Press, 193–200.
Shannon, C. E., 2001. A mathematical theory of communication. ACM SIGMOBILE Mobile Computing and Communications Review 5(1): 3–55.
Shapiro, S. S. & M. B. Wilk, 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611.
Solimini, A. G., A. Benvenuti, R. D’Olimpio, M. De Cicco & G. Carchini, 2001. Size structure of benthic invertebrate assemblages in a Mediterranean river. Journal of the North American Benthological Society 20(3): 421–431.
Star Consortium, 2003. The AQEM sampling method to be applied in STAR. Internet-URL: http://www.eu-star.at Link: Protocols.
Tachet, H., P. Richoux, M. Bournaud & P. Usseglio-Polatera, 2000. Invertébrés d’eau douce: systématique, biologie, écologie. CNRS éditions Paris.
Tachet, H., P. Usseglio-Polatera & C. Roux, 1994. Theoretical habitat templets, species traits, and species richness: Trichoptera in the Upper Rhône River and its floodplain. Freshwater Biology 31(3): 397–415.
Tomanova, S. & P. Usseglio-Polatera, 2007. Patterns of benthic community traits in neotropical streams: relationship to mesoscale spatial variability. Fundamental and Applied Limnology/Archiv für Hydrobiologie 170(3): 243–255.
Traversetti, L. & M. Scalici, 2014. Assessing the influence of source distance and hydroecoregion on the invertebrate assemblage similarity in central Italy streams. Knowledge and Management of Aquatic Ecosystems(414):02.
Traversetti, L., A. Manfrin & M. Scalici, 2013. Remapping hydroecoregion boundaries: a proposal for improving the base of the running water monitoring procedures. Journal of Basic & Applied Sciences 9: 533–537.
Traversetti, L., M. Scalici, V. Ginepri, A. Manfrin & S. Ceschin, 2014. Concordance between macrophytes and macroinvertebrates in a Mediterranean river of central Apennine region. Journal of Environmental Biology 35: 497–503.
Usseglio-Polatera, P., 1994. Theoretical habitat templets, species traits, and species richness: aquatic insects in the Upper Rhône River and its floodplain. Freshwater Biology 31(3): 417–437.
Usseglio-Polatera, P. & H. Tachet, 1994. Theoretical habitat templets, species traits, and species richness: Plecoptera and Ephemeroptera in the Upper Rhône River and its floodplain. Freshwater Biology 31(3): 357–375.
Wasson, J., A. Chandesris, A. G. Bautista, H. Pella & B. Villeneuve, 2006. Combined pressures and geographical context: hydro-ecoregions framework. Cemagref REBECCA project report.
Yandell, B. S., 1997. Practical data analysis for designed experiments. CRC Press, Boca Raton.
Acknowledgments
This study was technically supported by Roma Tre University. A. Manfrin was financially supported by the Department of Science, Roma Tre University, Rome, and Leibniz-Institute for Freshwater Ecology and Inland Fisheries, Berlin. S. Larsen was supported by an individual fellowship from the German Centre for Integrative Biodiversity Research (iDiv). We want also to thank T. Mehner and the participants of the workshop “Scientific Writing” at the Leibnitz-Institute of Freshwater Ecology and Inland Fisheries for helpful discussion on an early stage of the manuscript. We are indebted to Giorgio Pace, for his help during the field sampling. Finally, we want to thank two anonymous referees for their precious suggestions that helped to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sonja Stendera
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manfrin, A., Traversetti, L., Pilotto, F. et al. Effect of spatial scale on macroinvertebrate assemblages along a Mediterranean river. Hydrobiologia 765, 185–196 (2016). https://doi.org/10.1007/s10750-015-2412-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2412-0