Abstract
Anchialine ecosystems are groundwater-fed brackish coastal habitats that contain high percentages of endemic fauna and are at risk from anthropogenic stressors, introduced species, and sea level rise. Data on endemic species distribution, habitat condition, and species/habitat interactions in this ecosystem are scarce across large spatial scales. This study offers the most thorough regional perspective on anchialine pool habitat characteristics along the western and southern coastlines of the island of Hawaii since the 1970s. Daytime surveys of 398 anchialine pools documented the widespread distribution of two dominant endemic shrimp Halocaridina rubra and Metabetaeus lohena in a wide range of habitats. Introduced fishes (tilapia, poeciliids) were present in about 25% of pools. Generalized additive models were used to determine the relationship between shrimp occurrence and pool characteristics, invasive species, water properties, and land use. Introduced fishes had a strong negative effect on the occurrence of H. rubra and M. lohena. High benthic silt cover and adjacent development also had significant negative relationships with shrimp occurrence. Our results indicate that conservation efforts should include controlling introduced fishes, preventing new introductions, minimizing siltation, and protecting groundwater resources and low-lying coastal areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last century, multiple anthropogenic stressors including invasive species, elevated nutrient loads, and destructive land-use practices have caused widespread coastal aquatic ecosystem degradation and native biodiversity loss (Vitousek et al., 1997; Paine et al., 1998; Foley et al., 2005; Halpern et al., 2008). However, perturbation effects on endemic species can be difficult to determine due to natural variability, ecosystem complexity (Paine et al., 1998), and synergistic effects (Crain et al., 2008; Ormerod et al., 2010; Piggott et al., 2012). Examining multiple variables within a high number of habitats has proved useful for identifying the relative importance of various factors on endemic species occurrence (Knapp et al., 2003, Miro & Ventura, 2013).
Anchialine habitats are brackish, tidally influenced coastal water bodies without surface connection to the ocean (Holthuis, 1973). These ecosystems include caves and open pool habitats and are common in tropical coastal areas including Hawaii, the Indo-Pacific (Holthuis, 1973; Maciolek, 1983; Webb et al., 2010), and the Yucatan (Sanchez et al., 2002) where porous substrates, such as karst or lava, provide good hydrologic connectivity between groundwater and the ocean. Anchialine ecosystems in the United States exist only in Puerto Rico and Hawaii where they are prevalent on the younger islands of Maui and Hawaii (Brock & Kam, 1997). The global biological diversity of these habitats is just beginning to be recognized with more than 450 new species of anchialine organisms discovered and described in the past 25 years (Iliffe & Kornicker, 2009; Anker, 2010; Weese, 2012).
Hawaiian anchialine habitats support diverse endemic biota, including seven species listed as candidates for protection under the Endangered Species Act (US Fish and Wildlife, 2015). Anchialine pools are typically devoid of macroalgae, have clear water, and may include a unique microbial mat that covers the basalt substrate (Bailey-Brock & Brock, 1993). Pools may occur in bare basalt substrate with no associated vegetation or on older lava flows surrounded by trees or wetland vegetation (NPS, 2012). The endemic red shrimp Halocaridina rubra is a dominant species within this ecosystem with densities up to thousands of individuals per m2 (Sakihara et al., 2015). Halocaridina rubra graze on algae and diatoms are thought to keep macroalgae in check (Brock & Kam, 1997). Metabetaeus lohena is a candidate endangered shrimp that preys on H. rubra and other invertebrates (Holthuis, 1973). Other endemic shrimp as well as gastropods are occasionally seen in pools, with some rare species found in highly localized distributions (Maciolek & Brock, 1974; Chai et al., 1989; Sakihara, 2012). The larvae of H. rubra and other shrimp species may disperse as oceanic plankton, and all life stages are assumed to move through subterranean groundwater (Craft et al., 2008). Interestingly, Santos (2006) found high genetic structure among populations of H. rubra on the west coast of Hawaii indicating barriers to dispersal. However, M. lohena populations showed little evidence for genetic structure (Russ et al., 2010). Anchialine gastropods are thought to have the same mechanisms of dispersal (Kano & Kase, 2004).
The negative effects of introduced fish species on native biodiversity and habitat have been well documented in aquatic ecosystems (Gozlan et al., 2010; Cucherousset & Olden, 2011). Introduced predatory fishes such as tilapia (Oreochromis mossambicus and other species) and poeciliids (Poecilia reticulata, Gambusia affinis), along with the invasive prawn, Macrobrachium lar, are thought to be a primary cause of pool degradation in Hawaii where these taxa prey on the herbivorous H. rubra (Chai et al., 1989; Capps et al., 2009; Carey et al., 2010). Reduction of grazing by H. rubra has been suggested as the primary mechanism for ecosystem phase shifts resulting in rapid macroalgal accumulation and pool senescence (Brock & Kam, 1997).
Groundwater nutrient loading may also be a compounding factor in anchialine pool degradation. Nutrients and other pollutants can leach through porous basalt substrate into groundwater and flow into anchialine pools (Knee et al., 2008). Monitoring has shown statistically significant nutrient increases in pools that are in proximity to developments after project build-out (Brock et al., 1987; Brock & Kam, 1997; Weigner et al., 2006). Dalton et al. (2012) found that anchialine pools with poeciliids and higher nutrient loads had higher epilithon biomass. The long-term effects of elevated nutrients on anchialine pools, especially when combined with introduced predators, are presently unknown.
Since the initial island-wide survey of anchialine pools was completed in 1973 (Maciolek & Brock, 1974), shoreline development has modified Hawaii’s coastal environment, and nonnative species have invaded many pools (Brock & Kam, 1997). The primary goal of this study was to determine the effects of a wide variety of biotic and abiotic factors on the distribution of the dominant endemic shrimp species within anchialine pool habitats on Hawaii Island. Specifically, the objective was to determine the relative importance of pool characteristics, invasive species, water properties, and land use on endemic shrimp H. rubra and M. lohena occurrence. The presence of rarer endemic species was also documented. An understanding of the relationships between habitat characteristics, and the occurrence of endemic anchialine shrimp species is essential for prioritization of successful ecosystem restoration.
Materials and methods
Study area
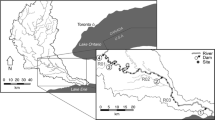
This study occurred along the Ala Kahakai National Historic Trail (Trail) corridor on the arid western and southern coastlines of the island of Hawaii (Fig. 1a). Annual rainfall in the study area ranges from 25 to 50 cm per year (Giambelluca et al., 2011). Ala Kahakai National Historic Trail passes through Kaloko-Honokōhau National Historical Park, Pu‘ukohola Heiau National Historical Site, Pu‘uhonua O Honaunau National Historical Park, and Hawai‘i Volcanoes National Park, as well as numerous state and county parks and private lands (Fig. 1a). These areas encompass one of the highest concentrations of anchialine pools in the world (Brock & Kam, 1997). Previous anchialine pool habitat assessment studies along the Trail corridor include localized baseline inventories on state conservation and national park lands (Chai et al., 1989; Brock & Kam, 1997; NPS, 2012; Sakihara, 2012), and state-mandated water quality monitoring near resort developments (Weigner et al., 2006). The most spatially extensive survey occurred in 1972–1973 (Maciolek & Brock, 1974; Fig. 1b).
Maps showing the (a) Island of Hawaii with locations of the five National Parks and Manuka Natural Area Reserve (State of Hawaii) shown, and (b) the distribution of anchialine pools within the Ala Kahakai National Historic Trail. Pools marked 2009–2013 are NPS surveys included in the study. Pools indicated as 1972 were documented by Maciolek & Brock (1974) but were not surveyed for this study. Parentheses indicate the number of pools surveyed at each location
Anchialine pool surveys
Data describing the habitat and faunal characteristics of 330 anchialine pools were collected during daylight hours on single site visits from July 12 to August 10, 2012 and July 2 to July 15, 2013. Some of the pools had not been surveyed since the 1970s (Maciolek & Brock, 1974) or had never been formally documented. Data for an additional 68 pools were added from previous surveys conducted by the National Park Service (NPS) between 2007 and 2009 (Jones et al., 2011; NPS, 2012). Pools were located within private, state conservation, and national park boundaries and were in proximity to a range of land uses including undeveloped, residential, resort, and urban. The objective was to visit as many pools as possible along the Trail, therefore we used maps, reports, and interviews with locals to identify sites. Geospatial layers of sites visited during the 1970s (Nature Conservancy, 1987) confirms that we revisited many of the same sections of coastline as Maciolek & Brock (1974; Fig. 1b). However, due to difficult or restricted access, we were not able to sample all known pools along the southern and western coastlines (Fig. 1b). Data collected for each pool included physical attributes, water properties, the presence of plants and animals, and land use.
Physical attributes
Physical attributes included location, surface area, maximum depth, substrate, and distance from the shoreline. Pool surface area was determined within ESRI’s ArcGIS 10.0 using a polygon of the pool perimeter collected in the field with a Trimble GeoXH Global Positioning System (GPS). Surface area for small pools or those with high canopy cover was calculated as pool length × width measured with a transect tape at the high water line. Maximum depth was recorded at each pool at the time of the survey. Substrate was categorized as percent cover of rock, sand, and silt using visual estimates. Distance from coastal shoreline was calculated in ArcGIS as the Euclidian distance between the shoreline and the closest edge of each pool.
Water properties
Salinity, pH, temperature, dissolved oxygen (DO), turbidity, and Chlorophyll a (Chl a) were collected at pools with a portable sonde (YSI 6500 or Hydrolab Quanta). Water measurements were collected at the surface of each pool to standardize for possible changes of salinity with depth (Jones et al., 2011). Recognizing that our water measurements represent a one-time snapshot, we assessed variability in surface water salinities within a subset of pools for inter-annual (n = 18 pools) and daily (n = 11 pools) time frames. Previous work has indicated that although pools may exhibit variability in water properties with depth (Holthuis, 1973; Havird et al., 2014), the surface of each pool remains relatively constant on annual and daily timescales (Bienfang et al., 2011; NPS, 2012).
Plant and animal species
Endemic pool species, including the shrimp H. rubra and M. lohena, as well as introduced fishes and the prawn M. lar were recorded as present or absent based on visual surveys during daylight hours. P. reticulata, Poecilia mexicana, and G. affinis were present in pools but were not always distinguishable in the field so were combined as poeciliids for statistical analysis. Tilapia species were also grouped together for this study. Oreochromis mossambicus probably makes up the majority of tilapia found on the island of Hawaii, but this majority is not certain because other tilapia species exist on Oahu and Kauai, and they are difficult to identify as juveniles (Mackenzie & Bruland, 2012). Kuhlia sandvicensis was the most common reef fish found in anchialine pools and was included in the analysis. Other reef fish were seen in less than 10 pools and were not included in the study.
Most pools were small and shallow enough to visually examine the entire water body from the pool edges. Larger pools, where the center and bottom were not visible from the surface, were examined using mask and snorkel. Macroalgae were recorded as present or absent. Terrestrial vegetation associated with pools was documented including visual estimates of percent canopy cover by species, percent cover of emerging vegetation by species, and percent cover of plants within 0.5 meters of pool periphery by species. Percent cover of vegetation was estimated in 10% increments or as less than 1% by a single observer.
Imperfect detection of organisms may cause biased results in habitat models, therefore multiple surveys per season are recommended for the most precise detection models (MacKenzie & Royle, 2005). Multiple visits per season were not feasible for many of the remote and privately managed sites within this study. Nevertheless, we found that repeated visits to a subset of pools show that introduced fishes, M. lar, and the endemic anchialine shrimp H. rubra and M. lohena occurred consistently within pools. For example, within 58 pools visited repeatedly (two or more times) between December 2007 and October 2008, H. rubra were consistently present (NPS, 2012). During 2013 surveys, H. rubra re-occurred in all but two of these same pools. Other pools visited multiple times between 2012 and 2013 throughout the entire study area showed consistent H. rubra presence unless shallow pools were visited at low tide when they appeared dry.
Land use
Land use was described using two categorical variables. One likely condition is that in populated areas, nutrient, and contaminant inputs to groundwater would be elevated due to septic tanks, irrigation with treated sewage water, golf courses, storm drains, or other activities (Weigner et al., 2006; Dalton et al., 2012). Because these contaminants often arrive in pulses to groundwater and may flow through the system quickly, they are difficult and expensive to measure across large areas. Land-use categories were used as a proxy for potential groundwater effects on pools associated with development. Rather than create separate categories for all possible land-use types, we created two binary factors that describe the proximity of pools to development. LU_PROXIMAL scores pools situated within resort or residential developments with a 1 and assigns all other pools a 0. LU_UPSLOPE assigns all pools with development within 1 km upslope a 1 and all other pools to 0. Land-use categories were identified using Quickbird satellite imagery (USDA, 2008). Although this is a very coarse measure of the potential impacts of development on groundwater flowing through anchialine pools, it is a starting point for examining regional patterns of endemic shrimp distribution.
Statistical analyses
To determine the importance of invasive species, pool characteristics, water properties, and land use on endemic shrimp occurrence, each explanatory variable was first tested individually to determine if it is related to a significant difference between shrimp occurrence categories (H. rubra present, both H. rubra and M. lohena present, and pools with neither shrimp present). Because M. lohena occurred with H. rubra in all but one pool, a category for only M. lohena present was not included. Percentage and continuous variables did not fulfill assumptions of normality therefore were examined with a non-parametric Kruskal-Wallace test instead of a one-way analysis of variance (Quinn & Keough, 2009). The null hypothesis was that there was no difference in the mean or median of the groups tested (H. rubra present, both H. rubra and M. lohena present, and pools with neither shrimp present). Categorical explanatory variables such as introduced fish presence or absence were tested for effects on the occurrence of different shrimp species using the contingency table Pearson Chi squared test with Yates correction (χ 2) and a significance level of P < 0.05.
We developed generalized additive models (GAMs) (Knapp, 2005; Miro & Ventura, 2013) to explore data relationships and determine the relative importance of factors on endemic shrimp occurrence. GAMs do not require constant variance or normally distributed errors, and therefore are useful when dependant variables are binary (i.e., presence/absence data). GAMs relax the assumption that the relationship between the dependent variable (on the logit scale) and the explanatory variables are linear. Nonparametric loess smoothing functions are used in GAMs to describe the relationships between the dependent and continuous explanatory variables (Hastie & Tibshirani, 1991). Appendix 1 describes our approach in more detail.
The dependent variables in the GAMs were the presence or absence of H. rubra or M. lohena. The explanatory variables that were examined for potential use in exploratory GAM models represented water properties, pool characteristics, land use, and co-occurring endemic and introduced species. Spatial autocorrelation was accounted for with a loess smoothed term that combined the latitude and longitude of each pool (Knapp et al., 2003, Dormann et al., 2007). Chlorophyll a (n = 201 pools), turbidity (n = 230 pools), salinity (n = 323 pools) and temperature measurements (n = 314 pools) were not available for all of the pools included in the dataset so were examined in separate GAM models using the subset of pools with data. None of the water property variables were a significant term for explaining H. rubra or M. lohena occurrence, and therefore, were not included in the full models.
Plots were created to depict the relationship between significant explanatory variables and the probability of species occurrence. For continuous variables, the response curve shows the relative influence of the explanatory variable on the probability of species occurrence. The response curves are based on partial residuals, are plotted on a log-scale, and are standardized to have an average value of 0.
Results
Pools represented a wide range of physical and biological characteristics (Table A2). Pools ranged in size from <1–952 m2 and were located 11–600 m from the shoreline. A majority of pools were in basalt bedrock and were exposed to open sky, but some were cave-like existing within collapsed lava tubes. Most pools (92%) were less than 1 m in depth with a mean depth of 0.38 ± S.D. 0.7 m at the time of measurement, although in one large cave the water was at least 10 m deep. Some shallow pools that became dry at low tide were populated with H. rubra when the groundwater surface rose at higher tides.
In terms of water characteristics, salinity values ranged from 1.3 to 26.6 ppt but had narrower ranges within areas relating to distinct aquifer sectors (Fig. A1). During the study period, a subset of pools were sampled multiple times to examine variability in surface salinity across annual and daily time scales. In 18 pools located across the study area, the mean difference in surface salinity for an individual pool between 2012 and 2013 sampling periods was 0.02 ppt and was not significant (Two sampled T test, T(2-tailed) = 0.01, df = 34, P = 0.99). In 11 individual pools, the mean difference in surface salinity between morning and night was also 0.02 ppt and was not significant (Two sample T test, T(2-tailed) = 0.05, df = 20, P = 0.96). Although many of the pools in this study were sampled once for water properties, the above comparisons increase our confidence that they represent relatively stable water conditions useful for comparison with other pools within the same time period. Throughout the study area, most pools had low turbidity (0.39 ± 2.5 S.D. NTUs) and Chl a (1.12 ± 5.5 S.D. mg/L) values.
Visible macroalgae and microbial crust were absent from a majority of pools, even in those with rocky substrate. Approximately 50% of the pools observed had substrates dominated by rocky substrate (≥70% rock) and contained no visible benthic algal or microbial crust growth. Macroalgae was visibly present within 10% of pools and was dominated by the genera Ulva, Cladophora, and Vaucheria. Of the pools with visible macroalgae, 63% (n = 25) contained H. rubra and 18% (n = 8) contained M. lohena. Macroalgae were present in 17% of the 237 pools with introduced fish.
Terrestrial vegetation occurred at 33% of pools and included endemic wetland and shoreline plants as well as introduced pickleweed (Batis maritima) and the introduced mesquite tree (Prosopis pallida). Percent silt cover was positively correlated with percent terrestrial vegetation cover including emergent vegetation (Pearson Correlation Coefficient (r) = 0.48, P < 0.0001), perimeter vegetation (r = 0.72, P < 0.0001), and canopy (r = 0.16, P < 0.002).
The daytime surveys showed that H. rubra and M. lohena occurred across the entire study area (Fig. A2). Both shrimp occupied pools representing the measured range of salinities (1.83–26.6 ppt), vegetation cover (0–100%), and substrate type (0–100%) (Fig. 2). Turbidity and Chl a were higher in pools without shrimp than with shrimp, and although this result was statistically significant, the measured differences were minor except for a few outliers (Fig. 2). Substrate type and percent vegetation cover (canopy, perimeter, and emergent) were significantly different between pool categories (Fig. 2). Compared to pools without shrimp, a majority of pools with H. rubra or both shrimp present had a higher percentage of rocky substrate. Compared to pools with no shrimp or H. rubra alone, pools with both H. rubra and M. lohena were associated with a higher percentage of canopy cover. Although H. rubra are usually a deep red color, a distinct white color morph occurred in pools with high amounts of terrestrial or aquatic vegetation throughout the study area (n = 18 pools). This white color morph was absent in pools without associated vegetation.
Boxplots comparing pool characteristics for pools with no shrimp observed (none), Halocaridina rubra present (hrub), and both H. rubra and Metabetaeus lohena present (mloh) during daytime surveys. Within each plot, the dark line represents the median, the box is the first and third quartiles of data, the vertical lines represent 1.5 times the interquartile range of data, and circles are outliers. Kruskal-Wallis test P values are at the top right of each plot. If P >0.05 there is no significant difference (NS) between groups
Tilapia occurred in 3.5% (n = 14), poeciliids in 23.9% (n = 95), and M. lar in 3.8% (n = 15) of the 398 pools surveyed (Fig. A2). All three taxa were found at sites on the western and southern coastlines in a variety of land-use areas including undeveloped, residential, resort, and near urban areas. Sites with these introduced taxa were located in private lands, National Parks, and state conservation lands. These introduced species also occurred in pools with substrates ranging from 100% rock to 100% sediment.
Other native species observed in anchialine pools during daytime surveys included gastropods (Nerita picea, Neritina vespertina, Theodoxus cariosus, and Thiaridae species), the endemic prawn Macrobrachium grandimanus, the glass shrimp Palaemon debilis, and the candidate endangered damselfly Megalagrion xanthomelas. During our surveys at Manuka National Area Reserve, we observed Antecaridina lauensis and Periclimenes pholeter but did not see these rarely observed shrimp in any other locations visited along the coastal corridor.
Factors related to shrimp occurrence
Halocaridina rubra
Halocaridina rubra were detected at 230 of the 398 pools (45%) and were distributed throughout the daylight survey area (Fig. A2). Univariate analysis indicated no significant difference in the probability of H. rubra occurrence between sites with or without nonnative M.lar (Table 1). There was also no significant difference in H. rubra occurrence between sites less than 1 km down slope from human development or farther away (Table 1). Halocaridina rubra occurrence was significantly less probable in pools that were immediately next to coastal development and in pools with tilapia, poeciliids, and K. sandvicensis (Table 1).
Nonnative fishes (tilapia, poeciliids) were the most important variables explaining the lower probability of H. rubra occurrence in anchialine pools during daytime surveys (explaining 21% of the deviance in GAMs; Table 2; Fig. 3a, b). The occurrence of the native reef fish K. sandvicensis explained an additional 3.5% of model deviance (Table 2; Fig. 3c). Of the eight additional explanatory variables, only silt substrate and location were significantly correlated with H. rubra occurrence (Table 2). The response curve describing the estimated effect of silt substrate on the probability of shrimp occurrence indicated a negative association, with higher probabilities of shrimp presence at low silt levels and lower probabilities above 80% silt cover (Fig. 3e). Although the percent of perimeter vegetation was not a significant predictor variable in the GAM, it did have a high percent deviance (12%) indicating it might have some negative effect on the probability of H. rubra occurrence.
Estimated effect of each of the highly significant (P ≤ 0.01) predictor variables on the probability of Halocaridina rubra occurrence, as determined from the generalized additive model. Note that perimeter vegetation has a P value of 0.08 but has a high deviance value so is included. Response curves are based on partial residuals and are standardized to have an average probability of zero. For continuous variables, the dashed lines represent the approximate 95% confidence intervals, and hatchmarks along the x-axis describe the frequency of data points along the gradient of the continuous variable. For categorical (binary) variables, the width of the horizontal lines in the boxplots are proportional to the frequency of data within each category not observed and present. Numbers in parentheses are the percentage of explained deviance of each variable
Metabetaeus lohena
Metabetaeus lohena occurred in 13% of pools surveyed during daylight hours and co-occurred with H. rubra in all but one pool. Univariate analysis confirmed a significant difference in the probability of occurrence of M. lohena in pools with and without H. rubra (Table 1). GAM results also showed that H. rubra occurrence was positively associated with the probability of occurrence for M. lohena, after accounting for the influence of other explanatory variables (Table 2; Fig. 4a).
Estimated effect of each of the highly significant (P ≤ 0.01) predictor variables on the probability of Metabetaeus lohena occurrence, as determined from the generalized additive model. Response curves are based on partial residuals and are standardized to have an average probability of zero. For continuous variables, the dashed lines represent the approximate 95% confidence intervals, and hatchmarks along the x-axis describe the frequency of data points along the gradient of the continuous variable. For categorical (binary) variables, the width of the horizontal lines in the boxplots are proportional to the frequency of data within each category not observed and present. Numbers in parenthesis are the percentage of explained deviance of each variable
Although fish effects on M. lohena occurrence were not significant in GAMs (Table 2), contingency table results indicated a significant negative relationship between poeciliids and M. lohena (Table 1). Furthermore, M. lohena were not found in any pools with tilapia or K. sandvicensis and in only one pool with M. lar. In these comparisons, category counts were too low for conclusive statistical results.
In the GAMs, canopy was the only other explanatory variable significantly correlated with the probability of M. lohena occurrence (Table 2). The response curve describing the estimated effect of canopy cover on the probability of M. lohena occurrence indicates a positive association between shrimp and canopy (Fig. 4b).
Discussion
This study offers the most comprehensive regional perspective on anchialine pool habitat characteristics along the western and southern coastlines of the island of Hawaii since the 1970s. Our data show that the endemic anchialine shrimp H. rubra and M. lohena are distributed throughout the study area. However, introduced fishes are present in over 25% of pools and have a strong negative relationship with shrimp occurrence. Based on their habitat associations, these endemic shrimp exhibit a range of tolerance for a number of physical and biological parameters including salinity and temperature. However, endemic H. rubra occurrence is negatively associated with human land use. This association was not as clear for M. lohena possibly because of the low number of pools observed with this species.
Comparisons between our data and data collected by Maciolek & Brock (1974) indicate that although H. rubra and M. lohena remain widespread across the study area, introduced fishes occur in a greater percentage of pools than 40 years ago. Restricting comparisons to the same sections of coastline, Maciolek and Brock (1974) observed introduced fishes (tilapia, poeciliids, or both) in 11% of 264 total pools, and our observations showed these fish occurred in 25% of 398 total pools. Additionally, Maciolek and Brock (1974) reported that M. lohena occurred in 32% and H. rubra in 59% of the 264 pools they surveyed. By comparison, M. lohena occurred in 13% and H. rubra in 58% of the 398 pools we surveyed. Pool sites from the same area differed between the two surveys because this study documented some pools that were not reported by Maciolek and Brock (1974), and other pools were filled in later years by shoreline development. Although not all pool locations were identical, most of the major pool complexes were revisited in this study. Overall, the comparison of the two surveys shows that introduced fishes have spread over time. However, it is difficult to draw conclusions about the effects of introduced fish on endemic shrimp from this historical comparison.
How do introduced and native fishes influence native anchialine pool biota?
Introduced fishes (tilapia, poeciliids) were the most important variables explaining the lower probability of H. rubra occurrence in anchialine pools during daytime surveys. M. lohena were also less likely to occur when tilapia and poeciliids were present. These results support previous studies that have hypothesized that introduced fishes are a primary reason native fauna are absent from some Hawaiian anchialine pools (Chai et al., 1989; Brock & Kam, 1997). Interestingly, the native reef fish K. sandvicensis, which may wash into pools during storms or may be placed in pools by people, also had a negative effect on H. rubra and M. lohena occurrence. Our results are novel in that they suggest that native reef fishes such as K. sandvicensis are likely to play a similar role as introduced fishes in reducing native shrimp in anchialine pools.
Previous studies in small subsets of pools from the study area showed that poeciliids have large effects on H. rubra abundance and behavior. In the presence of poeciliids, H. rubra avoid predators during daylight hours by hiding in the basalt substrate and become more active and abundant at night when fish are inactive (Capps et al., 2009; Carey et al., 2010; Dalton et al., 2012; Sakihara, 2012; Havird et al., 2013). In each of these studies, the predator avoidance behavior resulted in significantly fewer H. rubra during the day versus the night in pools with poeciliids. Gut content analysis of poeciliids (G. affinis and P. reticulata) showed that actual predation on H. rubra was low to nonexistent in the four pools examined, indicating that their movement out of the pools into the deeper groundwater during the day is a successful predator avoidance behavior (Havird et al., 2013). Although night surveys were not performed at each pool included in this study, our limited nighttime results support this conclusion. Diel predator avoidance behavior by H. rubra is likely to be common within Hawaiian anchialine habitats. Furthermore, this behavior may occur in other, less commonly observed anchialine shrimp species such as M. lohena.
Specific introduced fish taxa appear to have different effects on anchialine pool biota. Higher shrimp occurrence with poeciliids versus tilapia is likely due to gape limitation in poeciliids. Because poeciliids are gape limited in regards to H. rubra, these fish and larger shrimp may coexist (Dalton et al., 2012). Tilapia are larger fish and are not gape limited in relation to H. rubra or M. lohena, explaining the absence of shrimp in pools with tilapia.
Although the introduced prawn M. lar also preys on H. rubra and M. lohena, M. lar occurrence was not an important variable explaining the occurrence of either shrimp during daytime surveys. One explanation may be that H. rubra and M. lohena naturally co-occur with the native prawn M. grandimanus, which is similar in size and morphology to M. lar. Additionally, unlike the introduced fishes, there is some evidence that M. lar hunts at night causing a reverse diel behavior in prey so that H. rubra and M. lohena become most active in pools during the day (Carey et al., 2010). A reverse diel behavior in H. rubra and M. lohena due to M. lar would not have been detected by our daytime surveys.
Studies show that introduced fishes can affect food web dynamics and primary producer standing stocks within anchialine pools by reducing H. rubra abundances and changing H. rubra behavior. In a grazer exclusion experiment, Sakihara et al. (2015) found that grazing by H. rubra significantly decreased epilithon biomass on artificial tiles and that epilithon compositions varied with shrimp grazing pressure and salinity but were primarily composed of diatoms, cyanobacterium, and filamentous algae. Dalton et al. (2012) showed that pools containing poeciliids had significantly higher epilithon biomass than pools without fish, presumably due to reduced grazing by H. rubra. One might therefore expect macroalgae presence to be associated with fish presence due to suppression of shrimp grazing. However, results from this study showed that only 17% of pools with fish contained visible macroalgae and many appeared to contain bare rock substrate. Although epilithon biofilms and macroalgae are not identical metrics, they both represent primary producer standing stock that depend on nutrient availability and grazing pressures. Why might fish presence lead to greater primary producer standing stocks in some pools and not others? Algal consumption by fish and snails (Capps et al., 2009) may compensate for reduced H. rubra grazing and partially explain our observations. Additionally, H. rubra grazing during the night may control macroalgae in most pools. Finally, other parameters such as salinity, nutrient levels, and groundwater flow may determine which algal species grow in specific pools and at what rate (Sakihara et al., 2015).
What roles do the physical habitat and terrestrial vegetation play in structuring anchialine pool communities?
The second most important group of variables explaining shrimp occurrence included abiotic pool characteristics. A high percentage of silt substrate has a negative effect on H. rubra presence. Due to the porous basalt substrate that forms anchialine pools, anchialine shrimp are able to move freely between pool water and subterranean groundwater. This behavior was most evident in the shallowest pools. When groundwater levels dropped below the elevation of the pool substrate, shrimp appeared absent. However, as groundwater levels rose with higher tides, shrimp moved into pools and were observed actively grazing on the substrate. High silt cover, especially if it is thick, may block access to subterranean passages and restrict movement of shrimp between the subterranean groundwater and open pools. It may also reduce grazing surfaces for H. rubra. Higher silt pools were often at the edges of wetland habitat and had higher amounts of associated terrestrial vegetation.
As pools age and soils develop in surrounding substrate, terrestrial vegetation can encroach and may provide additional sources of nutrients for anchialine food webs (Dudley et al., 2014). Our results showed that higher canopy cover was strongly associated with a higher probability of M. lohena occurrence. Terrestrial subsidies may provide nutrient support for an additional trophic level above the grazing H.rubra. Stable isotope analysis indicates that H. rubra utilize terrestrial plant litter as a source of nutrients if it is available (Marrack, 2015). The highest observed density of M. lohena visited during the survey (>100 per m2) was in a pool that also had the highest density of H. rubra (~1000 per m2) (Marrack, 2015). This pool had 100% vegetation around the perimeter, and 30% tree canopy. M. lohena occurrence is also strongly explained by H. rubra presence. In pools with and without terrestrial vegetation, H. rubra are often the only apparent prey item available for M. lohena. These results suggest that M. lohena is dependent on H. rubra occurrence and that terrestrial subsidies support higher populations of both shrimp.
A potentially negative aspect of terrestrial vegetation for endemic shrimp is that as plant litter accumulates and decomposes, biogenic sediment may also accumulate. This buildup may create a positive feedback whereby sediment continues to infill pools providing more substrate for marsh plants until pools eventually become filled in completely. This natural process may be accelerated by the presence of rapidly growing introduced plant species such as Batis maritima. Currently, no published studies have examined the role that introduced plants play in driving anchialine pool senescence.
Salinity and temperature tolerances
Based on their habitat associations, these endemic shrimp exhibit tolerances for a number of physical and biological characteristics of anchialine pools including salinity and temperature. However, there are important distinctions to be made between an organism’s habitat associations and optimum niche requirements (Hutchinson, 1957; Sax et al., 2013). First, surface water measurements do not capture the range of spatial or temporal variability that may exist within the full extent of an anchialine shrimp’s above ground and subterranean habitat. Second, it is not clear if H. rubra and M. lohena are able to complete their entire life cycle within the full range of observed salinities and temperatures. In the lab, adult H.rubra may tolerate 0–56 ppt (Havird et al., 2014) while larvae have undergone development at 10–15 ppt (Couret & Wong, 1978), 15 ppt (Havird et al., 2014), and 20 ppt (Iwai, 2005). Data on tolerance levels are important for the long-term protection of anchialine pool endemic communities because pool salinities are projected to increase due to increases in anthropogenic groundwater withdrawal (Oki, 1999; Thornberry-Ehrlich, 2011), sea level rise (Marrack, 2014), and decreases in precipitation (Giambelluca et al., 2011).
What is the role of land use on anchialine pool ecosystems?
Proximal development was the only land-use category that showed significant effects on the probability of shrimp occurrences. Both land-use variables (proximal and upslope), used as a proxy for potential groundwater effects or other influences on pools due to development, were very broad and do not account for specific factors affecting individual sites. Furthermore, the variables used represent current land use and do not represent effects of past actions. Construction has filled in at least 10% of anchialine pools along the western coastline of the island of Hawaii (Brock et al., 1987). Potential threats to pool habitat related to proximal land use include pathways for further infestations of new and current introduced species to spread into uninfected pools, degradation of pool habitat from human use, over-collection of shrimp, and increases in nonpoint source pollution of groundwater. Pools with human development on their immediate periphery had significantly higher concentrations of ammonium, dissolved inorganic nitrogen, and soluble phosphorus (Dalton et al., 2012). Elevated nutrients (nitrogen, phosphorus), especially when combined with loss of grazer capacity, can result in increased primary producer biomass (Gruner et al., 2008) and major shifts in aquatic ecosystem structure and function (Carpenter et al., 1998; Jackson et al., 2001; Folke et al., 2004; U.S. EPA, 2004). These potential effects associated with land use have serious implications for the integrity of anchialine pool ecosystems, and they need to be monitored and addressed in more detail.
Conservation impacts
Introduced fishes are present in >25% of pools where they have a strong negative impact on shrimp occurrence. H. rubra and M. lohena move within subterranean groundwater and emerge in pools at night when fish are inactive (Capps et al., 2009; Carey et al., 2010; Sakihara, 2012), suggesting that fish removal efforts may result in rapid recovery of shrimp in pool habitats during daylight hours. Fish removal efforts are resource intensive (Britton et al., 2011), therefore targeting “the worst offender” may be the most cost-effective strategy. Tilapia may have the largest effect on shrimp occurrence and sediment accumulation, and therefore should be a priority for fish removal efforts.
High sedimentation was negatively associated with shrimp occurrence, while canopy cover had a positive effect on the presence of the candidate endangered shrimp M. lohena. Current anchialine pool restoration efforts often involve the removal of fine benthic sediments and clearing of surrounding terrestrial vegetation. Our results indicate that removal of sediments may increase the probability that shrimp will occur in pools. However, removal of terrestrial vegetation may have a mixed effect on pool fauna. Although vegetation may increase the rate of pool infilling, M. lohena is positively associated with canopy cover. Adult M. xanthomelas, the candidate endangered damselfly, are also known to associate with canopy cover around anchialine pools (Tango et al., 2012). Further investigations on the relationship of M. lohena and M. xanthomelas to canopy and proximity of vegetation are needed.
As sea levels rise and existing pools become inundated, high subsurface hydrologic connectivity will cause new anchialine habitats to emerge inland in the rugose lava terrain (Marrack, 2014). Endemic anchialine shrimp and mollusks will be able to populate new pools because adults and larvae move in brackish coastal groundwater through subterranean cracks (Craft et al., 2008). Future anchialine habitat in low-lying coastal areas should be identified and fully protected through vigilant land-use planning. Conservation efforts aimed at reducing nonnative fish populations, along with preventing new species introductions, nutrient loading to groundwater, and development of potential future anchialine habitat will ultimately allow the unique Hawaiian anchialine pool ecosystem to persist.
References
Anker, A., 2010. Metabetaeus Borradaile, 1899 revisited, with description of a new species from French Polynesia (Crustacea: Decapoda: Alpheidae). Zootaxa 2552: 37–54.
Bailey-Brock, J. H. & R. E. Brock, 1993. Feeding, reproduction, and sense organs of the Hawaiian anchialine shrimp Halocaridina rubra (Atyidae). Pacific Science 47: 338–355.
Bienfang, P., S. DeFelice & E. A. Laws, 2011. Quantitative environmental benchmarking in a hydrologically driven Hawaiian coastal system. Marine Technology Society Journal 45: 88–100.
Britton, J. R., R. Gozlan & G. Copp, 2011. Managing non-native fish in the environment. Fish and Fisheries 12: 256–274.
Brock, R. E. & A. K. H. Kam, 1997. Biological and water quality characteristics of anchialine resources in Kaloko-Honokohau National Historical Park. Honolulu, Hawai‘i: University of Hawai‘i at Manōa, Cooperative National Park Resources Studies Unit, Technical Report 112.
Brock, R. E., J. Norris, D. Zeimann & M. T. Lee, 1987. Characteristics of water quality in anchialine ponds of the Kona, Hawaii coast. Pacific Science 4l: 200–208.
Capps, K. A., C. B. Turner, M. T. Booth, D. L. Lombardozzi, S. H. McArt, D. K. Chai & N. G. Hairston Jr, 2009. Behavioral responses of the endemic shrimp Halocaridina rubra (Malacostraca: Atyidae) to an introduced fish, Gambusia affinis (Actinopterygii: Poeciliidae) and implications for the trophic structure of Hawaiian anchialine ponds. Pacific Science 63: 27–37.
Carey, C. C., M. P. Ching, S. M. Collins, A. M. Early, W. W. Fetzer, D. Chai & N. G. Hairston Jr, 2010. Predator-dependent diel migration by Halocaridina rubra shrimp (Malacostraca: Atyidae) in Hawaiian anchialine pools. Aquatic Ecology 45: 35–41.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8(3): 559–568.
Chai, D. K., L. W. Cuddihy & C. P. Stone, 1989. An inventory and assessment of anchialine pools in Hawai‘i Volcanoes National Park from Waha‘ula to Ka‘aha, Puna and Ka‘u, Hawai‘i. Honolulu, Hawai‘i: University of Hawai‘i at Manōa and the National Park Service, Technical. Report 69: 39p.
Couret, C. L. & D. Wong, 1978. Larval development of Halocaridina rubra Holthuis (Decapoda, Atyidae). Crustaceana (Leiden) 34: 301–309.
Craft, J. D., A. D. Russ, M. N. Yamamoto, T. Y. Iwai, S. Hau Jr, J. Kahiapo, C. T. Chong, S. Ziegler-Chong, C. Muir, Y. Fujita, D. A. Polhemus, R. A. Kinzie III & S. R. Santos, 2008. Islands under islands: the phylogeography and evolution of Halocaridina rubra Holthuis, 1963 (Crustacean: Decapoda: Atyidae) in the Hawaiian archipelago. Limnology and Oceanography 53: 675–689.
Crain, C. M., K. Kroeker & B. S. Halpern, 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11: 1304–1315.
Cucherousset, J. & J. D. Olden, 2011. Ecological impacts of nonnative freshwater fishes. Fisheries 36: 215–230.
Dalton, C. M., A. Mokiao-Lee, T. S. Sakihara, M. G. Weber, C. A. Roco, Z. Han, B. Dudley, R. A. MacKenzie & N. G. Hairston Jr, 2012. Density- and trait- mediated top-down effects modify bottom-up control of a highly endemic tropical aquatic food web. Oikos 122: 790–800.
Dormann, C. F., J. M. McPherson, M. B. Araújo, R. Bivand, J. Bolliger, G. Carl, R. G. Davies, A. Hirzel, W. Jetz, W. D. Kissling, I. Kühn, R. Ohlemüller, P. R. Peres-Neto, B. Reineking, B. Schröder, F. M. Schurr & R. Wilson, 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30: 609–628.
Dudley, B., R. A. MacKenzie, T. S. Sakihara, H. Dulaiova, C. A. Waters, R. F. Hughes & R. Ostertag, 2014. Influences of native and exotic vegetation and invasive fish on water chemistry of Hawaiian anchialine ponds. Pacific Science 68(4): 509–523.
Foley, J. A., R. DeFries, G. P. Asner, C. Barford, G. Bonan, S. R. Carpenter, F. S. Chapin III, M. T. Coe, G. C. Daily, H. K. Gibbs, J. H. Helkowski, T. Holloway, E. A. Howard, C. J. Kucharik, C. Monfreda, J. A. Patz, I. C. Prentice, N. Ramankutty & P. K. Snyder, 2005. Global consequences of land use. Science 309: 570–574.
Folke, C., S. Carpenter, B. Walker, M. Scheffer, T. Elmqvist, L. Gunderson & C. S. Holling, 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annual Review of Ecology, Evolution, and Systematics 35: 557–581.
Giambelluca, T. W., Q. Chen, A. G. Frazier, J. P. Price, Y. L. Chen, P. S. Chu, J. Eischeid & D. Delparte, 2011. The rainfall atlas of Hawai‘i. Honolulu, Hawai‘i: Geography Department, University of Hawai‘i, http://rainfall.geography.Hawaii.edu.
Gruner, D., J. Smith, E. Seabloom, S. Sandin, J. Ngai & H. Hillebrand, 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecology Letters 11: 740–755.
Gozlan, R., J. R. Britton & G. H. Copp, 2010. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology 76: 751–786.
Halpern, B. S., S. Walbridge, K. A. Selkoe, C. V. Kappel, F. Micheli, C. D’Agrosa, J. F. Bruno, et al., 2008. A global map of human impact on marine ecosystems. Science 319: 948–952.
Hastie, T. & R. Tibshirani, 1991. Generalized additive models. Chapman and Hall, New York, New York, USA.
Havird, J. C., S. R. Santos & R. P. Henry, 2014. Osmoregulation in the Hawaiian anchialine shrimp Halocaridina rubra (Crustacea: Atyidae): expression of ion transporters, mitochondria-rich cell proliferation, and hemolymph osmolality during salinity transfers. Journal of Experimental Biology, in press.
Havird, J. C., J. R. Weeks, S. Hau & S. R. Santos, 2013. Invasive fishes in the Hawaiian anchialine ecosystem: investigating potential predator avoidance by endemic organisms. Hydrobiologia 716: 189–201.
Holthuis, L. B., 1973. Caridean shrimps found in land-locked saltwater pools at four Indo-west Pacific localities (Sinai Peninsula, Funafuti Atoll, Maui and Hawai‘i Islands), with the description of one new genus and four new species. Zoologische Verhadenlingen 128: 3–55.
Hutchinson, G. E., 1957. Concluding remarks. Cold Spring Harbor Symposium- Quantitative Biology 22: 415–427.
Iliffe, T. M. & L. S. Kornicker, 2009. Woldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. Smithsonian Contributions to Marine Science. 38: 269–280.
Iwai, T. Y., 2005. Captive breeding of the endemic Hawaiian red shrimp, Halocaridina rubra, Part I: Reproduction, larval development, and first feeding. Division of Aquatic Resources, Hawaii Department of Land and Natural Resources, Honolulu, Hawaii, USA. 23 pp.
Jackson, J. B. C., M. X. Kirb, W. H. Berher, K. A. Bjorndal, L. W. Botsford, et al., 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293: 629–638.
Jones, T., K. DeVerse, G. Dicus, D. McKay, A. Farahi, K. Kozar & E. Brown, 2011. Water quality vital signs monitoring protocol for the Pacific Island Network:Volume 1; Version 1.0. Natural Resource Report NPS/PACN/NRR—2011/418. National Park Service, Fort Collins, Colorado.
Kano, Y. & T. Kase, 2004. Genetic exchange between anchialine cave populations by means of larval dispersal: the case of a new gastropod species Neritilia cavernicola. Zoologica Scripta 33: 423–437.
Knapp, R. A., 2005. Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biological Conservation 121: 265–279.
Knapp, R. A., K. R. Matthews, H. K. Preisler & R. Jellison, 2003. Developing probabilistic models to predict amphibian site occupancy in a patchy landscape. Ecological Applications 13: 1069–1082.
Knee, K., J. Street, E. Grossman & A. Paytan. 2008. Submarine ground-water discharge and fate along the coast of Kaloko-Honokohau National Historical Park, Island of Hawai’i–Part 2, Spatial and temporal variations in salinity, radium-isotope activity, and nutrient concentrations in coastal waters, December 2003-April 2006: U.S. Geological Survey Scientific Investigations Report 2008–5128.
MacKenzie, R. A. & G. L. Bruland, 2012. Nekton communities in Hawaiian coastal wetlands: the distribution and abundance of introduced fish species. Estuaries and Coasts 35: 212–226.
MacKenzie, D. I. & J. A. Royle, 2005. Designing occupancy studies: general advice and allocating survey effort. Journal of Applied Ecology 42: 1105–1114.
Maciolek, J. A., 1983. Distribution and biology of Indo-Pacific insular hypogeal shrimps. Bulletin of Marine Science 33: 606–618.
Maciolek, J. A. & R. E. Brock, 1974. Aquatic survey of the Kona coast ponds, Hawai‘i Island. Honolulu, Hawai‘i: University of Hawai‘i Sea Grant, Report AR74-04.
Marrack, L. 2014. Incorporating groundwater height into sea level detection models for Hawaiian anchialine pool ecosystems. Journal of Coastal Research. Online preview at: http://www.jcronline.org/doi/abs/10.2112/JCOASTRES-D-13-00043.1.
Marrack, L. 2015. Modeling the effects of sea level rise and introduced species on Hawaiian Anchialine Pool Ecosystems. PhD dissertation for University of California, Berkeley. 114 p.
Miro, A. & M. Ventura, 2013. Historical use, fishing management and lake characteristics explain the presence of non-native trout in Pyrenean lakes: implications for conservation. Biological Conservation 167: 17–24.
National Park Service (NPS), 2012. Integrated resource management applications. Pacific Island Network Anchialine Pool Dataset. Relational Database-2192748. https://irma.nps.gov/App/Reference/Profile/2192748.
Nature Conservancy, 1987. Biological database of rare species and natural communities in anchialine pools in the state of Hawai‘i. Natural Heritage Program, Nature Conservancy of Hawai‘i, Honolulu, HI.
Oki, D., 1999. Geohydrology and numerical simulation of the ground-water flow system of Kona, Island of Hawaii. Reston, Virginia: U.S. Geological Survey, Water-Resources Investigation Report 99-4073.
Ormerod, S. J., M. Dobson, A. G. Hildrew, D. L. Strayer & C. R. Townsend, 2010. Multiple stressors in freshwater ecosystems. Freshwater Biology 55: 1–4.
Paine, R. T., M. J. Tegner & E. A. Johnson, 1998. Compounded perturbations yield ecological surprises. Ecosystems 1: 535–545.
Piggott, J. J., K. Lange, C. R. Townsend & C. D. Matthaei, 2012. Multiple stressors in agricultural streams: a mesocosm study of interactions among raised water temperature, sediment addition and nutrient enrichment. PLoS ONE 7(11): e49873.
Quinn, G. & M. Keough, 2009. Experimental Design and Data Analysis for Biologists. Cambridge University Press, New York: 537p.
Russ, A., S. R. Santos & C. Muir, 2010. Genetic population structure of an anchialine shrimp, Metabetaeus lohena (Crustacea: Alpheidae), in the Hawaiian Islands. Revista de Biologia Tropical 58: 159–170.
Sakihara, T., 2012. A diel comparison of the unique faunal assemblage in remote anchialine pools on Hawai‘i Island. Pacific Science 66: 83–95.
Sakihara, T. S., B. D. Dudley, R. A. MacKenzie & J. P. Beets, 2015. Endemic grazers control benthic microalgal growth in eutrophic tropical brackish ecosystems. Marine Ecology Progress Series 519: 29–45.
Sanchez, M., J. Alcocer, E. Escobar & A. Lugo, 2002. Phytoplankton of cenotes and anchialine caves along a distance gradient from the northeastern coast of Quintana Roo, Yucatan Peninsula. Hydrobiologia 467: 79–89.
Santos, S. R., 2006. Patterns of genetic connectivity among anchialine habitats: a case study of the endemic Hawaiian shrimp Halocaridina rubra on the Island of Hawai’i. Molecular Ecology 15: 2699–2718.
Sax, D. F., R. Early & J. Bellemare, 2013. Niche syndromes, species extinction risks, and management under climate change. Trends in Ecology and Evolution 28: 517–523.
Tango, L. K., D. Foote, K. N. Magnacca, S. J. Foltz & K. Cutler, 2012. Biological inventory of anchialine pool invertebrates at Pu’uhonua o Hōnaunau National Historical Park and Pu’ukoholā Heiau National Historic Site, Hawai’i Island. University of Hawaii Pacific Cooperative Studies Unit. Technical Report #181.
Thornberry-Ehrlich, T., 2011. Kaloko-Honokōhau National Historical Park: geologic resources inventory report. Ft. Collins, Colorado: National Park Service, Natural Resource Report NPS/NRPC/GRD/NRR- 2011/384.
U.S. EPA (Environmental Protection Agency) 2004. National water quality inventory. Report to Congress. Office of Water. U.S. Government Printing Office, Washington, D.C., USA. EPA 841-R-08-001.
U.S. Department of Agriculture (USDA), 2008. Natural Color Orthophoto Mosaic of the Island of Hawaii, Hawaii County, Hawaii. Fort Worth, Texas: U.S. Department of Agriculture, Natural Resources Conservation Service, 11 June, 2008.
U.S. Fish and Wildlife, 2015. Endangered and Candidate Species Reports. Accessed at http://www.fws.gov/endangered/index.html.
Vitousek, P. M., J. D. Abler, R. W. Howarth, G. E. Likens, P. A. Matson, D. W. Schindler, W. H. Schlesinger, et al., 1997. Human alterations of the global nitrogen cycle: sources and consequences. Ecological Applications 7: 737–750.
Webb, J. A., K. G. Grimes & I. D. Lewis, 2010. Volcanogenic origin of cenotes near Mt Gambier, Southeastern Australia. Geomorphology 119: 23–35.
Weese, D., 2012. Revealing hidden diversity in a unique island ecosystem: the evolution, ecology and conservation of anchialine shrimp in the Pacific Basin. PhD Thesis, Auburn University.
Weigner, T., J. Beets, W. Dudley, L. Muehlstein & M. Parsons, 2006. A review of coastal monitoring data for developments in West Hawai‘i. Hilo, Hawai‘i: University of Hawai‘i, Hilo for the County of Hawaii.
Acknowledgements
We are grateful to Rick Gmirkin, Aric Arakaki, Nancy Erger, Vivian Varney, Nahaku Kalei, David Chai, Pi’i Laeha, Anne Brasher, Ann Farahi, Lindsey Kramer, and others for their field support and willingness to share their knowledge. We are grateful to Stephanie Carlson and Mary Power for their comments on the manuscript. We appreciate comments from Justin Havird and an unknown reviewer. Funding for this work was provided by the National Park Service George Melendez Wright Climate Change Fellowship and the UC Berkeley Coleman Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jonne Kotta
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marrack, L., Beavers, S. & O’Grady, P. The relative importance of introduced fishes, habitat characteristics, and land use for endemic shrimp occurrence in brackish anchialine pool ecosystems. Hydrobiologia 758, 107–122 (2015). https://doi.org/10.1007/s10750-015-2277-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2277-2