Abstract
Determining the habitat use and movements by fish is critical to our understanding of aquatic ecosystem function. The objective of this study was to assess the diel movements of Burbot (Lota lota) over the open water season. We employed a high-resolution acoustic telemetry positioning system to track the movements and activity of four Burbot during the ice-free season (between June and September) in a sub-Arctic lake. Burbot underwent diel bank migration (DBM), a benthic form of diel vertical migration, where depths are transitioned in close association with the bottom rather than through the water column. During daytime, Burbot occupied deeper water, at the transition of soft, low complexity substrates and ascended along the rocky bottom lake banks to shallower water habitats at night. Increased activity rates during shallow water forays suggest active feeding events. DBM was low at the start of summer with nearly 24 h of daylight, but increased towards the mid-summer with a more pronounced night cycle, coalescing towards the fall. The DBM of Burbot is dynamic, with proximate triggers of light and temperature, and ultimate causes likely being foraging opportunities, bioenergetics gain and predator avoidance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the movement of animals is a fundamental aspect of ecology and is key in developing and implementing conservation and management strategies (Monahan & Tingley, 2012). In aquatic systems, some organisms conduct a cyclical diel ascent and descent through the water column in what is referred to as diel vertical migration (DVM) (Alexander, 1972; Mehner, 2012). Because of this daily migration of biota, DVM is an important pathway for energy transfer from deep to shallow water habitats (Gorman et al., 2012b). It is widely accepted that the proximate causes or triggers for DVM are most commonly associated with illumination strength, temperature, and hydrostatic pressure (Mehner, 2012), where animals move from cool deep waters to warm shallow waters at dusk and then descend again at dawn (Appenzeller & Leggett, 1995; Scheuerell & Schindler, 2003; Busch & Mehner, 2009; Probst & Eckmann, 2009). The ultimate causes for DVM may include bioenergetics advantage (Sims et al., 2006; Busch et al., 2011), feeding opportunities (Clark & Levy, 1988; Mehner et al., 2007) or predatory avoidance (Scheuerell and Schindler, 2003; Hrabik et al., 2006; Gjelland et al., 2009). There is a growing consensus among researchers that more than one ultimate cause with appropriate multiple tradeoffs contribute to DVM and multi-factor hypotheses to explain DVM in fishes have been proposed (Clark and Levy, 1988; Scheuerell & Schindler, 2003; Gjelland et al., 2009; Donner & Eckmann, 2011; Mehner, 2012; Harrison et al., 2013).
The phenomenon of DVM is best described for marine and freshwater zooplankton (Ringelberg, 1995). Because zooplanktons are a key diet item of many pelagic fishes, DVM is also a wide-ranging phenomenon among icthyofuana. For example, it has been documented with the Oil fishes (Comephorous spp.) of Lake Baikal in Siberia (Eshenroder et al., 1999), Cichlids (Rhamaphochromis ssp.) of Lake Malawi in Africa’s Rift Valley (Thompson et al., 1996), and Ciscoes (Coregonus spp.) and Lake Trout (Salvelinus namaycush) in the Laurentian Great Lakes (Hrabik et al., 2006; Jensen et al., 2006; Stockwell et al., 2010). More recently, however, it has been observed that some aquatic organisms migrating vertically in a diel pattern follow the bottom contour rather than move through the water column (Gorman et al., 2012a). Specifically, Gorman et al. (2012a) described the diel movements of lean and siscowet forms of Lake Trout and Lake Whitefish (Coregonus clupeaformis) between deep and shallow demersal habitats and coined this “diel bank migration” (DBM). Burbot are known to transition between deep and shallow water habitats in a diel pattern (Carl, 1995; Harrison et al., 2013). Because Burbot are presumed to be in close association with benthic habitats at all depths they occupy, previous descriptions of DVM by adult Burbot may not fully describe the habitat niche of this species. It is plausible that DBM is a more accurate description of the habitat occupancy of adult Burbot, similar to other benthic-feeding fish, such as Lake Whitefish (Gorman et al., 2012a).
Burbot are a benthic top-level predator (McPhail & Paragamian, 2000; Stapanian et al., 2010; Cott et al., 2011) that has a predominantly fish-based diet as adults (Amundsen et al., 2003; Gallagher & Dick, 2015; Recknagel et al., 2015). Along with Northern Pike (Esox lucius), Burbot have the largest distribution of any freshwater fish in the world (Van Houdt et al., 2005). Unusual among freshwater fish, Burbot are the only freshwater representative of the extensive Family Gadidae (the codfishes) (Cohen et al., 1990; McPhail and Paragamian, 2000; Stapanian et al., 2010), which are thought to have transitioned from marine environments 5 to 15 million years ago (VanHoudt et al., 2005). Burbot are a physoclistous fish with a gas gland equipped with a swim bladder to regulate their buoyancy and allow them to transition depth strata while remaining close to the bottom. Their gas gland mass increases towards the winter, possibly to facilitate the increased movement required in searching for mates across pressure gradients (Cott et al., 2013a), with Burbot in deeper lakes having larger gas glands (Cott et al., 2013b). This benthic lifestyle, coupled with their aversion to light (Beatty, 1969) and warm water temperature (Hofmann and Fischer, 2002) make Burbot an interesting species to study the movements in relation to environmental variables.
In northern latitudes, the light regime shifts markedly throughout the year. The response in DVM behaviour of fishes has been documented during the long periods of day length associated with sub-Arctic summers (Gjelland et al., 2009; Kahilainen et al., 2009), as well as during the long-term darkness of the under-ice environment (Steinhart & Wurtsbaugh, 1999; Jurvelius & Marjomäki, 2008). In addition, advancements in animal tracking technology have made it possible to discover daily and seasonal patterns of fish movement in relation to their physical environment in ways that were previously impossible (Cooke et al., 2004). Here we incorporate a fine-scale acoustic telemetry positioning system in a high-latitude northern lake that allowed us to investigate, in detail, the spatial movements, activity rates (tail beat frequency transmitters), and corresponding depth occupancy of four adult Burbot against an extensive change in seasonal day length (i.e., > 10-hour decline in day length over 3-month study period).
Because Burbot are bottom-dwelling, light and warm water intolerant, we hypothesize that there will be a significant interactive effect of photoperiod (day/night), water temperature and day length on their daily movements and habitat occupancy. We predict that Burbot movement and activity level will be greater during low light periods relative to high light periods, and these disparities will become greater as the day and night periods become similar in duration (i.e. ~ 12 h each). We also expect that Burbot will stay deep and be less active nearest the summer solstice, after which they will exhibit greater movement and venture into shallower waters as day length draws shorter. Specifically, we (1) test for the interactive effect of light, diel period and water temperature on various movement, activity and habitat occupancy measures, (2) test for differences in the above measures between daily light and dark periods (diel periods) and (3) describe Burbot movement and habitat occupancy in detail, to explain why it is more appropriately described as DBM, rather than DVM.
Materials and methods
Study site
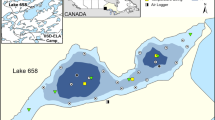
The study occurred at Alexie Lake, located approximately 30 km northeast of Yellowknife, Northwest Territories (NWT), Canada (Fig. 1). Alexie Lake is a medium-sized (area: 420 ha; maximum depth: 32 m; mean depth: 11.7 m), oligotrophic boreal lake, on the Pre-Cambrian Shield that undergoes thermal stratification during summer months (Healey & Woodall, 1973). The fish community comprises Burbot, Lake Trout, Northern Pike, Lake Whitefish, Cisco (Coregonus artedi), Lake Chub (Couesius plumbeus), Ninespine Stickleback (Pungitius pungitius), Trout Perch (Percopsis omiscomaycus), Slimy Sculpin (Cottus cognatus), Spoonhead Sculpin (Cottus ricie) and Deepwater Sculpin (Myoxocephalus thompsoni) (Cott et al., 2011). The opossum shrimp, Mysis diluvania, is also present. Alexie Lake is a scientific research reserve and is closed to public fishing.
Lake bathymetry and bottom habitat classification
A high-resolution hydroacoustic survey was performed in June 2012 (Milne Technologies, Keene, ON, Canada) to characterize the bathymetry and the associated habitat characteristics of Alexie Lake. Hydroacoustic data were collected during daytime periods using a 120 kHz Simrad EK60 7.0° × 7.0° split-beam echo-sounder system. The transducer was attached to a 5.3-m aluminium boat, and data were collected in a systematic parallel survey design, with transects spaced 25 m apart. Echo-sounder data were then initially processed using Echoview (Myriax Pty Ltd., version 5.3.39.22429) and then exported out to Quester Tangent Corporation’s Impact Bottom Classification software (QTC). Bathymetry was determined using point data generated using the “Best Bottom Candidate” line pick algorithm in Echoview, using a bottom back step of −0.15 m. QTC Impact software was used to measure and cluster proprietary characteristics of the bottom echo waveform to identify 18 acoustically distinct classes of bottom substrate, which were ground truthed with Eckman sediment grabs and a tethered drop-style underwater low-light video camera (Ocean Systems, Inc. 3901 Smith Avenue Everett, WA 98201) with a weight attached. The QTC Impact data were then imported into QTC CLAMS to develop spatially interpolated plots of bottom substrate complexity, which represent a measure of habitat diversity. A feature complexity map (hereafter referred to as “bottom substrate complexity”) was developed by calculating the total variance of all available QTC Impact feature data (“Q-values”) within a 60 m search radius of each 3 × 3 m grid cell of Alexie Lake. In Alexie Lake, substrate complexity values range from < 0.1 to 1.7. Relative bottom substrate hardness, expressed as a nautical area backscattering coefficient (m2/nmi2), was determined through integration of acoustic backscatter through the secondary echo region (Siwabessy et al., 1999; Kloser et al., 2001). For both bottom complexity and hardness, a higher value represented more complex and harder bottom substrates.
Fish acoustic telemetry
A total of six adult Burbot (total length range 528–663 mm) were captured using hook-less long lines baited with Cisco from 15–18 June, 2013. Burbot sampling sites were in < 10 m of water to reduce the potential for barotrauma (Bruesewitz et al., 1993. Burbot were brought to shore in holding containers, lightly anesthetized in a solution of 90 mg/l Tricaine Methanesulfonate buffered with sodium bicarbonate, and implanted with a coded, pressure/accelerometer acoustic transmitter that randomly emitted an acoustic signal every 290–490 s (V13AP-1L, tail beat algorithm; Vemco, Ltd., Bedford, NS, Canada). See Blanchfield et al. (2005) for a detailed description of surgical procedures. Transmitters were 13 mm in diameter, 44 mm in length and weighed ~ 6 g in water. Prior to implantation, the depth sensor of each transmitter was individually calibrated at 4-m depth intervals from surface to bottom in Alexie Lake.
The depth and spatial positions of individual acoustic-implanted fish were monitored using a Vemco Positioning System (VPS; Vemco Ltd.) consisting of 72 underwater omni-directional acoustic receivers (VR2 W 69 kHz) (Fig. 1) with overlapping detection ranges (as determined by a range test performed prior to the study). Each receiver was outfitted with an acoustic transmitter or “sync tag” (V16-1L) with an average transmission delay of 20 min, located 1–2 m above the receiver used to synchronize the internal clocks of the receivers. An additional 11 sync tags were distributed throughout the acoustic array to aid in system synchronization and positioning accuracy (Fig. 1). Fish positions and associated errors (see below) are determined by the telemetry company and provided as latitude and longitude coordinates. The acoustic transmitters provide depth readings in meters and tail beat acceleration measured in m/s2. The depth sensor is accurate to ± 1.7 m at depths of 34 m or less with a resolution of 0.15 m. For a detailed description on the methods of VPS telemetry, see Espinoza et al. (2011) and Smith (2013).
Lake water temperature and light conditions
Water temperature and light conditions in Alexie Lake were monitored over the duration of the study using a string of data loggers (Onset HOBO Pendant Temp/Light, 64 k model UA-002-64) deployed over the deepest point of Alexie Lake. Data loggers were set at 0.5 m, 1-m depth intervals from 1 to 20 m, and 25 and 30 m below the water surface. Water temperatures (°C) and light (lx) were recorded hourly, providing a detailed record of the thermal profile of the lake. We used spline interpolation to produce a mean daily temperature and light penetration value for each 0.1-m depth interval from the water surface to lake bottom. Interpolated hourly profile data were used to estimate the water temperature and light conditions occupied for each depth reading from acoustically tagged Burbot, under the assumption that thermal regime was constant across the area of the lake. We acknowledge that prevailing winds, seiche effects and warming of shallow bays may have resulted in variations in the thermal regime in Alexie Lake to some degree. However, the study lake is relatively small, includes numerous islands and the watershed is forested down to the shoreline so we assume that influence of wind on the thermal regime lake would be modest (Fee et al., 1996).
Day length and diel period classification
Length of daylight, which was used as a proxy for season (spring–fall), and assignment of diel period for each day of the study were determined using sunrise and sunset times for Yellowknife (30 km to the southwest) as determined by the United States Naval Observatory (http://aa.usno.navy.mil/index.php). For each individual fish position, we assigned a day length value and diel period classification (day or night) that corresponded to the date and time for each individual telemetry fish position.
Statistical analyses
All data processing and statistical analyses were performed using the R statistical computing package (hereafter “R”; Version 3.1.1 Development Core Team, 2014). Prior to analyses, telemetry data were filtered for data quality assurance using the following method. First, time series plots of fish depth and spatial positions were used to eliminate fish that had died, shed their tag or had tag malfunctions. Next, all fish positions outside the lake or had a depth outside the depth range of Alexie Lake (0–32 m) were eliminated from the dataset. We then used the methods of Meckley et al. (2014) to calculate twice the distance root mean square (2DRMS) for the measured easting and northing error of each sync tag position within each one unit interval bin of the unitless hyperbolic positioning error (HPE; for details see Smith, 2013). A linear regression was then developed between the 1 unit interval HPE bins and their corresponding 2DRMS of measured error and overlaid on a plot of HPE and measured error (HPEm) for all sync tag positions during the study period. The regression line indicated that an average measured error of approximately 10 m corresponded to a cut-off value of HPE = 20, and we therefore removed all fish positions that had an HPE > 20. Next we removed successive positions that had timestamps less than the minimum tag ping rate (for each individual fish) as these positions were assumed to be false positions. For data used to assess spatial movement, we applied an extra filter, where step lengths between successive detections that travelled at speeds well beyond the maximum swimming speed of Burbot were deleted (> 30 m/min). As a result of this extra filter, the number of points used for spatial analyses was slightly less than the number of depth and acceleration positions combined. To reduce the chances that fish behaviour was altered by tag implantation, we removed all data collected within the first 2 days from the date of the final tag implantation from our analyses. Typically, 14 days is accepted as a conservative approach (Rogers & White, 2007), however, because we wanted to examine Burbot behaviour during the summer solstice, we used a shorter acclimation period.
Vertical habitat occupancy measures included depth, water temperature and light. These values were calculated using depth measurements provided by the pressure sensors of the acoustic transmitters. For depth occupancy, we calculated a daily mean value, in meters, for each fish in each daily diel period. For water temperature and light occupancy, we converted each fish-depth position to a water temperature or light value based on the interpolated hourly data, resulting in a value with units of °C or lx, respectively. We then used these temperature or light values to calculate a daily mean value for each fish for each daily diel period. For graphing purposes, we combined the data from individual fish to calculate a population mean ± standard error of the mean (SEM) for each of depth, temperature and light occupancy for each daily diel period.
Using spatial position data, we calculated the sum of horizontal movement rates (HMR) per hour in each daily diel period (day/night) for each individual fish. This was achieved by calculating the sum of the absolute distance (m) between successive positions for each fish within each diel period of each day, divided by the duration of each diel period (h), resulting in a daily value (m/h) for each fish. Vertical movement rate (VMR) was estimated using the depth readings provided by the acoustic transmitter. Similar to HMR, VMR was calculated as the absolute difference in depth between successive depth values for each fish and corrected for the length of each daily diel period. We calculated a mean acceleration value for each fish in each daily diel period based on the accelerometer data associated with each spatial position and used this as our estimate of activity. For graphing purposes, we combined the data from individual fish to calculate a population mean ± SEM for each of HMR, VMR and tail beat acceleration for each diel period.
To evaluate differences in spatial habitat use between diel periods (day vs. night) and duration of daylight (a proxy for season), we estimated mean daily depth contour, bottom hardness and bottom substrate complexity occupancy for each individual fish. This was achieved by converting each set of spatial position of an acoustic-tagged Burbot to a corresponding depth off bottom, bottom hardness or bottom complexity value as determined from raster maps developed from hydroacoustic surveys. For graphing purposes, we again combined the data from individual fish to calculate a population mean ± SEM for each of depth contour, bottom hardness and substrate complexity occupancy for each diel period.
We used a combination of linear mixed effect models (LMM; R package: nlme) and generalized linear mixed effect models (GLMM; R package: glmmADMB) to model the interactive effect of photoperiod, surface water temperature and duration of daylight (season) on Burbot habitat use and movement. We used marginal conditional F tests and likelihood ratio tests with Chi squared distributions to test for significance effects of fixed factors in LMM and GLMM, respectively, and pairwise least squares mean post hoc tests (R package: lsmeans) to compare differences in mean Burbot depth, temperature and habitat use between diel periods. Differences in light occupancy between diel periods were evaluated using a non-parametric pairwise comparison test (R package: nparcomp). Only daily diel periods with a minimum of four positions were included in the analyses. A LMM was also used to quantify how well Burbot depth predicted depth at location (DBM quantification). To account for potential effects of individual fish behaviour in each model, individual fish were treated as random intercepts (Zuur et al., 2009). The proportion of variance attributed by the random effect (individual fish behaviour) was estimated using intraclass correlation coefficients (ICC) (Raudenbush &d Bry, 2002). Coefficients of determinations (conditional R 2) were estimated using the methods of Nakagawa & Schielzeth (2013) (R package: MuMIn).
Multicollinearity among predictor variables was assessed using variance inflation factors (VIF; R package: usdm). Because all predictor variables had a VIF < 4, none were excluded from analyses (Zuur et al., 2009). Assumptions of normality and heteroscedasticity were tested graphically using Q–Q plots, boxplots and histograms of standardized residuals (Zuur et al., 2009). Where data deviated from normality or heteroscedasticity, data transformations were used to meet assumptions of LMM. Data requiring transformation included temperature occupancy, depth off bottom, bottom hardness (log10-transformation) and HMR, VMR and tail beat acceleration (cube root transformation). Because light occupancy data were zero-inflated and could not be transformed to meet assumptions of LMM, these data were modelled using a negative binomial GLMM. In the LMM and GLMM, temporal autocorrelation was accounted for using autoregressive correlation structures (R function: corARMA) with the model order represented by p and with the moving average component (q) set to zero (Pinheiro & Bates, 2000). The optimum correlation structures were determined by plotting the autocorrelation and partial autocorrelation structures of the residuals (Zuur et al., 2009). The optimum correlation structure for depth occupancy, temperature occupancy, HRM and VRM were p = 1, q = 0 and for tail beat acceleration was p = 2, q = 0. A correlation structure was not included when modelling light occupancy (Table 1).
Results
Lake water temperature and light conditions
During the 3-month study period (June 20–Sept. 26, 2013), the number of daylight hours declined from a peak of > 20 to < 12 h. The ice came off Alexie Lake on May 27, 2013, and the lake stratified in mid-June, resulting in a upper layer of warm water > 15°C that extended from the surface to a depth of ~ 8 m during peak stratification (Fig. 2a). Apart from this strong seasonal variability in water temperatures in the upper several meters of the water column, the deeper waters of Alexie Lake were stable throughout the open water season. Much of the volume of Alexie Lake (8 m and below) contained cool water temperatures preferred by Burbot (≤ 10°C) throughout the ice-free season. Likewise, light penetration into the water column of Alexie Lake showed a strong pattern over the study period (Fig. 2b). High light penetration occurred throughout the first 2 months of the open water season, but rapidly declined as day length shortened in mid-August. The maximum daily depth of light penetration ranged from 21.7 to 26.4 m throughout the study period, with a daily mean ± SEM maximum depth of 24.5 ± 0.11 m. The day length ranged from 24 h at the start to 13.4 h by the end of the study.
Telemetry data
Using sync tag data collected over the 97 days study period we were able to estimate the spatial positioning accuracy of our VPS array. After removing all sync tag positions with HPE > 20, the average measured error (HPEm) of the sync tags in our VPS array was 4.61 ± 0.01 m (SEM), based on 396,072 positions. The proportion of filtered sync tag positions that had measured errors less than 10, 5 and 1 m were 89.8, 66.8 and 10.0%, respectively. We applied the same filter to the fish spatial position data.
Two of the six Burbot implanted with acoustic transmitters were not included in our analyses (one tag malfunction and one mortality), resulting in a sample size of four fish. These four fish had a total of 24,172 post-filtered spatial positions, 13,373 depth positions and 13,279 tail beat acceleration positions. The number of days each fish was detected ranged from 49 to 97 days. The number of post-filtered depth, acceleration and spatial positions per fish ranged from 1,563 to 5,538, from 1,606 to 5,558 and from 2,808 to 10,478, respectively.
Fish water column occupancy
A total of 525 mean values for each of depth, temperature and light occupancy (per fish per diel period) were calculated from a total of 13,373 post-filtered depth positions. Burbot showed distinct daily and seasonal patterns in depth, water temperature and light occupancy. Overall, mean depths occupied by Burbot were significantly shallower at night (mean ± SE: 14.13 ± 0.39 m; lsmeans: est = −2.22, z = −2.64, P = 0.01) than during the day (16.25 ± 0.35) (Fig. 3a). The maximum rate of ascent represented by the 95th quantile of all ascents during the study period was 2.4 m/h. When the study commenced, the sun was at the zenith, and Burbot occupied the deepest waters of the study period. At this time, depths occupied by Burbot were similar during day and night. As the season progressed, Burbot showed a clear separation in their diel depth occupancy, whereby they were found in shallower water during the night. The overall trend of inhabiting shallower water progressed over the summer, with Burbot occupying the shallowest water by late September. The gap between the depths occupied in the day and night peaked by late August (mean difference of ≈ 4 m), then coalesced by late September. By late September, Burbot occupied water that was nearly 10 m shallower than at the start of June (Fig. 3a).
Comparison of mean daily (± SEM) a depth, b water temperature and c light occupancy of four acoustic-tagged Burbot during dark and light photoperiods in Alexie Lake, NWT, Canada during the period 20 June–24 September 2013. The lines with grey bands represent a LOESS trend line and its 95% confidence interval
Acoustically tagged Burbot occupied significantly warmer water temperatures (est = 0.06, z = 3.88, P < 0.001) at night (7.39 ± 0.16°C) compared to during the day (6.16 ± 0.11°C) over the duration of the study (Fig. 3b). This trend in night time occupancy of warmer water was evident by analysing the total proportion of Burbot positions occurring in > 11.1°C (the upper 5th quantile of all positions), which was 10.9% at night and only 2.8% during the day. Over the study period, the trend of temperature occupancy by Burbot is similar to that of depth; there was a pronounced diel separation in water temperature occupied peaking by late August (mean difference of ≈ 3.5°C), then narrowed by about 2°C by late September (Fig. 3b).
Similarly, Burbot occupied portions of the water column that contained significantly greater light levels (est = 0.90, t = 15.49, P < 0.001) during the day (118.85 ± 14.16 lx) than at night (0.50 ± 0.12 lx) (Fig. 3c). The difference in light regime occupied by Burbot between day and night and over the course of the study period is very subtle, considering that light measured at 1 m was often > 10,000 lx (Fig. 2b).
Fish activity
Overall, acoustically tagged Burbot were much more active at night than during daylight hours (see Fig. 4 for an example). Burbot had significantly higher rates of vertical movement (est = 0.35, z = 8.30, P < 0.001) at night (1.49 ± 0.08 m/h) that were more than double that relative to during daylight (0.52 ± 0.04 m/h) (Fig. 5a). Likewise, Burbot rates of horizontal movement were significantly higher (est = 0.72, z = 5.68, P < 0.001) at night (56.78 ± 3.39 m/h) relative to daylight (29.91 ± 1.93 m/h) (Fig. 5b). The mean daily horizontal distance travelled by Burbot was 852.8 ± 40.2 m and ranged 1881.1–1948.0 m. Greater night time movement by Burbot was reflected by significantly higher rates of mean tail beat acceleration (est = 0.13, z = 14.89, P < 0.001) at night (0.04 ± 0.00 m/s2) relative to daylight (0.01 ± 0.00 m/s2) (Fig. 5c). Trends in Burbot vertical movement, horizontal movement and acceleration show greater activity occurring at dark periods than light periods for most of the study period, but becoming more similar by late September (Fig. 5a–c). See Table 1 for a summary of model parameters.
An example illustrating how Burbot move during DBM, including a spatial movements and corresponding b depth (dashed line) and tail beat acceleration (solid line) over 3 days, 16 August–19 August 2013. In a, b, each circle (day) and square (night) represent a spatial position estimated by the VPS, and black lines connecting each point represent the horizontal or vertical distance travelled between successive positions. c Represents an illustration of Burbot DBM, where Burbot move up and down steep drop-offs between diel periods, occupying more rocky, shallow habitat at night
Comparison of mean ± SEM a sum of daily vertical movement (m), b sum of daily horizontal movements (m), and c daily tail beat acceleration of four acoustic-tagged Burbot during dark and light photoperiods in Alexie Lake, NWT, Canada during the period 20 June–24 September 2013. The lines with grey bands represent a LOESS trend line and its 95% confidence interval
Fish habitat occupancy
Burbot showed a strong association with the lake bottom. Lake bottom depth was found to be a strong predictor of fish depth at location (F 1,13438 = 56055.32, R 2 = 0.89, P < 0.001). Overall, the average difference between fish depth in the water column and the bathymetric depth contour for each fish position was 0.47 ± 0.02 m, demonstrating that burbot stayed close to the lake bottom. The upper and lower 95% quantiles of the difference in depth and depth at location were 2.7 and −3.7 m, respectively. The close association with the bottom by Burbot was held during both day (0.46 ± 0.13 m) and night (0.05 ± 0.09 m) and did not significantly differ between these periods (est = 0.31, z = 1.47, P = 0.14) (Fig. 6a). Further, the fact that water temperature, light and season were poor predictors of depth off bottom (Table 1) indicates that Burbot maintained their benthic behaviour throughout the open water season. Burbot occupied areas with more complex bottom substrate (est = 0.04, z = 2.70, P = 0.01) at night (0.30 ± 0.01) compared to the day (0.26 ± 0.01) (Fig. 6b). Burbot occupied areas with significantly harder bottom substrates (est = 0.44, z = 2.36, P = 0.02) at night (10.5 × 103 ± 1.3 × 103 m2/nmi2) compared to the day (6.7 × 103 ± 1.0 × 103 m2/nmi2) (Fig. 6c). Burbot occupied habitats with harder bottoms at the beginning and end of the study period compared to mid-summer (Fig. 6b). There was a trend of more complex habitat use towards the end of the study period (Fig. 6c). This corresponds to initial occupancy by Burbot of softer and more homogenous substrates in the deeper regions of the lake, with gradual shoreward movements during the open water season that resulted in inhabiting shallower, rocky areas (see Fig. 4 for an example of DBM). See Table 1 for a summary of model parameters.
Comparison of mean daily ± SEM a depth off bottom (m), b bottom substrate hardness (m2/nmi2), and c bottom substrate complexity occupancy of four acoustic-tagged Burbot during dark and light photoperiods in Alexie Lake, NWT, Canada during the period 20 June–24 September 2013. The lines with grey bands represent a LOESS trend line and its 95% confidence interval
Discussion
As predicted, we found that Burbot moved vertically in a diel pattern that followed the bottom contour rather than through the water column. Gorman et al. (2012a) coined the term “diel bank migration” (DBM) to describe diel vertical movements of lean and siscowet forms of Lake Trout and Lake Whitefish along lake banks from deep to shallow demersal habitats. A key assumption of DBM is that fish are associated with the lake bottom; however, there have been no quantitative data to support that diel migration in Burbot takes place on or near the bottom. The use of high-resolution positioning telemetry allowed us to demonstrate that Burbot were closely associated with the bottom at our study site, a sub-Arctic boreal lake. The Burbot in study stayed along the lake bottom during the day, moved up the bank at dusk to shallower water closer to the shore, before descending at dusk (Fig. 4c). We feel that DBM is an accurate term describing the diel movement of Burbot and adopt this terminology hereafter. On average, Burbot were less than half a metre from the lake bottom (Fig. 6a). The negative values shown are either the result of the slight positioning error (4.6 m ±) associated with our telemetry system (e.g. if a fish was positioned a bit shallower than it actually was), or the Burbot was below the lake bed hiding in a crevasse or burrow.
Burbot showed clear patterns of diel movement between deep and shallow habitats, which were most pronounced when distinct dark and light periods existed. Further, the habitat occupied by Burbot changed over the course of the ice-free season, corresponding to the prevailing light conditions and water column temperature regimes. During periods where there was a distinct day and night (mid-summer), Burbot made unambiguous movements to shallower, warmer areas of the lake at night where they were substantially more active before retreating to cooler, deeper habitats during the daytime where they were more sedentary (Fig. 4). Such movements from resting and digesting in deeper, cooler waters during the day to actively foraging in shallower, warmer waters at night would offer Burbot a bioenergetic advantage (Sims et al., 2006; Busch et al., 2011; Harrison et al., 2013). Carl (1995) observed Burbot that ventured into 20°C water, presumably to feed. This is very warm for a cold water stenotherm, such that foraging into suboptimal thermal habitat must be worth the trade-off from a bioenergetics perspective. Perhaps a function of our study being at much higher latitude than that of Carl (1995), Burbot did not enter 20°C water, and only two out of 13,373 depth positions were recorded in 19°C water. In fact, 95% of all positions occurred in water temperatures < 11°C, demonstrating a strong thermal habitat preference.
Our study period commenced shortly after ice out when the water temperatures in depths that Burbot occupy (~ 5–25 m) are very similar to those in September. However, Burbot stay deep early in the season when light levels are higher, and move shallower later in the season, when due to the low angle of the sun, the amount of light entering the water column of lakes is greatly reduced (Blanchfield et al., 2009). This suggests that although temperature is an important proximate cause, light is likely the primary trigger in the diel and seasonal cycles in Burbot movements. Light as a primary trigger for diel movements makes sense for a photophobic animal. Similar to marine codfishes, Burbot are well adapted to low light conditions (Beatty, 1969), with developed chemosensory (Hinkens & Cochran, 1988), and auditory capabilities (Cott et al., 2013c), and communicate acoustically (Cott et al., 2014). Seasonal changes in diel movements of Burbot corresponding to light conditions are also reflected in changes in their eye physiology. The proportion of paired retinal pigments increase in the fall and winter when light is almost absent under the ice, and decrease in the spring and summer as light penetration increases (Beatty, 1969). The eye physiology of marine gadoids respond in a similar way to seasonal changes in light, although their retinal pigments are mono rather than paired (Beatty, 1969). Perhaps the seasonal variation in retinal pigments is another adaptive holdover from its marine ancestry.
As pelagic larvae, Burbot undergo typical DVM, feeding in the epilimnion during the night and descending into the hypolimnion through the water column by day (Fischer, 1999; Miller & Fischer, 2004; Probst & Eckmann, 2009; Donner & Eckmann, 2011). DVM in larval Burbot is thought to be triggered by light (Probst & Eckmann, 2009). As has been documented for adult Burbot (Harrison et al., 2013), larval Burbot diel migration is in response to multiple ultimate causes, including predator avoidance, feeding opportunities, and bioenergetic gain. At approximately 15–30 mm in length, Burbot undergo an ontogenetic shift to an epi-benthic stage and are highly associated with structure in streams and the shores of lakes (Ryder & Pesendorfer, 1992). Little is known about the movement of juvenile Burbot, particularly after they first become benthic, but from trawling surveys in Lake Superior, DBM does not appear to be exhibited in 1–2 year old juvenile Burbot (O. T. Gorman, personal communication).
During the day, Burbot occupied the deepest habitat with softer and less complex habitat, and at night they ascended along the bottom to shallower areas with harder and more complex habitats, such as boulders intermixed with cobble (Fig. 4). As light levels decreased, Burbot moved from the interface of the soft sediments deeper in the lake, but remained in rocky areas at the toe of the slope, perhaps attracted to shade and crevasses, before ascending along the rocky bank to shallower waters during periods of low light. Burbot are highly associated with structure as adults (Edsall et al., 1993) and juveniles (Fabricius, 1954; Ryder & Pesendorfer, 1992), and are even known to make biogenic structures or burrows (Boyer et al., 1989; McPhail, 2007). During our study, Burbot were observed hiding in rock crevasses along drop-offs during the day. The association with this habitat may have several benefits including foraging opportunities and predator avoidance, while avoiding light. Trawling conducted in deep offshore areas of Lake Superior (100–300 m depth, > 20 km from shore) showed much higher catch rates of adult Burbot during night time than daytime (USGS unpublished data). In these offshore regions, there are no banks for Burbot to express DBM. This suggests that during the day, adults rest in areas of complex bottom structure or burrows where bottom trawls are ineffective in capturing Burbot. At night, Burbot emerge from hiding and move about in search of prey, and are vulnerable to bottom trawls (O. T. Gorman, personal communication).
Overall Burbot were quite motile, with the average horizontal distance travelled over a 24-h period being almost a kilometre. The Burbot in our study increased their daytime activity levels towards the fall, and they occupied shallower habitats during both day and night. Similarly, Carl (1995) observed an increase in swimming speed of tagged Burbot in the fall compared to the summer. In an assessment of the circannual cycle of reproductive development, gonad mass was seen to increase sharply beginning in October, and peak in January, for populations of Burbot that spawn in February (Cott et al., 2013a), including our study lake (Cott et al., 2013b). The increased activity we observed in our fall data was likely was related, in part, to foraging required to fuel the energetic demands of gonad production. Burbot are active predators (Amundsen et al., 2003; Knudsen et al., 2010; Gallagher & Dick, 2015; Recknagel et al., 2015) and are most likely moving into shallower areas closer to shore at night to feed. This notion is corroborated by stable isotope analysis. Burbot in Alexie Lake hold a trophic position similar to that of Lake Trout; however, they derive their energy intermediate of that the limnetic-feeding Lake Trout and the littoral-feeding Northern Pike (Cott et al., 2011) and matched the intermediate spatial and depth habitat use relative to these species (Guzzo et al., unpublished manuscript).
Seasonal shifts in activity have been reported for other populations of Burbot in northern latitudes. In a study of Burbot in a lake in northern Sweden, a distinct diel shift in activity was observed across seasons (Müller, 1973). During the winter, Burbot were active primarily during the day, this switched to strictly nocturnal by April through October, when they adopted a split day–night activity period, after which day length of < 12 h triggered Burbot to adopt a diurnal activity phase again (Müller, 1973). Our study period ended in late September, at that time Burbot became equally active during the day and night also. The same seasonal movement pattern in response to long sub-Arctic day length has also been reported for coregonid fishes (Gjelland et al., 2009; Kahilainen et al., 2009). Continuing this investigation into the winter would be an interesting avenue for future study. The circadian rhythm of activity may be partially controlled by the pineal gland—a photosensory organ in teleost fishes. When the pineal gland was surgically removed from Burbot, their activity period lengthened in the winter and decreased in the summer (Kavaliers, 1980).
The DBM of Burbot may also be a function of predator avoidance. Alexie Lake supports three large-bodied piscivores: Lake Trout, Burbot and Northern Pike (Cott et al., 2011). All of these species are known to prey on the other and each other; however, due to the size it attains in Alexie Lake, Northern Pike are the only species that is a predatory threat to adult Burbot, Lake Trout, and other pike. We have collected pike in Alexie Lake excess of 1,100 mm (M. Guzzo, unpublished data), whereas the maximum size of Burbot collected in Alexie Lake was 631 mm (Cott et al., 2011). We have witnessed large pike attack Lake Trout and other pike that were being angled for mark and recapture surveys. Of the seven Lake Trout observed with pike-inflicted injuries during a mark-recapture study, the size range was 415–623 mm (L. Brekke unpublished data), indicating that pike were actively targeting fish of this size. The nocturnal habits and large body size of adult Burbot would offer some protection from Northern Pike. Telemetry data (presented elsewhere) demonstrated moderate overlap of the 3D habitats of Burbot on those of Northern Pike, but this was predominately at night (Guzzo et al., unpublished manuscript). Harrison et al. (2013) found larger Burbot occupying significantly shallower water than smaller Burbot, and attributed this to a reduced risk of predation on larger fish. While juvenile Burbot were not represented in our study, we assume they would use similar predation avoidance behaviour. This depth separation between juveniles and adults would be particularly important considering the cannibalistic nature of Burbot (Gallagher & Dick, 2015; Recknagel et al. 2015).
Although our study occurred over a single open-water season and our sample of tagged Burbot was low, we believe that the detailed nature of the study presents several advantages that advance our understanding of Burbot movement, behaviour and habitat selection. For example, the combination of detailed bathymetric and habitat mapping with high-precision acoustic positioning telemetry allowed us to effectively demonstrate that Burbot remain in close association to the lake bottom, and do indeed perform DBM. Furthermore, in situ vertical light and temperature sensors allowed us to demonstrate the important role of light as a daily trigger for DBM and the seasonal migration to shallower DBM. We recognize that the small sample size may have limited the ability to capture some of the variability associated with individual behaviour. For example, Harrison et al. (2014) found within-population variability of Burbot movements in a large river–reservoir system, with individuals broadly categorized as “resident” or “mobile”. Ideally, we would have preferred a larger sample size and to have tagged Burbot of known sex, as sex-based differences in habitat occupancy and activity have been documented in other Burbot populations (Stapanian et al., 2013), and may have implications in contaminant uptake (Madenjian et al., 2014).
Conclusions
This study confirms that Burbot perform DBM, a benthic form of DVM, a behaviour that has been often suggested but never quantitatively documented in Burbot. As is the case for DVM for many fish species, light also appears to be the proximate cause for DBM for adult Burbot. The proximate and ultimate causes of DBM in Burbot would likely vary somewhat among systems, as it does for DVM for other fishes (Mehner, 2012); however, we feel that our study lake represents “typical” Burbot habitat in the core of the species range and generally applicable for lake systems of similar size. Taken collectively, our results and observations (i.e. hunt warm, rest cool behaviours; avoidance of pike; and littoral fish foraging opportunity) support the multi-factor hypothesis for adult Burbot diel movements (Harrison et al., 2013), as has been suggested for other fish species (Clark & Levy, 1988; Scheuerell & Schindler, 2003; Gjelland et al., 2009; Mehner, 2012). Our approach would be useful for studying other life history types (e.g. fluvial and adfluvial) or life stages. The diel movement of juvenile (1–2-year old) Burbot remain largely unknown and would be an interesting avenue for further study. Because Burbot are a top-level predator and undergo DBM, they play an important role in cycling energy between offshore and nearshore habitats in sub-arctic lakes. Understanding such interactions is the key in our understanding of food web dynamics of lake ecosystems.
References
Alexander, R. M., 1972. The energetics of vertical migration by fishes. In Sleigh, M. A. & A. G. MacDonald (eds), The Effects of Pressure on Living Organisms Symposia of the Society for Experimental Biology. Academic Press, New York, NY: 273–294.
Amundsen, P., T. Bøhn, O. A. Popova, F. J. Staldvik, Y. S. Reshetnikov, N. A. Kashulin & A. A. Lukin, 2003. Ontogenetic niche shifts and resource partitioning in a subarctic piscivore fish guild. Hydrobiologia 497: 109–119.
Appenzeller, A. R. & W. C. Leggett, 1995. An evaluation of light-mediated vertical migration of fish based on hydroacoustic analysis of the diel vertical movements of rainbow smelt (Osmerus mordax). Canadian Journal of Fisheries and Aquatic Sciences 52(3): 504–511.
Beatty, D. D., 1969. Visual pigments of the burbot, (Lota lota), and seasonal changes in their reative proportions. Vision Research 9: 1173–1183.
Blanchfield, P. J., L. S. Flavelle, T. F. Hodge & D. M. Orihel, 2005. The response of lake trout to manual tracking. Transactions of the American Fisheries Society 134: 346–355.
Blanchfield, P. J., L. S. Tate, J. M. Plumb, M.-L. Acolas & K. G. Beaty, 2009. Seasonal habitat selection by lake trout (Salvelinus namaycush) in a small Canadian shield lake: constraints imposed by winter conditions. Aquatic ecology 43: 777–787.
Boyer, L. F., R. A. Cooper, D. T. Long & T. M. Askew, 1989. Burbot (Lota lota) biogenic sedimentary structures in Lake Superior. Journal of Great Lakes Research 15: 174–185.
Bruesewitz, R. E., D. W. Coble & F. Copes, 1993. Effects of deflating the expanded swim bladder on survival of Burbot. North American Journal of Fisheries Management 13: 346–348.
Busch, S. & T. Mehner, 2009. Hydroacoustic estimates of fish population depths and densities at increasingly longer time scales. International Review of Hydrobiology 94(1): 91–102.
Busch, S., B. M. Johnson & T. Mehner, 2011. Energetic costs and benefits of cyclic habitat switching: a bioenergetics model analysis of diel vertical migration in coregonids. Canadian Journal of Fisheries and Aquatic Science 68: 706–717.
Carl, L. M., 1995. Sonic tracking of burbot in Lake Opeongo, Ontario. Transactions of the American Fisheries Society 124: 77–83.
Clark, C. W. & D. A. Levy, 1988. Diel vertical migrations by juvenile sockeye salmon and the antipredation window. The American Naturalist 131: 271–290.
Cohen, D. M., T. Inada, T. Iwamato & N. Scialabba, 1990. FAO species catalogue. v. 10: Gadiform fishes of the world (Order Gadiformes). FAO, Rome.
Cooke, S. J., S. G. Hinch, M. Wikelski, R. D. Andrews, L. J. Kuchel, T. G. Wolcott & P. J. Butler, 2004. Biotelemetry: a mechanistic approach to ecology. Trends in Ecology & Evolution 19: 334–343.
Cott, P. A., T. A. Johnston & J. M. Gunn, 2011. Food web position of burbot relative to lake trout, northern pike, and lake whitefish in four sub-Arctic boreal lakes. Journal of Applied Ichthyology 27: 49–56.
Cott, P. A., T. A. Johnston & J. M. Gunn, 2013a. Sexual dimorphism in an under-ice spawning fish: the burbot (Lota lota). Canadian Journal of Zoology 91: 732–740.
Cott, P. A., T. A. Johnston & J. M. Gunn, 2013b. Stability in life history characteristics among burbot populations across environmental gradients. Transactions of the American Fisheries Society 142: 1746–1756.
Cott, P. A., T. A. Johnston, J. M. Gunn & D. M. Higgs, 2013c. Hearing sensitivity of the burbot. Transactions of the American Fisheries Society 142: 1699–1704.
Cott, P. A., A. Hawkins, D. Zeddies, B. Martin, T. A. Johnston, J. D. Reist, J. M. Gunn & D. M. Higgs, 2014. Song of the burbot: under-ice acoustic signalling by a freshwater gadoid fish. Journal of Great Lakes Research 40: 435–440.
Donner, M. T. & R. Eckmann, 2011. Diel vertical migration of larval and early-juvenile burbot optimises survival and growth in a deep, pre-alpine lake. Freshwater Biology 56(5): 916–925.
Edsall, T. A., G. W. Kennedy & W. H. Horns, 1993. Distribution, abundance, and resting microhabitat of burbot on Julian’s Reef, southwestern Lake Michigan. Transactions of the American Fisheries Society 122: 560–574.
Eshenroder, R. L., V. G. Sideleva & T. N. Todd, 1999. Functional convergence among pelagic sculpins of Lake Baikal and deepwater ciscoes of the Great Lakes. Journal of Great Lakes Research 25: 847–855.
Espinoza, M., T. J. Farrugia, D. M. Webber, F. Smith & C. G. Lowe, 2011. Testing a new acoustic telemetry technique to quantify long-term, fine-scale movements of aquatic animals. Fisheries Research 108: 364–371.
Fabricius, E., 1954. Aquarium observations on the spawning behaviour of the burbot, Lota vulgaris L. Report of the Institute of Freshwater Research 35: 51–57.
Fee, E. J., R. E. Hecky, S. E. M. Kasian & D. R. Cruikshank, 1996. Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnology and Oceanography 41: 912–920.
Fischer, P., 1999. Otolith microstructure during the pelagic, settlement and benthic phases in burbot. Journal of Fish Biology 54: 1231–1243.
Gallagher, C. P. & T. A. Dick, 2015. Winter feeding ecology and the importance of cannibalism in juvenile and adult burbot (Lota lota) from the Mackenzie Delta, Canada. Hydrobiologia 38: 798–805.
Gjelland, K. Ø., T. Bøhn, J. K. Horne, I. Jensvoll, F. R. Knudsen & P. A. Amundsen, 2009. Planktivore vertical migration and shoaling under a subarctic light regime. Canadian Journal of Fisheries and Aquatic Sciences 66(4): 525–539.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012a. Habitat use by fishes of Lake Superior. I. Diel patterns of habitat use in nearshore and offshore waters of the Apostle Islands region. Aquatic Ecosystem Health & Management 15: 333–354.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012b. Habitat use by fishes of Lake Superior. II. Consequences of diel habitat use for habitat linkages and habitat coupling in nearshore and offshore waters. Aquatic Ecosystem Health & Management 15: 355–368.
Guzzo, M. M., A. J. Chapelsky, P. A. Cott & P. J. Blanchfield, accepted. Resource partitioning among piscivores in a sub-Arctic lake during thermal stratification. Journal of Great Lakes Research. Unpublished manuscript GLR-D-14-00248.
Harrison, P. M., L. F. G. Gutowsky, E. G. Martins, D. A. Patterson, A. Leake, S. J. Cooke & M. Power, 2013. Diel vertical migration of adult burbot: a dynamic trade-off among feeding opportunity, predation avoidance, and bioenergetic gain. Canadian Journal of Fisheries and Aquatic Science 70: 1765–1774.
Harrison, P. M., L. F. G. Gutowsky, E. G. Martins, D. A. Patterson, S. J. Cooke & M. Power, 2014. Personality-dependent spatial ecology occurs independently from dispersal in wild burbot (Lota lota). Behavioral Ecology. doi:10.1093/beheco/aru216.
Healey, M. C. & W. L. Woodall, 1973. Experimental cropping of lakes: II. physical and chemical features of the lakes. Fisheries Research Board of Canada TechnicalReport 348: 19.
Hinkens, E. & P. A. Cochran, 1988. Taste buds on the pelvic fin rays of the burbot, Lota lota (L.). Journal of Fish Biology 32: 975.
Hofmann, N. & P. Fischer, 2002. Temperature preferences and critical thermal limits of burbot: implications for habitat selection and ontogenetic habitat shift. Transactions of the American Fisheries Society 1311: 1164–1172.
Hrabik, T. R., O. P. Jensen, S. J. D. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. I. Changes in vertical migration of coregonids in response to varying predation risk. Canadian Journal of Fisheries and Aquatic Sciences 63: 2286–2295.
Jensen, O. P., T. R. Hrabik, S. J. D. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. II. Modeling trade-offs at an intermediate trophic level. Canadian Journal of Fisheries and Aquatic Sciences 63: 2296–2307.
Jurvelius, J. & T. J. Marjomäki, 2008. Night, day, sunrise, sunset: do fish under snow and ice recognize the difference? Freshwater biology 53(11): 2287–2294.
Kahilainen, K. K., T. Malinen & H. Lehtonen, 2009. Polar light regime and piscivory govern diel vertical migrations of planktivorous fish and zooplankton in a subarctic lake. Ecology of Freshwater Fish 18(3): 481–490.
Kavaliers, M., 1980. Circadian locomotor activity rhythms of the burbot, Lota lota: seasonal differences in period length and the effect of pinealectomy. Journal of Comparative Physiology A 136: 215–218.
Kloser, R. J., N. J. Bax, T. Ryan, A. Williams & B. A. Barker, 2001. Remote sensing of seabed types in the Australian South East Fishery; development and application of normal incident acoustic techniques and associated ‘ground truthing’. Marine and Freshwater Research 52(4): 475–489.
Knudsen, R., P.-A. Amundsen & A. Klemesten, 2010. Arctic charr in sympatry with burbot: ecological and evolutionary consequences. Hydrobiologia 650: 45–54.
Madenjian, C. P., M. A. Stapanian, P. A. Cott, R. R. Rediske & J. P. O’Keefe, 2014. Polychlorinated biphenyl concentrations of burbot Lota lota from Great Slave Lake are very low but vary by sex. Archives of Environmental Contamination and Toxicology 66: 529–537.
McPhail, J. D., 2007. The Freshwater Fishes of British Columbia. The University of Alberta Press, Edmonton.
McPhail, J. D. & V. L. Paragamian, 2000. Burbot biology and life history. In Willis, D. W. & V. L. Paragamian (eds), Burbot: Biology, Ecology, and Management, Vol. 1. Fisheries Management Section of the American Fisheries Society, Spokane, WA: 11–23.
Meckley, T. D., C. M. Holbrook, C. Wagner & T. R. Binder, 2014. An approach for filtering hyperbolically positioned underwater acoustic telemetry data with position precision estimates. Animal Biotelemetry 2: 7.
Mehner, T., 2012. Diel vertical migration of freshwater fishes–proximate triggers, ultimate causes and research perspectives. Freshwater Biology 57(7): 1342–1359.
Mehner, T., P. Kasprzak & F. Hölker, 2007. Exploring ultimate hypotheses to predict diel vertical migrations in coregonid fish. Canadian Journal of Fisheries and Aquatic Sciences 64(6): 874–886.
Miller, O. & P. Fischer, 2004. Distribution and onshore migration behaviour of burbot larvae in Lake Constance, Germany. Journal of Fish Biology 64: 874–886.
Monahan, W. B. & M. W. Tingley, 2012. Niche tracking and rapid establishment of distributional equilibrium in the house sparrow show potential responsiveness of species to climate change. PloS one 7: e42097.
Müller, K., 1973. Seasonal phase shift and the duration of activity time in the burbot, Lota lota (L.)(Pisces, Gadidae). Journal of Comparative Physiology 84: 357–359.
Nakagawa, S. & H. Schielzeth, 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4: 133–142.
Pinheiro, J. C. & D. M. Bates, 2000. Mixed effects models in S and S-Plus. Springer, New York. doi:10.1198/tech.2001.s574.
Probst, W. N. & R. Eckmann, 2009. The influence of light on the diel vertical migration of young-of-the-year burbot Lota lota in Lake Constance. Journal of Fish Biology 74(1): 150–166.
R-Development-Core-Team, 2014. R: a language and environment for statistical computing [online]. R package version 3.1.1 edn. R Foundation for Statistical Computing, Vienna.
Raudenbush, S. W. & A. S. Bryk, 2002. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage, London.
Recknagel, H., A. Amos & K. R. Elmer, 2015. Morphological and ecological variation among populations and subspecies of Burbot (Lota lota [L, 1758]) from the Mackenzie River Delta, Canada. The Canadian Field-Naturalist 128(4): 377–384.
Ringelberg, J., 1995. Changes in light intensity and diel vertical migration: a comparison of marine and freshwater environments. Journal of the Marine Biological Association of the United Kingdom 75(01): 15–25.
Rogers, K. B. & G. C. White, 2007. Analysis of movement and habitat use from telemetry data. In Brown, M. & C. S. Guy (eds), Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society, Bethesda: 625–676.
Ryder, R. A. & J. Pesendorfer, 1992. Food, growth, habitat, and community interactions of young-of-the-year burbot, Lota lota L., in a Precambrian Shield lake. Hydrobiologia 243(244): 211–227.
Scheuerell, M. D. & D. E. Schindler, 2003. Diel vertical migration by juvenile sockeye salmon: empirical evidence for the antipredation window. Ecology 84(7): 1713–1720.
Sims, D. W., V. J. Wearmouth, E. J. Southall, J. M. Hill, P. Moore, K. Rawlinson, N. Huthinson, G. C. Budd, D. Righton, J. D. Metcalfe, J. P. Nash & D. Morritt, 2006. Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. Journal of Animal Ecology 75(1): 176–190.
Siwabessy, J., J. Penrose, R. Kloser & D. Fox, Seabed habitat classification. Shallow Survey ‘99. In: International Conference on High Resolution Surveys in Shallow Water, Sydney, 1999.
Smith, F., 2013. Understanding HPE in the VEMCO Positioning System (VPS). Bedford, NS, Canada. http://vemco.com/wpcontent/uploads/2013/09/understanding-hpe-vps.pdf.
Stapanian, M. A., V. L. Paragamian, C. P. Madenjian, J. R. Jackson, J. Lappalainen, M. J. Evenson & M. D. Neufeld, 2010. Worldwide status of burbot and conservation measures. Fish and Fisheries 11: 34–56.
Stapanian, M. A., L. D. Witzel & A. Cook, 2013. Temporal changes and sexual differences in spatial distribution of Burbot in Lake Erie. Transactions of the American Fisheries Society 142(6): 1724–1732.
Steinhart, G. B. & W. A. Wurtsbaugh, 1999. Under-ice diel vertical migrations of Oncorhynchus nerka and their zooplankton prey. Canadian Journal of Fisheries and Aquatic Sciences 56(S1): 152–161.
Stockwell, J. D., T. R. Hrabik, O. P. Jensen, D. L. Yule & M. Balge, 2010. Empirical evaluation of predator-driven diel vertical migration in Lake Superior. Canadian Journal of Fisheries and Aquatic Sciences 67(3): 473–485.
Thompson, A. B., E. H. Allison & B. P. Ngatunga, 1996. Distribution and breeding biology of offshore cichlids in Lake Malawi/Niassa. Environmental Biology of Fishes 47: 235–254.
Van Houdt, J. K. J., L. de Cleyn, A. Perretti & F. A. M. Volckaert, 2005. A mitogenic view on the evolutionary history of the Holarctic freshwater gadoid, burbot (Lota lota). Molecular Ecology 14: 2445–2457.
Zuur, A., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
Acknowledgements
The authors acknowledge Kim McDonald, Donna Patterson, Dave Callaghan, Lee Hrenchuck, Larry Dow, Bruce Hanna, Gerald Fillatre, Joe Acorn, Lorraine Brekke and Diego Cott for their assistance throughout the project. Thanks to Marty Stapanian and Chris Myrick for chairing the 5th International Symposium on Burbot, where this study was first presented. Two anonymous reviewers and Marty Stapanian are thanked for comments that have improved the manuscript. Alex Hood and DeBeers Canada, and Todd Slack and the Yellowknifes Dene First Nation are thanked for being supportive of this project. Funding was provided by DeBeers Canada, Fisheries and Oceans Canada (DFO), Natural Sciences and Engineering Research Council (NSERC) Strategic Grants (PJB), and NSERC PGF, Fish Futures Inc., and the University of Manitoba to MMG. Fish were collected under the authority of the License to Collect Fish for Scientific Purposes (DFO) and the Canadian Animal Care Committee Protocol through DFO and the University of Manitoba.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter A. Cott and Mathew M. Guzzo have equally contributed to this work.
Guest editors: Martin A. Stapanian & Christopher A. Myrick / Ecology, Culture, and Management of Burbot
Rights and permissions
About this article
Cite this article
Cott, P.A., Guzzo, M.M., Chapelsky, A.J. et al. Diel bank migration of Burbot (Lota lota). Hydrobiologia 757, 3–20 (2015). https://doi.org/10.1007/s10750-015-2257-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2257-6