Abstract
Harmful algal blooms can adversely affect fish communities, though their impacts are highly context-dependent and typically differ between fish species. Various approaches, comprising univariate and multivariate analyses and multimetric Fish Community Indices (FCI), were employed to characterise the perceived impacts of a Karlodinium veneficum bloom on the fish communities and ecological condition of the Swan Canning Estuary, Western Australia. The combined evidence suggests that a large proportion of the more mobile fish species in the offshore waters of the bloom-affected area relocated to other regions during the bloom. This was indicated by marked declines in mean species richness, catch rates and FCI scores in the bloom region but concomitant increases in these characteristics in more distal regions, and by pronounced and atypical shifts in the pattern of inter-regional similarities in fish community composition during the bloom. The lack of any significant changes among the nearshore fish communities revealed that bloom impacts were less severe there than in deeper, offshore waters. Nearshore habitats, which generally are in better ecological condition than adjacent offshore waters in this system, may provide refuges for fish during algal blooms and other perturbations, mirroring similar observations of fish avoidance responses to such stressors in estuaries worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harmful algal blooms (HABs) occur when algal cells proliferate to concentrations sufficient to cause harm to other species, as a result either of their production of endogenous toxins or their physical shape and/or accumulated biomass (Anderson et al., 2002). Such blooms occur globally and in a range of aquatic environments, including freshwater lakes and rivers (Harper, 1992; Smith, 2003), coastal and oceanic marine waters (Anderson et al., 2008a; Fu et al., 2012) and estuaries (Burkholder & Glasgow, 2001; Glibert et al., 2001). The occurrence of HABs can have a wide range of deleterious impacts on the biotic communities of aquatic ecosystems, including their fish faunas. Acute adverse effects of HABs may occur due to the accompanying development of hypoxic conditions [dissolved oxygen (DO) <2 mg l−1], release of ichthyotoxins or direct physical impairment such as gill damage and clogging (Burkholder et al., 1992; Deeds et al., 2002; Landsberg, 2002; Glibert et al., 2005; Anderson et al., 2012). These effects may induce localised fish kills (Portnoy, 1991; Glasgow & Burkholder, 2000) or, particularly for more mobile species, emigration to more hospitable areas (Potter et al., 1983; Eby et al., 2005; Lamberth et al., 2010). Associated reductions in the quality and/or quantity of available prey (e.g. benthic invertebrates) or structural habitat (such as via shading of seagrass beds by overlying blooms) may also cause declines in the occurrence of particular fish species by impeding their ability to feed, shelter from predators and/or reproduce (Szedlmayer & Able, 1996; Levin & Hay, 2003). Given that such responses typically vary among fish species, interpretations of the effects of HABs on fish communities and estuarine ecology, and particularly those beyond the short term, are often complex.
The microtidal (maximum tidal range c. 1 m) Swan Canning Estuary, located on the south-west coast of Western Australia (WA; Fig. 1), exemplifies the impacts of HABs on estuarine ecology. This estuary and its large (121,000 km2) catchment have undergone substantial anthropogenic change since European settlement during the early- to mid-1800s, and consequently, the system is now classified as highly modified (Commonwealth of Australia, 2002). The relative contribution of land-derived surface runoff to river flows has been enhanced by extensive catchment clearing, shoreline modification and the growth of surrounding urban and agricultural activity, thereby magnifying the sediment, nutrient and pollutant loads in the estuary (Gerritse et al., 1998; Jakowyna et al., 2000; Rate et al., 2000). This is further exacerbated by stormwater inputs and the extensive network of wastewater drains that discharge into the estuary.
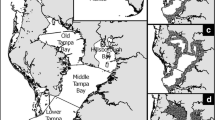
Locations of offshore (open circle) and nearshore (closed circle) sites in the lower, middle-downstream, middle-upstream and upper Swan River regions of the Swan Canning Estuary, at which the fish community was sampled before (‘summer’), during (‘bloom’) and after (‘autumn’) a Karlodinium veneficum bloom that occurred in the middle-upstream region of the Swan River during March 2004. Locations of phytoplankton sampling sites are denoted by closed squares: NIL Nile Street, STJ St John of God Hospital, MAY Maylands, RON Ron Courtney Island, KIN Kingsley Street, SUC Success Hill
One of the more obvious ecological responses of the Swan Canning Estuary to these numerous anthropogenic changes has been an increase in the frequency and density of large phytoplankton blooms, particularly in its middle and upper reaches (Twomey & John, 2001; Chan et al., 2002). The nutrient-enriched waters and sediments of that part of the estuary (SRT, 2009), in combination with pronounced vertical stratification of salinity and DO and reduced flushing linked to declining annual rainfall and river flows (Cottingham et al., 2014), encourage the formation of algal blooms. These blooms are particularly prevalent during the Austral summer and autumn when temperatures are elevated, river flows are low, water clarity is high and nutrient concentrations are increased by influxes from cyclonic storm events and enhanced sedimentary release under hypoxic conditions (Thompson, 1998; Horner Rosser & Thompson, 2001; Twomey & John, 2001).
Blooms of dinoflagellates, which may produce toxins that are potentially harmful to mammals and/or fish (Deeds et al., 2002; Landsberg, 2002; Place et al., 2012), have occurred more frequently and with particularly high cell densities in the middle to upper Swan Canning Estuary over the last decade or so (Thompson & Hosja, 1996; Twomey & John, 2001; Chan et al., 2002). For example, the ichthyotoxic dinoflagellate Karlodinium veneficum formed large blooms in this system during the late autumn/early winter of 2003, exceeding c. 100,000 and 140,000 cells ml−1 in the Swan and Canning rivers, respectively (Kidd & Srdarev, 2003). An estimated 300,000 fish were killed as a consequence of this bloom event. Fish kills have also been associated with several other phytoplankton blooms documented previously in the Swan Canning Estuary during summer or autumn, e.g. those in 1983, 1992, 1994, 2000 and 2005 (Hosja & Deeley, 1994; Place et al., 2012).
Despite widespread concern from the general public, recreational fishers and natural resource managers about the implications of HABs for the fish fauna of the Swan Canning Estuary, there are no previous published studies demonstrating their impacts on fish in this system. This study thus sought to employ (i) univariate analyses of fish species richness and abundance, (ii) multivariate analyses of fish community composition and (iii) ecological condition assessments using fish-based multimetric indices developed recently for the Swan Canning Estuary (Hallett et al., 2012a, b), to characterise the impacts of a large K. veneficum bloom on the fish communities and ecological integrity of this system. This bloom event, which occurred in the middle-upstream region of the tidal Swan River (Fig. 1) during March 2004, coincided with an existing fish sampling programme being undertaken in the estuary and thus provided a valuable opportunity to examine the impact of such HABs on the fish communities and ecological health of this system.
Materials and methods
Characterising the Karlodinium veneficum bloom
Phytoplankton data from the WA Department of Water (DoW) WIN database (DoW, unpublished data) were plotted to examine patterns in the abundance of K. veneficum cells in the lower (LS), middle-downstream (MD) and middle-upstream (MU) Swan River regions of the estuary (Fig. 1; note that no such data were available for the upper Swan River [US] region). These phytoplankton data (K. veneficum cell densities; cells ml−1) were collected c. weekly between January and May 2004 at six routine monitoring stations along the Swan River (Fig. 1), and also during additional sampling of selected sites, as per standard departmental protocols in the event of a HAB. According to these protocols, depth-integrated phytoplankton samples were collected with 50-mm diameter hose-pipe and immediately preserved with Lugol’s iodine solution, prior to enumeration and identification via light-microscopy (Cosgrove et al., 2015).
Sampling fish communities
Fish were sampled at various shallow nearshore (depth <2 m) and deeper offshore (depth >2 m) sites throughout the LS, MD, MU and US regions of the estuary (Fig. 1). Samples were collected during (i) January 2004 (‘summer’), c. 6 weeks prior to the bloom, (ii) the peak of the bloom in March 2004 (‘bloom’) and (iii) April 2004 (‘autumn’), c. 4 weeks after the peak of the bloom.
Fish sampling during the current study employed methods that have been applied extensively since the 1970s to characterise the fish fauna of the nearshore and offshore waters of this system (Loneragan et al., 1989; Loneragan & Potter, 1990; Hoeksema & Potter, 2006; Hallett & Hall, 2012). Sampling of fish in nearshore waters was carried out during daylight hours using a seine net that was 41.5-m long, 2-m deep and had two 20-m-long wings made of 25-mm mesh and a 1.5-m-wide central bunt made of 9-mm mesh. This net, which swept an area of c. 274 m2, was laid by boat in a semi-circle from the bank and then hauled on to the beach. Seven nearshore sites were sampled, three of which were located in each of the LS and MD regions, with one site in the MU region (Fig. 1). The total number of individuals of each species caught in each sample was recorded and expressed as a density, i.e. number 250 m−2, for reporting purposes.
Fish in the offshore waters were collected at three sites in each of the LS, MD, MU and US regions using composite sunken gill nets. Each of these nets comprised six 20 m long × 2 m high panels of varying stretched mesh size (i.e. 38, 51, 63, 76, 89 and 102 mm) and was laid parallel to the shoreline at dusk and retrieved after 3 h. The total number of individuals of each species caught in all mesh sizes in each sample was recorded and expressed as a catch rate, i.e. number of fish h−1.
All fish collected were immediately euthanised in an ice slurry and then taken to the laboratory where the total number of individuals of each species in each sample was recorded. Each species was also allocated to a functional ecological guild under each of three categories (i.e. ‘Habitat’, ‘Estuarine use’ and ‘Feeding mode’), as detailed by Hallett et al. (2012a).
Water quality parameters
Salinity, temperature (°C) and DO (mg l−1) were measured at each site on each sampling occasion using a YSI 556 water quality meter (Yellow Springs, Ohio). Measurements were made at the water surface at nearshore sites and at both the surface and bottom at offshore sites. These data were supplemented, where available, with complementary measurements of Secchi depth and chlorophyll a recorded by the WA DoW (DoW, unpublished data), and used to aid interpretation of any potential fish community responses to the algal bloom.
Catch rates, densities and numbers of species
Fish samples collected in the nearshore and offshore waters were treated separately for all of the following data analyses in this and subsequent subsections.
Two-way analysis of variance (ANOVA) was used to determine whether the number of species and overall density/catch rate of fish differed significantly among sampling periods (hereafter ‘period’, i.e. summer, bloom, autumn) and regions of the estuary (excluding the US and MU regions for the nearshore data set, as they lacked a sufficient number of replicate sites). Both factors were considered fixed and crossed with each other. Prior to undertaking these tests, the data for each variable were examined to ascertain the type of transformation required (if any) to satisfy the test assumptions of constant variance and normality. This was achieved by determining the extent of the slope between the log(mean) and log10(SD) of groups of replicate samples then employing the following decision rule: slope 0–0.25 = no transformation required; 0.25–0.5 = √; 0.5–0.75 = √√; 0.75–1 = log (Clarke & Warwick, 2001). Number of species required no transformation for both the nearshore and offshore waters, whilst both density and catch rate were log-transformed.
When ANOVA detected a significant difference (significance level, P ≤ 0.05) between periods and/or regions and there was no significant interaction, Scheffé’s a posteriori test was used to ascertain the nature of those differences. Alternatively, when the region × period interaction was significant, inter-period and inter-regional differences were examined using plots of the marginal means and their 95% confidence intervals (95% CI, which had been back-transformed where necessary).
Fish community composition
The following analyses were performed using the PRIMER v6 multivariate statistics package (Clarke & Gorley, 2006) with the PERMANOVA+ add-on module (Anderson et al., 2008b), with two exceptions. Two novel multivariate analyses, i.e. a modified non-metric multidimensional scaling method and ‘shade plots’, were performed using an alpha test version of PRIMER v7. Detailed descriptions of these novel procedures are provided below.
Statistical testing of differences
The replicate fish species counts were initially dispersion weighted (Clarke et al., 2006a) to downweight the contributions of those species with large and erratic differences in abundance within groups of replicate samples. These data were then square-root transformed to ‘balance’ the contributions of highly abundant and less abundant species, an effective combination of data pre-treatment for fish abundance data of this type (Clarke et al., 2013). The pretreated data were then used to construct a Bray–Curtis similarity matrix containing all pairs of replicate samples.
The above matrix was initially subjected to a two-way Permutational Multivariate Analysis of Variance (PERMANOVA) test (Anderson, 2001) to ascertain whether the fish assemblage composition differed significantly among periods and/or regions. Both factors were considered fixed and crossed with each other, and the relative importance of each significant term (P ≤ 0.05) was assessed by the magnitude of its components of variation (COV). Given the significant period × region interaction detected by this test (see “Results” section), inter-period differences were then further explored separately for each region (and vice versa) by subjecting relevant subsets of the above Bray–Curtis matrix to one-way analysis of similarities tests (ANOSIM; Clarke & Green, 1988). The extent of any significant differences (again P ≤ 0.05) was determined by the magnitude of the test-statistic R, i.e. values close to 0 indicate little difference among groups, while those close to +1 indicate large group differences (Clarke & Green, 1988). Note that the P values for pairwise comparisons in these tests were not interpreted, given the insufficient number of possible permutations to ensure a reliable significance test.
A modified non-metric multidimensional scaling method
To summarise and illustrate any spatio-temporal differences in ichthyofaunal composition, a Bray–Curtis similarity matrix was next constructed from the pretreated fish species abundances averaged for each period × region combination. This matrix was initially subjected to non-metric multidimensional scaling (nMDS) ordination (Kruskal, 1964) but, due to the influence of an extreme outlier, resulted in a ‘collapsed’ plot that effectively comprised only two opposing points, one being the outlier sample and the other containing all remaining samples. This common outcome results from the purely non-metric nature of this approach; it is a function only of the ranks of the inter-point resemblances and thus the positioning of an extreme outlier is not well defined. Any position for the outlying point which places it further from the set of remaining points than the largest dissimilarity within that set is equally satisfactory, because it obeys all the rank conditions placed on the outlier in just the same way. The iterative process inherent in finding an nMDS solution is then increasingly able to reduce the stress value of the ordination by placing the outlier at ever larger distances from the set of remaining points, and the iteration converges on a two-point, zero stress solution. Standard practice is to remove the outlier and repeat the nMDS for the remaining set (Clarke & Gorley, 2006). Whilst this is understandable from the viewpoint of satisfying purely rank-order relationships, it can be unhelpful in visualising the macro-pattern across all samples, and particularly in discerning the ‘direction’ in which an outlier is positioned in relation to the remaining samples.
The solution lies in utilising a small amount of information from metric MDS (mMDS; Cox & Cox, 2001), which is sufficient to constrain the position of any outliers in relation to the main group(s) of points, since there is now a quantitative measurement scale of dissimilarities that can be exploited. The metric analogue to nMDS has not been widely used because, on its own, mMDS is too inflexible and often unsuccessful. The Shepard (1962) diagram of inter-point distances from a mMDS ordination, plotted against the corresponding dissimilarities from the resemblance matrix, needs to be fitted by a straight line. Yet, such relationships are rarely linear, leading to high stress or departure from the fitted line. Thus, for example, mMDS seeks to place two samples with a dissimilarity of 60% at precisely twice the distance of two samples with a dissimilarity of 30%, and difficulties are inevitable with a dissimilarity scale which has an upper limit of 100% (or 1), as for all the widely used ‘biological’ resemblance coefficients (Clarke et al., 2006c).
Successful low-dimensional ordinations are achieved by nMDS because the Kruskal (1964) algorithm permits a general monotonic-increasing relationship to be fitted between ordination distance and dissimilarity. This non-parametric regression must therefore remain at the core of a modified nMDS procedure. The stress function of such a procedure is mixed in proportions of about 0.95 nMDS to 0.05 mMDS, and the combined stress minimised using precisely the same iterative steps as outlined previously. The small degree of constraint supplied by the metric stress function is enough to locate an outlier on the plot mensuratively, thus preventing the plot from collapsing in the presence of one or more outliers yet retaining the (non-metric) flexibility of the arrangement of points for the remaining samples. Thus, in the current study, the modified nMDS approach, which was carried out using an alpha test version of PRIMER v7, placed the outlier at a distance from its most similar sample that was only marginally greater than the maximum distance among the remaining samples (as demonstrated by nearly identical respective dissimilarities of 77.4 and 77.3; unpublished data). Had these two values been the other way around, the standard nMDS plot would not have collapsed at all, demonstrating the importance of this innovation in avoiding an unbalanced picture of the inter-sample relationships. Furthermore, the evidence to date suggests that this procedure is not sensitive to the precise balance of the two stress functions, with identical plots and stress values resulting in this case from mixing non-metric to metric proportions of 0.95, 0.05 and 0.75, 0.25.
Further multivariate analyses of community composition
Second-stage nMDS ordination (Clarke et al., 2006b) was then used to explore whether the pattern of inter-regional similarity in fish community composition during the bloom differed markedly from that in all other sampling periods. Data collected during winter and spring 2004 were also included in these analyses to better determine any perturbing effects of the bloom within the context of a ‘typical’ seasonal cycle. These analyses were achieved by subjecting the Bray–Curtis similarity matrix constructed from the period × region averages to the 2STAGE procedure, using region as the ‘inner’ factor and period as the ‘outer’ factor (Clarke & Gorley, 2006; Clarke et al., 2006b). Spearman’s rank correlation coefficient (ρ) was used to calculate the correlations between all pairs of sub-matrices, and the subsequent values were then subjected to nMDS ordination to illustrate any differences in inter-regional relationships during the bloom period.
The fish species that were most responsible for any such differences between the bloom and the preceding summer and subsequent autumn periods were identified using a ‘shade plot’ analysis (Clarke et al., 2013), which was again carried out using an alpha test version of PRIMER v7. The output of this routine is a visual display of the pretreated and averaged fish species abundances in each period × region combination. The y-axis represents the most prevalent fish species (i.e. those accounting for >10% of the abundances in any period × region group), the x-axis comprises the samples ordered sequentially by period then region and the corresponding cells contain greyscale shading that increases with fish abundance (ranging from white = absent to black = maximum abundance). Additionally, the fish species on the y-axis are presented as they appear in hierarchical agglomerative clustering (using group-average linkage) applied to a resemblance matrix defined between species as Whittaker’s index of association (Whittaker, 1952; Somerfield & Clarke, 2013).
Ecological condition, as quantified by the Fish Community Index
The well-documented response of biological communities to environmental stress has spawned a multitude of approaches for quantifying the degree to which the structural and functional integrity of these communities deviates from a pristine or ‘best attainable’ state, thus providing a measure of the ecological ‘health’, ‘condition’ or ‘status’ of aquatic ecosystems (Karr, 1981; Gibson et al., 2000). For example, multimetric indices based on fish communities are employed globally to assess the ecological health or integrity of aquatic ecosystems, including estuaries (Whitfield & Elliott, 2002; Borja et al., 2012; Pérez-Domínguez et al., 2012). These tools integrate information on various biological variables (‘metrics’), each of which quantify an aspect of the structure and/or function of biotic communities and responds to a range of stressors that may affect the ecosystem. Commonly employed metrics include the number or diversity of fish species, the number of species or proportion of fish belonging to specific feeding groups (e.g. trophic specialists versus omnivorous or opportunistic feeders) or to specific habitat or life history guilds (Pérez-Domínguez et al., 2012). As the integrity of ecosystem structures, functions and processes is impacted by an algal bloom or other putative stressor, the effects of these impacts are reflected in the biotic community metrics and index scores, which thus can be used to quantify changes in the broader ecological condition of the ecosystem.
One such multimetric index, the ‘Fish Community Index’ (FCI), has been recently developed for assessing the ecological condition of nearshore and offshore waters of the Swan Canning Estuary. The development and detailed methodology of the FCI, including the mechanism by which estuarine condition is scored and graded, has been described extensively elsewhere (Hallett & Hall, 2012; Hallett et al., 2012a, b; Hallett, 2014). A brief summary of the steps undertaken to calculate the FCI is provided below.
-
1.
The species abundances in each sample were used to calculate values for each of the fish metrics comprising the nearshore and offshore FCIs (Hallett & Hall, 2012; Hallett et al., 2012a).
-
2.
Metric values were converted to metric scores (0–10) by comparing them with their appropriate ‘best available’ reference conditions, tailored to each region and season (Hallett & Hall, 2012; Hallett et al., 2012b).
-
3.
Metric scores were combined into an index score (0–100) for each sample (Hallett et al., 2012b).
-
4.
The index score was compared to objective, statistically derived thresholds based on the observed distributions of historical index scores (Hallett, 2014) to determine the ecological condition grade for the sample, i.e. A = very good to E = very poor.
For the current study, spatial and temporal patterns in index scores and condition grades during the bloom, summer and autumn periods were assessed visually to examine any effects of the HAB on the ecological integrity of the estuary.
Results
Water quality parameters
Due to the very low rainfall in early autumn of 2004, the water column in the upper reaches of the Swan River was relatively unstratified throughout the study period, as shown by generally negligible differences between the values for temperature and salinity that were measured from the surface and bottom of the water column (supplementary material). The water column was also generally well oxygenated (>3 mg l−1) at all depths throughout March and April (supplementary material; DoW, unpublished data). Maximal water temperatures of 27–30°C were recorded in summer, declining to 21–23°C by autumn, and temperatures in any particular season were fairly uniform across the four studied regions of the estuary (supplementary material). Salinities increased downstream in any given season, and increased from summer to autumn (supplementary material) due to the lack of any notable rainfall during the study period.
Characterising the Karlodinium veneficum bloom
The abundance of K. veneficum in the MD and MU regions initially increased from almost zero to >10,000 cells ml−1 in mid- to late-February 2004, with a subsequent bloom (>25,000 cells ml−1) becoming established throughout March, centred on the MU region (Fig. 2). Peak K. veneficum densities were recorded on March 8th at Success Hill (MU; c. 56,000 cells ml−1), Kingsley Street (MU; c. 34,000 cells ml−1) and Ron Courtney Island (MD; c. 28,000 cells ml−1), with a clear decreasing pattern downstream from the uppermost site of Success Hill. During the study period, K. veneficum densities did not exceed 10,000 cells ml−1 at any site in the LS region and those at the lowermost site, Nile Street, remained near zero. Cell densities in the MU and MD regions declined rapidly in early April, although those at Success Hill remained elevated until April 19th.
Cell densities (cells ml−1) of Karlodinium veneficum from depth-integrated samples of the water column collected at routine monitoring sites in the lower (LS), middle-downstream (MD) and middle-upstream (MU) Swan River regions between January and May 2004. See Fig. 1 for site codes
Catch rates, densities and numbers of species of fish
The mean number of nearshore fish species or densities did not differ significantly among either periods or regions. In contrast, the mean number of offshore species differed significantly between periods (F 2,24 = 5.23, P = 0.013) and regions (F 3,24 = 3.59, P = 0.028), with the influence of the former factor being greater, i.e. mean squares (MS) of 9.0 and 6.2, respectively. Scheffé’s a posteriori test demonstrated that the number of species in the offshore waters was significantly higher during summer than during either the bloom or autumn periods, and that significantly higher values were recorded in the LS region than in the MU region (Fig. 3). The mean number of species in offshore waters declined between the summer and bloom periods in all regions except the LS, and this decrease was particularly pronounced in the MU (i.e. from 4 to 0.3 species; Fig. 3).
Mean catch rates in the offshore waters also differed significantly between periods and regions (F 2,24 = 5.71, P < 0.001 and F 3,24 = 2.72, P = 0.003, respectively), and the interaction between these factors was also significant (F 6,24 = 3.35, P < 0.001). However, period again exerted the largest influence (Fig. 4). Mean catch rates declined by 99.7% between summer and the height of the bloom in the MU region, and by 85 and 50%, respectively, in the adjacent MD and US regions (Fig. 4). In contrast, mean catch rates in the more distant LS region increased roughly five-fold, from 2.7 fish h−1 in summer to 14 fish h−1 during the bloom. By mid-autumn, when the bloom had begun to subside, catch rates in the MU region had increased slightly whilst those in the LS, MD and US regions decreased.
Fish community composition
No significant differences in the composition of the nearshore fish fauna were detected among periods or for the period × region interaction (P = 0.33–0.68). As such, no further multivariate analyses were undertaken on this nearshore dataset.
In contrast, the offshore fish faunal composition differed significantly among periods and regions and the interaction between these factors was significant (P = 0.001–0.002). Each of these terms was equally important in explaining the variability in the fish fauna, as demonstrated by their highly similar COV values (Table 1). Subsequent one-way ANOSIM tests undertaken separately for each period demonstrated that a major cause of the above interaction was the notably different patterns of inter-regional similarities during the bloom than in summer and autumn. This is reflected in Fig. 5, in which the pairwise R statistic values display an expected trend in summer and autumn of being greatest between regions that are relatively far apart (i.e. LS vs US, LS vs MU and MD vs US), whereas this was not always the case in the bloom period. For example, the R values for both the MD versus US and MD versus LS comparisons were far lower in the bloom than in either summer or autumn. It is also of interest to note that in autumn, the pairwise R values for almost all regional comparisons were considerably lower than in summer and that, in contrast to both summer and the bloom, only small ichthyofaunal differences occurred between the MU and US regions (Fig. 5).
The different patterns of inter-regional similarities during the bloom are well summarised by the second-stage nMDS plot (Fig. 6), in which the point representing the bloom period is clearly distinct, not only from those in summer and autumn, but also from those representing independent spring and winter samples. Furthermore, the modified nMDS plot (Fig. 7) specifically demonstrates the notable shift in ichthyofaunal composition in the bloom period and region (MU), with the representative point being markedly distinct from all other period × region combinations. It is also of interest to note that, in both the MD and US regions that are adjacent to the bloom region, the fish compositions in the bloom and subsequent autumn period were more similar than those in the bloom and preceding summer period (Fig. 7).
Modified nMDS ordination plot derived from the Bray–Curtis similarities calculated between averaged samples of the offshore fish fauna in each region of the Swan River during the summer (S), bloom and autumn (A) sampling periods. LS lower Swan River, MD middle-downstream Swan River, MU middle-upstream Swan River, US upper Swan River
The shade plot clearly indicates that the distinctness of samples from the bloom period and region (MU) was due to the fact that they contained almost no fish (Fig. 8). This contrasts markedly with most other samples, where moderate to relatively high numbers of various species were recorded. Figure 8 also highlights the regional shifts in abundance of several species from the summer to bloom periods, and particularly those that were caught in their highest numbers in the MU region during summer. One such species, Acanthopagrus butcheri, increased markedly during the bloom in the US region just upstream of the MU bloom area, reaching almost the maximal abundance on the shading scale. In contrast, others such as Nematalosa vlaminghi and Amniataba caudavittata were recorded in higher numbers in the LS during the bloom than in summer. While the regional patterns of these species during autumn tended to be similar to those in summer, their abundances were consistently lower than before the bloom. Further still, species such as Mugil cephalus, which were recorded in moderate to very high numbers in most regions during summer, were almost entirely absent from all regions during the bloom and were then found only in moderate numbers in the US in autumn. Several other species such as Torquigener pleurogramma, Rhabdosargus sarba and Gerres subfasciatus were recorded almost exclusively during the bloom, but only in the LS and in low to moderate numbers.
Shade plot illustrating differences in the abundance of the most prevalent offshore fish species during the summer, bloom and autumn sampling periods and in each region of the Swan River. LS lower Swan River, MD middle-downstream Swan River, MU middle-upstream Swan River, US upper Swan River. Shading intensity increases with fish abundance on the dispersion-weighted scale (see the shade key). Fish species are ordered by a cluster analysis of their mutual associations across period × region groups
Ecological condition, as quantified by the Fish Community Index
Mean FCI scores for offshore sites in the LS, MD and MU regions ranged between c. 58 and 65 points in summer, which equated to fair to good (C/B to B) ecological condition. However, the condition of offshore waters in the US region was poor (D; mean score = 47) during this period (Fig. 9). By the mid-point of the bloom, the condition across all offshore sites had become poor (D; mean score = 45), driven largely by declines in the MD region (D; mean score = 43) and most notably in the MU region (E; mean score = 17; Fig. 9). In contrast, the ecological condition of offshore waters in the LS and US regions increased slightly from summer to the bloom period, by 2 and 10 points, respectively. During autumn, the offshore FCI scores in each region recovered towards their pre-bloom levels, such that most regained fair to good ecological condition (B/C to B; Fig. 9). However, although the mean FCI score for the MU region increased by 31 points from the bloom period to autumn, the condition of this bloom-affected region remained poor (mean = 47.5; D).
Mean offshore Fish Community Index scores recorded from the lower (LS), middle-downstream (MD), middle-upstream (MU) and upper (US) Swan River regions (and across all sites sampled) during the summer, bloom and autumn periods. The average SE observed across all regions and periods is plotted for clarity, whilst grey lines denote condition grade boundaries
The nearshore FCI scores responded in a far less conspicuous manner than the offshore scores over the summer, bloom and autumn periods. Mean scores in the LS and MD regions were c. 74 in summer, indicating good/very good (B/A) condition prior to the onset of the bloom (Fig. 10). Similarly, the mean score across all nearshore sites in this period was also 74. By the mid-point of the bloom, the nearshore condition of most regions had declined slightly, and far less markedly than in the offshore waters (cf. Figs. 9, 10). Thus, the nearshore waters in the LS and MD regions still remained in good condition (B) at the height of the bloom, with mean scores of 65 and 70, respectively (Fig. 10). Notably, the score at the single nearshore site in the MU region increased from 72 (B) in summer to 81 (A) during the bloom, as the condition of the adjacent offshore waters plummeted. As for the offshore waters, scores in the nearshore waters of each region recovered towards their pre-bloom levels by mid-autumn (Fig. 10).
Mean nearshore Fish Community Index scores recorded from the lower (LS) and middle-downstream (MD) Swan River regions (and across all sites sampled, including the one site from the middle-upstream Swan River region) during the summer, bloom and autumn periods. The average SE observed across all regions and periods is plotted for clarity, whilst grey lines denote condition grade boundaries
Discussion
The timing of the K. veneficum bloom in the upper reaches of the Swan Canning Estuary in March 2004 coincided with an existing fish sampling programme in this system, thus providing a rare opportunity to investigate the potential impacts of such HABs on its fish communities. Given the opportunistic nature of this investigation, two important limitations are apparent. First, in characterising the HAB, we utilised the only available phytoplankton and water quality data, which were recorded independently by the WA DoW in their regular monitoring programme. These data were collected at different spatio-temporal scales and for different purposes than the fish data. The phytoplankton data were derived from depth-integrated samples, precluding any interpretation of lateral (i.e. bank to bank) or vertical patterns in the densities of K. veneficum throughout the estuary during the period of interest. Also, the sampling of water quality during daylight hours only precluded any understanding of diel cycling of DO during the bloom period. Second, the current study lacks a replicated before–after control-impact (BACI) design that would have been required to demonstrate definitively that the bloom caused the observed changes in the fish fauna.
In spite of these limitations, this study provides in the first instance a relatively detailed description of the spatial and temporal characteristics of the K. veneficum bloom. The available phytoplankton data effectively illustrate the longitudinal extent of the bloom throughout the upper Swan Estuary and enable quantification of changes in K. veneficum abundance from summer (i.e. pre-bloom) to autumn (post-bloom), thus providing adequate context for interpreting the spatial and temporal changes in fish communities that accompanied the HAB. Additionally, and despite an inability to demonstrate causality, the changes in the fish community observed during this study represent a pronounced departure from the annual cyclical changes that typically occur in this system, and are thus more likely a consequence of the bloom and its broader effects on the estuary as a whole.
The HAB coincided with marked changes in the fish communities inhabiting the middle and upper reaches of the estuary, including pronounced spatial and temporal changes in the number of species and catch rates of fish in the offshore waters. Multivariate and multimetric analyses revealed further and more subtle responses of the fish communities and broader ecological condition of the system, and particularly of its offshore waters, at the time of the bloom. The remainder of this discussion details evidence of the perceived impacts of the K. veneficum bloom on the fish communities, and evaluates the potential mechanisms and implications of these effects.
Fish communities, fish movements and ecological condition
FCI scores at offshore sites within the bloom-affected MU region of the estuary exhibited a clear decrease from summer to the bloom period, reflecting a decline in ecological condition from fair/good (C/B; mean FCI score of 58) to poor (E; mean FCI score of 17).
Given the absence of a large-scale fish kill during the bloom, these observed decreases in FCI scores, together with concomitant increases in the scores for more distal regions and corroborating evidence of changes in fish abundance, species richness and community composition, suggest that a large proportion of the fish inhabiting the MU region in summer relocated to other regions during the bloom. For example, the peak abundance of Black Bream, A. butcheri, shifted from the MU region in summer to the US region during the HAB, likely reflecting the emigration upstream of this large and highly mobile species away from the bloom centre. Other large and highly mobile species, including N. vlaminghi and A. caudavittata, were recorded in higher numbers in the LS during the bloom than in summer, likely reflecting their emigration downstream to avoid bloom conditions. Such movements undoubtedly contributed to the marked decreases in species richness and catch rates in the MU region to effectively zero during the bloom, and to the pronounced shifts in ichthyofaunal composition over the pre- to post-bloom periods. The available evidence also suggests that several other larger and fast swimming species moved away from the MD and US regions adjacent to the bloom centre, e.g. N. vlaminghi, A. caudavittata, Platycephalus endrachtensis and Pelates octolineatus in the MD and the former two species and M. cephalus in the US. This contributed to the decreases in the mean numbers of species and catch rates in these regions during the HAB and their concomitant increases, particularly in catch rates, in the LS.
The compositions of fish communities in the nearshore and offshore waters of the Swan Canning Estuary generally undergo well-documented cyclical changes throughout the year. These changes reflect seasonal variations in water quality (e.g. salinity and temperature) due largely to highly seasonal rainfall and freshwater discharge into the estuary, as well as differences in the timing of the reproductive cycles and juvenile recruitment of different fish species (Loneragan et al., 1989; Loneragan & Potter, 1990; Hoeksema & Potter, 2006). Fish community composition in the bloom period and region was, however, clearly distinct from that in all other period × region combinations, and second-stage nMDS ordination further showed that the inter-regional similarities in fish composition during the bloom were highly dissimilar to any of those in the standard seasonal cycle from summer to spring. Given that, with the exception of the bloom period, environmental conditions during the remainder of the study were fairly typical for the time of year and not markedly extreme in any known respect (e.g. pronounced stratification of the water column), our findings provide strong circumstantial evidence that the bloom was responsible for the pronounced changes in fish community composition and spatial distribution observed at that time.
Following the collapse of the bloom, there were various indications that some of the fish that had emigrated from the MU during the bloom subsequently returned by mid-autumn (c. 3–4 weeks later), suggesting a degree of ecological recovery. For example, A. butcheri once again characterised the faunas in this region yet no longer characterised those of the US where it was prevalent during the bloom, and offshore FCI scores for the MD and MU regions increased from the bloom period to autumn. Nonetheless, it was also apparent that some of the effects of the HAB still persisted in mid-autumn. For example, in both the MD and US regions adjacent to the bloom’s centre, fish faunal compositions in this season were more similar to those during the bloom than in the preceding summer period. Moreover, the mean number of species, catch rates and offshore FCI scores in these regions and/or in the MU had not yet returned to pre-bloom levels.
Although the available phytoplankton data precluded any understanding of lateral or vertical patterns in K. veneficum abundance, the lack of any significant period differences in fish species richness, catch rates or community composition in the nearshore waters, allied with the far less pronounced decrease in nearshore than offshore FCI scores during the bloom, indicates that the effects of the HAB were far more severe in the deeper waters of the estuary. These findings are consistent with existing evidence that the offshore waters of the Swan Canning Estuary are generally in poorer ecological condition, and are more susceptible to the effects of perturbations such as algal blooms, than the adjacent shallower habitats (Hallett, 2010; Cottingham et al., 2014). This may be attributable to several factors, but is most likely driven by the more frequent stratification and deoxygenation of those deeper waters. Moreover, the slight increase in the nearshore FCI score in the MU during the bloom, paralleled with the precipitous drop in the accompanying offshore FCI scores, supports the contention that the shallows act as refugia for fish escaping inhospitable conditions in the deeper waters.
The types of movement responses exhibited by the fish fauna in the Swan Canning Estuary during the 2004 HAB are similar to those noted by many other authors in response to algal blooms and their associated stressors, including hypoxia. For example, Potter et al. (1983) demonstrated that larger and more active fish species emigrated from areas affected by blooms of the blue-green alga Nodularia spumigena in the Peel-Harvey Estuary, Western Australia, and Lamberth et al. (2010) documented the movement of numerous fish species into normoxic refuges in the Berg River Estuary, South Africa, following a low DO event linked to a non-toxic ‘red tide’ in the adjacent marine environment. There is a growing body of evidence that many more mobile fish species are able to detect and actively avoid hypoxia. For example, Eby & Crowder (2002, 2004) noted that several fish species avoided hypoxic zones associated with high primary productivity in the Neuse River Estuary (USA) and instead remained in shallower, more highly oxygenated waters, only returning to previously hypoxic areas once conditions improved. Similar behavioural patterns were documented for demersal fish by Pihl et al. (1991) in the lower York River, Chesapeake Bay and by Craig (2012) in the Gulf of Mexico.
The above avoidance behaviours can also lead to ‘habitat compression’ effects, which may have significant physiological and ecological implications (Wannamaker & Rice, 2000; Wu, 2002; Kidwell et al., 2009; Brady & Targett, 2013). For example, elevated fish densities in refuge areas during perturbation events may lead to detrimental consequences for benthic habitat quality and trophic interactions, sublethal physiological impacts on fish health and growth (Eby & Crowder, 2002; Craig & Crowder, 2005; Eby et al., 2005), and ultimately have broader effects on ecosystem function, productivity and fisheries (Breitburg, 2002; Baird et al., 2004; Craig, 2012). The longitudinal and, to a lesser extent, lateral movements of fish that were observed during the HAB in the current study indicate that such habitat compression also occurs in the Swan Canning Estuary, albeit on a smaller scale than some of the above examples. It is thus highly relevant that the abundance of Black Bream in the deeper offshore waters of this system has declined between the mid 1990s and late 2000s whilst that in the shallow nearshore waters has increased, reflecting a perceived decline in the ecological condition of the former environment (Cottingham et al., 2014). The consequent density-dependent effects of this nearshore habitat compression are thought to have contributed to the notable declines in growth and body condition of this species (Cottingham et al., 2014).
Potential stressors associated with the 2004 K. veneficum bloom
It is often difficult to tease apart the roles of the numerous and largely confounded stressors that may accompany HAB events, and thus to determine the definitive cause of any fish avoidance responses or mortalities. K. veneficum may induce fish mortalities via both direct and indirect means, and blooms of this species have caused recorded fish kill events throughout the world (Deeds et al., 2002; Kempton et al., 2002; Hallegraeff et al., 2011), including in the Swan Canning Estuary (Hallett et al., 2012b; Place et al., 2012; Adolf et al., 2015). Indeed, approximately 300,000 fish were estimated to have died in this system during autumn and winter of 2003, following blooms of this species in the upper estuary (Kidd & Srdarev, 2003; Adolf et al., 2005). K. veneficum can be toxic to fish if algal cells lyse and release karlotoxin, a group of compounds which target the chloride cells in the gill tissues of fish and cause cellular lysis (Deeds et al., 2002; Mooney et al., 2010; Place et al., 2012). This dinoflagellate may also kill fish by clogging their gills and causing asphyxia (Deeds et al., 2002). Furthermore, K. veneficum blooms may indirectly lead to fish deaths via deoxygenation of the water column, which often occurs due to the sharp increases in biological oxygen demand associated with nocturnal respiration of the bloom and/or enhanced microbial activity during decomposition of a senescing bloom (Paerl et al., 1998; Anderson et al., 2012). This can lead to the development of hypoxic and even anoxic conditions, particularly at night and in the bottom waters of frequently-stratified estuaries (Paerl et al., 1998; Zhang et al., 2010), of which the Swan Canning is an example. K. veneficum blooms do not always result in fish kills, however, as shown by a study in Maryland, USA, where densities approached 1,000,000 cells ml−1 but did not lead to any known fish mortalities (Place et al., 2012).
Some species suffered mortality during the 2004 bloom in the Swan Canning Estuary, and approximately 170 dead fish, many of them Black Bream, were collected from the MU region where K. veneficum was most dense (T. Rose and J. Latchford, Water and Rivers Commission, pers. comm.). However, the scale of the fish kill that accompanied this HAB was comparatively negligible, and particularly so when compared to that in 2003. It is therefore unlikely that the observed ichthyofaunal responses in the present study were related to a significant release of karlotoxin, but more likely that various indirect effects of the HAB interacted to reduce the habitability of the bloom-affected region.
Indications from the individual metrics comprising the offshore FCI showed that the declines in ecological condition from summer to the bloom period in the MU and MD regions were driven by marked decreases in species diversity and the proportion of individuals belonging to benthic species, and by increases in the proportion of detritivores. In contrast, the increases in offshore FCI scores for the LS and US regions over the same period reflected an increase in the proportion of benthic individuals (Hallett, unpublished data). These patterns in the benthic species abundance suggest a putative role of bottom-water hypoxia as a stressor mechanism mediating fish community responses during the bloom. It is likely that respiration of the bloom and oxidative decomposition of settling organic matter in the MU region would have led to hypoxic bottom waters, at least nocturnally in the absence of photosynthesis (Tyler et al., 2009). While multivariate analyses [BIOENV (Clarke & Ainsworth, 1993; Clarke et al., 2008), data not shown] did not detect a significant correlation between the spatial patterns in offshore fish communities and those in DO concentrations recorded during fish sampling (although they did with chlorophyll a concentration in the surface and bottom waters and temperature in the latter, i.e. P = 0.001; ρ = 0.871), this most likely reflects the fact that these water quality measurements were made soon after nightfall and thus before hypoxia would have become established. Similarly, the generally well-oxygenated conditions (>3 mg l−1) recorded during the bloom period (supplementary material; DoW, unpublished data) only reflect day-time conditions and thus do not account for any bloom-induced hypoxia that may have developed throughout the night. The future characterization of diel cycling of DO concentrations via high-frequency measurements at multiple locations (Tyler et al., 2009) is essential to better understand the effects of algal blooms on oxygen dynamics in this system, and thus to determine whether hypoxia is responsible for observed HAB effects on its fish communities.
Although the specific causal mechanism(s) underlying the observed fish responses to this HAB remain unclear, it is evident that the bloom coincided with numerous significant changes in the ichthyofauna and the broader ecological condition of the offshore waters of the estuary. Given that rainfall and river flows in south-western Australia are predicted to continue to decline in coming decades (CSIRO, 2007), and that the prevalence of K. veneficum is thought to be linked with stratification of the water column that persists under dryer conditions (Hallegraeff et al., 2011; Cosgrove et al., 2015), the frequency and ecological impacts of these HABs are liable to intensify in this and similar microtidal systems that are susceptible to stratification (Acharyya et al., 2012).
Conclusion
This study has documented both conspicuous and more subtle, species-specific responses among the fish communities in the microtidal Swan Canning Estuary during a notable bloom of the dinoflagellate K. veneficum. These included (i) marked reductions in the number of species and catch rates, (ii) significant changes in community composition and the spatial distribution of more mobile fish species and (iii) declines and partial recovery of the broader ecological condition of the estuary, as measured by a FCI. Examination of these fish responses using multiple lines of evidence spanning univariate, multivariate and multimetric index approaches provides a fuller, more nuanced picture of both community-level and species-specific effects resulting from the HABs that frequently impact eutrophic estuaries.
References
Acharyya, T., V. V. S. S. Sarma, B. Sridevi, V. Venkataramana, M. D. Bharathi, S. A. Naidu, B. S. K. Kumar, V. R. Prasad, D. Bandyopadhyay, N. P. C. Reddy & M. D. Kumar, 2012. Reduced river discharge intensifies phytoplankton bloom in Godavari estuary, India. Marine Chemistry 132–133: 15–22.
Adolf, J. E., T. R. Bachvaroff, J. R. Deeds, A. Begum, W. Hosja, T. Reitsema, P. Ringeltaube, M. Robb & A. R. Place, 2005. Ichthyotoxic Karlodinium micrum in the Swan River Estuary (Western Australia): an emerging threat in a highly eutrophic estuarine system. 3rd Symposium on Harmful Algae in the US, Asilomar, California.
Adolf, J. E., T. R. Bachvaroff, H. A. Bowers, J. R. Deeds & A. R. Place, 2015. Ichthyotoxic Karlodinium veneficum in the Upper Swan River estuary (Western Australia): synergistic effects of karlotoxin and hypoxia leading to a fish kill. Harmful Algae (in press).
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Anderson, D. M., A. D. Cembella & G. M. Hallegraeff, 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring and management. Annual Reviews in Marine Science 4: 143–176.
Anderson, D. M., P. M. Glibert & J. M. Burkholder, 2002. Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25: 704–726.
Anderson, D. M., J. M. Burkholder, W. P. Cochlan, P. M. Glibert, C. J. Gobler, C. A. Heil, R. M. Kudela, M. L. Parsons, J. E. J. Rensel, D. W. Townsend, V. L. Trainer & G. A. Vargo, 2008a. Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39–53.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008b. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth.
Baird, D., R. R. Christian, C. Peterson & G. A. Johnson, 2004. Consequences of hypoxia on estuarine ecosystem function: energy diversion from consumers to microbes. Ecological Applications 14: 805–822.
Borja, A., A. Basset, S. Bricker, J.-C. Dauvin, M. Elliott, T. Harrison, J.-C. Marques, S. Weisberg & R. West, 2012. Classifying ecological quality and integrity of estuaries. In Wolanski, E. & D. McLusky (eds), Treatise on Estuarine and Coastal Science. Academic Press, Waltham: 125–162.
Brady, D. C. & T. E. Targett, 2013. Movement of juvenile weakfish Cynoscion regalis and spot Leiostomus xanthurus in relation to diel-cycling hypoxia in an estuarine tidal tributary. Marine Ecology Progress Series 491: 199–219.
Breitburg, D. L., 2002. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries and Coasts 25: 767–781.
Burkholder, J. M. & H. B. Glasgow, 2001. History of toxic Pfiesteria in North Carolina estuaries from 1991 to the present. Bioscience 51: 827–841.
Burkholder, J. M., E. J. Noga, C. H. Hobbs & H. B. Glasgow, 1992. New ‘phantom’ dinoflagellate is the causative agent of major estuarine fish kills. Nature 358: 407–410.
Chan, T. U., D. P. Hamilton, B. J. Robson, B. R. Hodges & C. Dallimore, 2002. Impacts of hydrological changes on phytoplankton succession in the Swan River, Western Australia. Estuaries 25: 1406–1415.
Clarke, K. R. & M. Ainsworth, 1993. A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series 92: 205–219.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth.
Clarke, K. R. & R. H. Green, 1988. Statistical design and analysis for a ‘biological effects’ study. Marine Ecology Progress Series 46: 213–226.
Clarke, K. R. & R. M. Warwick, 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed. PRIMER-E, Plymouth.
Clarke, K. R., M. G. Chapman, P. J. Somerfield & H. R. Needham, 2006a. Dispersion-based weighting of species counts in assemblage analyses. Marine Ecology Progress Series 320: 11–27.
Clarke, K. R., P. J. Somerfield, L. Airoldi & R. M. Warwick, 2006b. Exploring interactions by second-stage community analyses. Journal of Experimental Marine Biology and Ecology 338: 179–192.
Clarke, K. R., P. J. Somerfield & M. G. Chapman, 2006c. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. Journal of Experimental Marine Biology and Ecology 330: 55–80.
Clarke, K. R., P. J. Somerfield & R. N. Gorley, 2008. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366: 56–69.
Clarke, K. R., J. R. Tweedley & F. J. Valesini, 2013. Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. Journal of the Marine Biological Association of the United Kingdom 94: 1–16.
Commonwealth of Australia, 2002. Australian Catchment, River and Estuary Assessment 2002, Vol. 1. National Land and Water Resources Audit, Canberra.
Cosgrove, J., S. Hoeksema & A. Place, 2015. Ichthyotoxic Karlodinium cf. veneficum in the Swan-Canning Estuarine system (Western Australia): towards management through understanding. Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 2014.
Cottingham, A., S. A. Hesp, N. G. Hall, M. R. Hipsey & I. C. Potter, 2014. Changes in condition, growth and maturation of Acanthopagrus butcheri (Sparidae) in an estuary reflect the deleterious effects of environmental degradation. Estuarine, Coastal and Shelf Science 149: 109–119.
Cox, T. F. & M. A. A. Cox, 2001. Multidimensional Scaling, 2nd ed. Chapman and Hall, London.
Craig, J. K., 2012. Aggregation on the edge: effects of hypoxia avoidance on the spatial distribution of brown shrimp and demersal fishes in the Northern Gulf of Mexico. Marine Ecology Progress Series 445: 75–95.
Craig, J. K. & L. B. Crowder, 2005. Hypoxia-induced habitat shifts and energetic consequences in Atlantic croaker and brown shrimp on the Gulf of Mexico shelf. Marine Ecology Progress Series 294: 79–94.
CSIRO – Commonwealth Scientific and Industrial Research Organisation, 2007. Climate change in Australia – Technical Report 2007 [available on internet at http://www.climatechangeinaustralia.gov.au; Accessed 20 June 2014].
Deeds, J. R., D. E. Terlizzi, J. E. Adolf, D. K. Stoecker & A. R. Place, 2002. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae) - a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 1: 169–189.
Eby, L. A. & L. B. Crowder, 2002. Hypoxia-based habitat compression in the Neuse River estuary: context-dependent shifts in behavioral avoidance thresholds. Canadian Journal of Fisheries and Aquatic Sciences 59: 952–965.
Eby, L. A. & L. B. Crowder, 2004. Effects of hypoxic disturbances on an estuarine nekton assemblage across multiple scales. Estuaries 27: 342–351.
Eby, L. A., L. B. Crowder, C. M. McClellan, C. H. Peterson & M. J. Powers, 2005. Habitat degradation from intermittent hypoxia: impacts on demersal fishes. Marine Ecology Progress Series 291: 249–261.
Fu, F. X., A. O. Tatters & D. A. Hutchins, 2012. Global change and the future of harmful algal blooms in the ocean. Marine Ecology Progress Series 470: 207–233.
Gerritse, R. G., P. J. Wallbrink & A. S. Murray, 1998. Accumulation of phosphorous and heavy metals in the Swan-Canning Estuary, Western Australia. Estuarine, Coastal and Shelf Science 47: 165–179.
Gibson, G. R., M. L. Bowman, J. Gerritsen & B. D. Snyder, 2000. Estuarine and coastal marine waters: bioassessment and biocriteria technical guidance. USEPA report 822-B-00-024, Office of Water, Washington DC.
Glasgow, H. B. & J. M. Burkholder, 2000. Water quality trends and management implications for a five-year study of a eutrophic estuary. Ecological Applications 10: 1024–1046.
Glibert, P. M., R. Magnien, M. W. Lomas, J. Alexander, C. Fan, E. Haramoto, M. Trice & T. M. Kana, 2001. Harmful algal blooms in the Chesapeake and coastal bays of Maryland, USA: comparison of 1997, 1998, and 1999 events. Estuaries 24: 875–883.
Glibert, P. M., D. M. Anderson, P. Gention, E. Graneli & K. G. Sellner, 2005. The global, complex phenomena of harmful algal blooms. Oceanography 18: 130–141.
Hallegraeff, G., B. Mooney, K. Evans & W. Hosja, 2011. What triggers fish-killing Karlodinium veneficum dinoflagellate blooms in the Swan Canning River system? Swan Canning Research and Innovation Program Final Report, SRT Project no. RSG09TAS01. University of Tasmania, Hobart.
Hallett, C. S., 2010. The development and validation of an estuarine health index using fish community characteristics. Ph.D. thesis, Murdoch University, Perth.
Hallett, C. S., 2014. Quantile-based grading improves the effectiveness of a multimetric index as a tool for communicating estuarine condition. Ecological Indicators 39: 84–87.
Hallett, C. S. & N. G. Hall, 2012. Equivalence factors for standardising catch data across multiple beach seine nets to account for differences in relative bias. Estuarine, Coastal and Shelf Science 104–105: 114–122.
Hallett, C. S., F. J. Valesini & K. R. Clarke, 2012a. A method for selecting health index metrics in the absence of independent measures of ecological condition. Ecological Indicators 19: 240–252.
Hallett, C. S., F. J. Valesini, K. R. Clarke, S. A. Hesp & S. D. Hoeksema, 2012b. Development and validation of a fish-based, multimetric index for assessing the ecological health of Western Australian estuaries. Estuarine, Coastal and Shelf Science 104–105: 102–113.
Harper, D., 1992. Eutrophication of Freshwaters - Principles, Problems and Restoration. Chapman and Hall, New York.
Hoeksema, S. D. & I. C. Potter, 2006. Diel, seasonal, regional and annual variations in the characteristics of the ichthyofauna of the upper reaches of a large Australian microtidal estuary. Estuarine, Coastal and Shelf Science 67: 503–520.
Horner Rosser, S. M. J. & P. A. Thompson, 2001. Phytoplankton of the Swan-Canning Estuary: a comparison of nitrogen uptake by different bloom assemblages. Hydrological Processes 15: 2579–2594.
Hosja W. & D. Deeley, 1994. Harmful phytoplankton surveillance in Western Australia. Waterways Commission Report No. 43. Waterways Commission, Perth.
Jakowyna, B., R. Donohue & M. Robb, 2000. River Science 6 - The Delivery of Nutrients to the Swan and Canning Rivers has Changed Over Time. Water and Rivers Commission, Perth.
Karr, J. R., 1981. Assessment of biotic integrity using fish communities. Fisheries 6: 21–27.
Kempton, J. W., A. J. Lewitus, J. R. Deeds, J. M. Law & A. R. Place, 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae 1: 233–241.
Kidd, A. & R. Srdarev, 2003. Integrated phytoplankton status: Swan River Estuary sites 1–9, 9th June 2003. Phytoplankton Report. Phytoplankton Ecology Unit, Department of Environment, Perth.
Kidwell, D. M., A. J. Lewitus, E. B. Jewett, S. Brandt & D. M. Mason, 2009. Ecological impacts of hypoxia on living resources. Journal of Experimental Marine Biology and Ecology 381: S1–S3.
Kruskal, J. B., 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29: 1–27.
Lamberth, S. J., G. M. Branch & B. M. Clark, 2010. Estuarine refugia and fish responses to a large, anoxic, hydrogen sulphide “black tide” event in the adjacent marine environment. Estuarine, Coastal and Shelf Science 86: 203–215.
Landsberg, J. H., 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science 10: 113–390.
Levin, P. S. & M. E. Hay, 2003. Selection of estuarine habitats by juvenile gags in experimental mesocosms. Transactions of the American Fisheries Society 132: 76–83.
Loneragan, N. R. & I. C. Potter, 1990. Factors influencing community structure and distribution of different life-cycle categories of fishes in shallow waters of a large Australian estuary. Marine Biology 106: 25–37.
Loneragan, N. R., I. C. Potter & R. C. J. Lenanton, 1989. Influence of site, season and year on contributions made by marine, estuarine, diadromous and freshwater species to the fish fauna of a temperate Australian estuary. Marine Biology 103: 461–479.
Mooney, B. D., A. R. Place & G. M. Hallegraeff, 2010. Ichthyotoxicity of four species of gymnodinioid dinoflagellates (Kareniaceae, Dinophyta) and purified karlotoxins to larval sheepshead minnow. Harmful Algae 9: 557–562.
Paerl, H. W., J. L. Pinckney, J. M. Fear & B. L. Peierls, 1998. Ecosystem responses to internal and watershed organic matter loading: consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Marine Ecology Progress Series 166: 17–25.
Pérez-Domínguez, R., S. Maci, A. Courrat, M. Lepage, A. Borja, A. Uriarte, J. M. Neto, H. Cabral, V. St. Raykov, A. Franco, M. C. Alvarez & M. Elliott, 2012. Current developments on fish-based indices to assess ecological-quality status of estuaries and lagoons. Ecological Indicators 23: 34–45.
Pihl, L., S. P. Baden & R. J. Diaz, 1991. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Marine Biology 108: 349–360.
Place, A. R., H. A. Bowers, T. R. Bachvaroff, J. E. Adolf, J. R. Deeds & J. Sheng, 2012. Karlodinium veneficum - the little dinoflagellate with a big bite. Harmful Algae 14: 179–195.
Portnoy, J. W., 1991. Summer oxygen depletion in a diked New England estuary. Estuaries 14: 122–129.
Potter, I. C., N. R. Loneragan, R. C. J. Lenanton & P. J. Chrystal, 1983. Blue-green algae and fish population changes in a eutrophic estuary. Marine Pollution Bulletin 14: 228–233.
Rate, A., A. E. Robertson & A. T. Borg, 2000. Distribution of heavy metals in near-shore sediments of the Swan River Estuary, Western Australia. Water, Air and Soil Pollution 124: 155–168.
Shepard, R. N., 1962. The analysis of proximities: multidimensional scaling with an unknown distance function. Psychometrika 27: 125–140.
Smith, V. H., 2003. Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environmental Science and Pollution Research International 10: 126–139.
Somerfield, P. J. & K. R. Clarke, 2013. Inverse analysis in non-parametric multivariate analyses: distinguishing groups of associated species which covary coherently across samples. Journal of Experimental Marine Biology and Ecology 449: 261–273.
SRT – Swan River Trust, 2009. Swan Canning Water Quality Improvement Plan. Swan River Trust, Perth [available on internet at http://www.swanrivertrust.wa.gov.au; Accessed 25 Nov 2014].
Szedlmayer, S. T. & K. W. Able, 1996. Patterns of seasonal availability and habitat use by fishes and decapod crustaceans in a southern New Jersey estuary. Estuaries 19: 697–709.
Thompson, P. A., 1998. Spatial and temporal patterns of factors influencing phytoplankton in a salt wedge estuary, the Swan River, Western Australia. Estuaries 21: 801–817.
Thompson, P. A. & W. Hosja, 1996. Nutrient limitation of phytoplankton in the upper Swan River Estuary. Marine and Freshwater Research 47: 659–667.
Twomey, L. & J. John, 2001. Effects of rainfall and salt-wedge movement on phytoplankton succession in the Swan-Canning Estuary, Western Australia. Hydrological Processes 15: 2655–2671.
Tyler, R. M., D. C. Brady & T. E. Targett, 2009. Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuaries and Coasts 32: 123–145.
Wannamaker, C. M. & J. A. Rice, 2000. Effects of hypoxia on movements and behaviour of selected estuarine organisms from the southeastern United States. Journal of Experimental Marine Biology and Ecology 249: 145–163.
Whitfield, A. K. & M. Elliott, 2002. Fishes as indicators of environmental and ecological change within estuaries: a review of progress and some suggestions for the future. Journal of Fish Biology 61(Suppl A): 229–250.
Whittaker, R. H., 1952. A study of summer foliage insect communities in the Great Smoky Mountains. Ecological Monographs 22: 1–44.
Wu, R. S. S., 2002. Hypoxia: from molecular responses to ecosystem responses. Marine Pollution Bulletin 45: 35–45.
Zhang, H., S. A. Ludsin, D. M. Mason, A. T. Adamack, S. B. Brandt, X. Zhang, D. G. Kimmel, M. R. Roman & W. C. Boicourt, 2010. Hypoxia-driven changes in the behaviour and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. Journal of Experimental Marine Biology and Ecology 381: S80–S91.
Acknowledgments
Thanks are due in particular to Ray Gorley of PRIMER-E for his coding of the shade plots and modified nMDS algorithms in the developmental PRIMER software. Gratitude is expressed to the WA Department of Fisheries and Murdoch University for funding this research. KRC acknowledges his honorary adjunct and fellowship positions at Murdoch University and Plymouth Marine Laboratory. This work was carried out under Murdoch University animal ethics permit number W1006/03 and under permits from the WA Department of Fisheries and the WA Department of Environment and Conservation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Gideon Gal
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hallett, C.S., Valesini, F.J., Clarke, K.R. et al. Effects of a harmful algal bloom on the community ecology, movements and spatial distributions of fishes in a microtidal estuary. Hydrobiologia 763, 267–284 (2016). https://doi.org/10.1007/s10750-015-2383-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2383-1