Abstract
Fish can have an important role in the passive dispersal of freshwater zooplankton. In the Paraná River system the migratory fish Prochilodus lineatus (Valenciennes) constitutes about 60% of the fish biomass, the adult individuals have an ilyophagous feeding mode adapted to feed on soft bottom sediments. We hypothesize that P. lineatus ingests resting stages of zooplankton along with bottom sediments and that these resting stages are able to hatch after passing through the digestive tract. Forty adult P. lineatus individuals were caught in a lake located in the floodplain of Middle Parana River. Content of the last part of intestine was removed and divided into two equal portions and stored (3 months) at 4°C and at room temperature. Later, both portions were incubated for 27 weeks at 25°C. Hatching was controlled at 4-day intervals during the first 9 weeks of the experiment and later less frequently. At the end of the experiment, 8016 individuals were recorded, belonging to 18, mostly littoral species (15 rotifers, 2 cladocerans, and one copepod). Incubation preceded by a cooling period resulted in hatching of more species and individuals. Our results show that migratory fish may be an important vector for zooplankton dispersal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dispersal of individuals plays an important role in shaping the diversity, structure, and dynamics of aquatic communities. For example, species that occupy temporary habitats may be regulated by traits related to special dynamics such as dispersal (Harrison & Taylor, 1997), and evidence exists that dispersal is significant for species interactions (Bilton et al., 2001; Havel & Shurin, 2004). This is a basic component of the metacommunity concept, defined as a set of local communities linked by the dispersal of potentially multiple interacting species (Wilson, 1992; Leibold et al., 2004).
In floodplain river systems, hydrological connectivity, activated by flood pulse, has considerable influence on the exchange of material between the main river channel and the floodplain (Junk et al., 1989) and is also an important force in the dispersal of zooplankton organisms whose active locomotion is limited. However, zooplankton populations may also be spatially dispersed as resting stages. Indeed, resting eggs can remain viable over extended time periods in water body (Hutchinson, 1967; Brendonck & De Meester, 2003).
It has been found that the wall structure of the resting eggs enables them to withstand extreme environmental conditions (Gyllström & Hansson, 2004). The pool of resting stages deposited on the bottom of the lakes constitutes the so-called ‘egg banks’ (Hairston et al., 1995) that may hatch in the water body or be transported to a new location, so the dormant stages may also constitute an efficient component of dispersal in time (Havel & Shurin, 2004). Experimental studies show that the dispersal of zooplankton resting stages may include hydrochory (Shurin & Havel, 2003), anemochory (Cáceres & Soluk, 2002), antropochory (Duffy et al., 2000), and zoochory (Proctor et al., 1967; Mellors, 1975; Jarnagin et al., 2000).
The process of dispersal by fish (Ichthyochory) has mostly been studied in relation to seed dispersal (Cook, 1988; Correa et al., 2007; Pollux, 2011), and the studies suggest that this dispersal mechanism may play a significant role in the spatial distribution of aquatic and riparian plants. In contrast, ichthyochory, as a dispersal mode for zooplankton, has not been extensively studied. Therefore, the information of this process is more limited (Simberloff & Rejmanek, 2010), and the researches were only performed on planktivorous and carnivorous fish (Mellors, 1975; Jarnagin et al., 2000).
Mellors (1975) observed that Daphnia ephippia were able to survive digestion by fishes, their main dispersal agent thus being fish-eating birds and mammals. On the other hand, Jarnagin et al. (2000, 2004) showed that resting eggs of the cladoceran Bythotrephes survived passage through fish guts, promoting its dispersal. For rotifers, one of the most important groups of freshwater zooplankton, successful hatching of resting stages after passage through the digestive tract of vertebrate animals has not yet been demonstrated (Segers & De Smet, 2008).
In the floodplain of river systems, fishes may act as good vectors of dispersal of dormant zooplankton stages, mainly those that feed on the organic matter present in the mud (ilyophagous) and those that feed on active zooplankton (zooplanktophagous). Ilyophagous fishes belonging to the genus Prochilodus Agaziss (Characiformes, Prochilodontidae) are widely distributed in South America (Leccia, 1972), particularly in the La Plata River basin, the second largest basin in South America (Del Barco et al., 2007).
In this systems, Prochilodus lineatus (Valenciennes) represents the most abundant fish species and constitutes about 60% of the total fish biomass (Bonetto et al., 1969; Espinach Ros & Fuentes, 2000). Its life cycle is considered to be an adaptation to the environmental changes produced by the flood pulse, which is the main structuring force of the Middle Paraná River system (Bayo & Cordiviola de Yuan, 1996; Del Barco et al., 2007). At its early developmental stage, P. lineatus is zooplanktophagous (Rossi, 1992) but shifts to an ilyophagous feeding mode as an “adult” and is thus morphologically and functionally adapted to feed on soft bottom sediments (Angelescu & Gneri, 1949). Another important factor of dispersal is the extensive migration of P. lineatus along the river, upstream for reproductive purposes and downstream for food seeking during which it enters the lakes of the floodplain (lateral migration) (Bonetto et al., 1981; Tablado & Oldani, 1984; Bonetto & Castello, 1985).
Being a key species and due to its status as trophic web support, ilyophagous P. lineatus plays an important role in the bioeconomy of aquatic systems by exploiting an important part of the organic matter deposited in the sediment, thus acting as an extractor of organic detritus and accumulator of these in your stomach (Angelescu & Gneri, 1949; Bowen, 1983). These special features of the behavior and life cycle of P. lineatus may allow it to act as a potential vector in the endozoochoric dispersal of zooplankton resting stages found in the bottom sediment. Knowledge of the role of P. lineatus as a vector for zooplankton dispersal may help us to understand the integrative and sustaining mechanisms of the diversity of metacommunities in intricate ecological systems such as tropical and subtropical floodplains.
Our aims were (i) to analyze the possible role of Prochilodus lineatus as a dispersing vector of zooplankton via its ingestion of zooplankton resting stages present in bottom sediments, (ii) to test two methods of storage of gut contents prior to incubation, and (iii) assessment of the habitat membership (limnetic/littoral) of hatched species. We hypothesized that: (i) P. lineatus ingests zooplankton resting stages along with bottom sediments and that (ii) the resting stages may hatch after passing through the digestive tract of the fish; (iii) hatched species are mainly limnetic.
Materials and methods
Field sampling
Specimens of P. lineatus were caught using a multifilament nylon gill net (25 × 2 m) of mesh size 80 mm in October 2011 in Lake Feller (31°10′27.40″S, 60°1′59.60″W) located in the Santa Fe Province (Argentina). This is a semi-lentic water body belonging to the Middle Paraná River floodplain and connected to the main channel (Fig. 1). The Middle Paraná River system, including the main channel and the floodplain, with a surface area of 21,000 km2, constitutes an important part of the La Plata River basin (Iriondo, 1993).
Only adults and young specimens (> 13 cm) were selected for this study since at these levels of ontogenetic development the diet is constrained to organic matter of bottom sediments (Angelescu & Gneri, 1949; Rossi, 1992). The selected individuals had a standard length of 13–47 cm (mean = 28.95 ± 7.16 cm) and weighed 68–3640 g (mean = 910.85 ± 812.90 g).
The complete digestive system was dissected and placed on ice for transportation from the field to the laboratory. Half of the 40 selected specimens had empty intestines, leaving intestine contents from 20 individuals for experimental analysis.
Hatching experiment
We used the ex situ emergency assessment method (Brendonck & De Meester, 2003; Torres & Zoppi de Roa, 2010) to estimate the amount and composition of the pool of zooplankton dormant stages in the gut contents of P. lineatus and the dispersal of zooplankton. The emergency assessment method is commonly used to study egg banks in sediments (Garcia Roger et al., 2008). The method generally involves two steps: storage and incubation; but also other factors, such as time, light, and temperature, may be relevant. Several authors have successfully used a methodology including a cold storage period (Vandekerkhove et al., 2005; Palazzo et al., 2008; Allen, 2010; Santangelo et al., 2011). However, it must be emphasized that some studies of zooplankton resting stages using the cold storage methodology did not find a high diversity of cladocerans and copepods in tropical and subtropical environments, as compared with those found in active population in the same habitat (Maia-Barbosa et al., 2003; Palazzo et al., 2008; Battauz et al., 2014). Due to the absence of methodological consensus, in this study we used two different methods of storage for the purpose of comparison. In the laboratory, we extracted the bottom sediment content of the last part of the intestines, which is fecal matter waiting to be expelled. The sediment was placed in plastic circular trays (38.47 cm2, each) and covered with a gauze disk in order to prevent contamination by outside particles or organisms after which the sediment was dried at room temperature for 16 days. After drying, the intestine contents were divided into two equal parts which were then subjected to different storage treatments: cold dark (CD) where the gut contents of 10 P. lineatus specimens were stored in opaque boxes and kept in a refrigerator (4°C) for 3 months and ambient dark (AD) where the gut contents of 10 P. lineatus specimens of were stored in opaque boxes at room temperature for 3 months (≅ 21°C).
The CD and AD treatments were applied to 18.8 and 22.5 g intestinal content, respectively, with ten replicates for each treatment. Each tray contained the intestinal content of one individual and was covered with 2–3 cm of sterile water from Lake Feller (first filtered through a 20-μm mesh and then boiled). The trays were placed in an incubator at 25°C with a dark:light photoperiod of 8:16 h. The photoperiod used for the experiments is similar to that of the summer time in the region. Afterwards we controlled hatching at 4-day intervals for 9 weeks, the experiment being continued for 18 weeks where recordings were made every 25 days to record any late hatchlings. The supernatant water in the trays was filtered using a 20-μm mesh and mixed in a single sample fixed in 10% formalin.
The temperature of the hatching experiment was set to 25°C because the temperature in the region is relatively high during summer. During the last 30 years, from December to March, average regional temperature, has ranged between 23.1 and 29°C.
The samples were stained with erythrosine and analyzed under an optical Nikon Eclipse (E100) microscope. Analyses of abundance and richness of hatchlings were carried out in a 1-ml Kolwictz cell. Taxonomic determinations were made using the keys mainly; Koste (1978) and Segers (1995) for Rotifera, Korovchinsky (1992) and Kořínek (2002) for Cladocera and, Battistoni (1995) and Alekseev (2002) for Copepoda.
Data analysis
Similarity of taxonomic composition of hatchlings between the two storage treatments was calculated using the Jaccard similarity index (Magurran, 1988) and Sørensen index (quantitative) (Sørensen, 1948). The species with the greatest contribution to dissimilarity between treatments were determined using the SIMPER analysis (Clarke & Warwick, 1994), and the non parametric multivariate analysis of variance (NPMANOVA) was used to elucidate if those differences found in the SIMPER analysis were statistically significant.
The limnetic and littoral species listed were separated (Shiel et al., 1982; own knowledge of the regional fauna). A Chi square (χ 2) test was used to assess heterogeneity among storage treatments regarding the relative abundance of hatchlings of littoral and limnetic species. Accumulation curve of species hatched in both treatments was calculated.
The index for timing of hatching (ITH) was calculated to assess the incubation time (days) required to break the resting phase of each specific taxon (Vandekerkhove et al., 2004). Statistical analyses were performed using the Past 2.14 statistical package (Hammer et al., 2001).
Results
During the 193 incubation days of both treatments, a total of 18 taxa, 14 Rotifera Monogononta, 1 Rotifera Bdelloidea, 2 Cladocera, and 1 Copepoda were recorded. In the CD storage treatment a total of 17 taxa, 13 Rotifera Monogononta, 1 Rotifera Bdelloidea, 2 Cladocera, and 1 Copepoda were hatched, while in the AD storage treatment a total of 13 taxa, 11 Rotifera Monogononta, 1 Rotifera Bdelloidea, 1 Cladocera, and no Copepoda were hatched (Table 1).
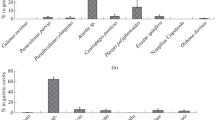
In the CD treatment, the dominant species were, in decreasing order of rank, Lecane inermis, Cephalodella sp., Colurella sp., Lepadella sp., and Lecane nana, constituting 94% of the individuals hatched out of a total of 6481 hatchlings.
In the AD storage treatment in decreasing order of rank Cephalodella sp., Lepadella sp., L. nana, L. inermis, and Colurella sp. showed higher abundance, representing 90% of the individuals that hatched out of a total of 1535 hatchlings. In both the treatments the rotifers dominated (> 96% of the total hatch). As depicted in Fig. 2, almost all species were more abundant than in the AD treatment.
There is close similarity in the composition of hatchlings between the two treatments (Jaccard Index: 0.7). However, if we analyze the abundance of species hatched the similarity between treatments is low (Sørensen index: 0.4). The SIMPER analysis showed that the dissimilarity was 60% (NPMANOVA: F = 2.59, P = 0.038), being six species (Cephalodella sp., L. inermis, Lepadella sp., L. nana, Colurella sp., Coronatella poppei) (Table 2) responsible for 77% of total dissimilarity.
Moreover, both treatments recorded higher hatching of littoral than of limnetic species (Fig. 3) (χ 2 = 9.267, P = 0.002). In the AD storage treatment a significant number of species broke their dormancy in the early stages of incubation, approximately before day 28. From this day onwards, there was an increase in species hatching in the CD treatment which behaved similar to the AD treatment, being stable with small variations until the completion of the experiment. However, accumulation of species was higher in the CD treatment. Richness was on average 5.1 ± 2.3 species per sampling in the CD treatment and 4.3 ± 2.0 in the AD treatment (Fig. 4).
The calculation of ITH showed no statistical differences in the zooplankton species between treatments (t = 1.54, P = 0.13), however some species suggested to be affected by the treatment types, regarding timing of hatching and the abundance (Table 3) (Fig. 5).
Analysis of the hatchings and abundance of the most common species per storage treatment. The index for timing of hatching (ITH) proposed by Vandekerkhove et al. (2004) was calculated; the parallel lines representing the ITH of the species of the cold-dark (CD) treatment and the parallel dashed lines represent the ITH of the species of the ambient-dark (AD) treatments
Discussion
Zooplankton eggs in bottom sediments and passage through fish guts
The zooplankton resting stages cannot only be ingested by ilyophagous Prochilodus lineatus but are able to maintain their hatching viability. Hence, an important number of resting stages present in bottom sediments may survive in the gut passage of P. lineatus. This is likely due to the strong structure of the egg walls (Gyllström & Hansson, 2004) as well as the digestive physiology of this fish whose pyloric stomach helps food compaction and removes the water in the sediment (Bonetto et al., 1989) and the cardiac stomach whose main function being food storage (Bowen, 1983) (Fig. 5).
The number of species hatched from fish gut contents was remarkably high in comparison with the number recorded from the dispersal studies of other vertebrates using similar methodologies of storage and incubation. Jenkins & Underwood (1998) collected duck feces in Washington Park Pond (Milwaukee Country, Wisconsin, USA), which was refrigerated for 7 days and incubated at 20°C. No zooplankton species appeared in the incubated feces. However, previous studies involving water flows under controlled laboratory conditions demonstrated different results (Proctor, 1964; Mellors, 1975). Jenkins & Underwood (1998) suggests that such events of hatching are not common and that natural dispersal depends on factors such as ingestion of viable propagules, survival of propagules in the gut, transport from one site to another within the gut passage, and deposition times. Previous studies have evidenced that resting stages are resistant to consumption by planktivorous and carnivorous fish (Jarnagin et al., 2000, 2004). In this sense, Mellors (1975) reported selective ingestion of Daphnia ephippia by Lepomis gibbosus (Linnaeus) and Perca flavescens (Mitchill) in the Fuller Pond (Massachusetts, USA), the Daphnia having a hatching success of 1.5% following passage through the guts. On the other hand, in an experiment conducted by Jarnagin et al. (2000) on controlled laboratory feeding experiment involving storage at 4°C and incubation at 10°C, removal of 61 mature resting eggs of the cladoceran Bythotrephes cederstroemi from fish fecal pellets of L. macrochirus, Micropterus dolomieu, and P. flavescens (Michigan Lake, USA) resulted in development of 57% and hatching of 41% of the resting eggs. Also, in another laboratory experiment Jarnagin et al. (2004) found that out of 113 mature resting eggs of B. cederstroemi consumed by P. flavescens, L. macrochirus, L. gibbosus, and M. dolomieu 94% survived gut passage. It should be noted, though, that none of the studies on survival of gut passage were assemblage based (including the rotifers, copepods, and cladocerans) but used only a single planktonic species preyed upon by fish.
The results of our study suggest that the survival of zooplankton resting stages after passage through the fish gut could be important for dispersal via P. lineatus migration and that, this migratory ilyophagous fish may widely disperse zooplankton resting stages.
Hatching of zooplankton and treatment effects
The results of our laboratory study show that diversity and abundance of zooplankton species are affected by the storage conditions of the gut contents prior to exposure. The highest richness and abundance were found in the assemblage of zooplankton hatchlings from gut content kept under cold-dark conditions. The high abundance of rotifers hatching during the experiment may be due to in situ reproductive events that take place over a period of 4 days at 25°C (Edmondson, 1965). Similarly, studies undertaken by Chittapun et al. (2005) on rotifers in dried sediments in Thailand, exposed to similar storage conditions for a 2-year period, recorded 15 species after cold-dark treatment and 9 after ambient-dark treatment. The authors argue that the effect of exposure conditions only becomes significant after 6 months; however, we found that an effect can in fact be observed after three months. It should be noted that in both treatments the highest abundance and species richness were recorded for rotifers. Moreover, the hatching of cladocerans and copepods was clearly favored by the exposure of gut contents to cold-dark storage. In the case of copepods, cold storage appeared to be critical for emergence as they were only recorded after exposure to this stimulus.
The emergence of cladocerans was very low, particularly seen in the light of the current knowledge of the cladocerans fauna in the Argentinean Parana River basin, which holds 42 genera and about 100 species of Anomopoda and Ctenopoda (Paggi, 2004). Studies on sediments from the wetlands of floodplain river systems in Brazil and Argentina performed by Maia-Barbosa et al. (2003), Palazzo et al. (2008), and Battauz et al. (2014) also recorded a low hatching rate of cladocerans. The preincubation treatment involving drying and exposure to low temperature was a suggestion as an explanation of this, but such behavior in relation to temperature was not observed in our experimental hatching of intestinal contents, and we observed that cold-dark conditions allow for the hatching of rotifers, copepods, and cladocerans.
The dominance of littoral species in the gut content of P. lineatus is seemingly related to its feeding habits. It often feeds in very shallow waters, < 30 cm deep, potentially due to the high nutritional value of shallow water detritus (Bowen, 1983). Another possible interpretation of our results is that littoral species are more likely to survive the passage through the digestive tract or respond more readily to hatching stimuli; this result may be caused by multiple factors as temperature, photoperiod, water pH, oxygen, nutrients, and bacteria not feasible to respond. Regarding the timing of hatching of the different species in the two treatments, suggestions have been forwarded that cool and dark conditions extend the diapause and increase the viability of stored resting eggs, thus preventing degradation of compounds and inhibiting bacterial development that may damage the resting eggs (Chittapun et al., 2005).
Our study provides significantly supports the hypothesis that ilyophagous fish P. lineatus may act as a dispersing vector of zooplankton by its ingestion of zooplankton resting stages present in bottom sediments. The movement of the fish in the aquatic network of a floodplain allows for passive dispersion of zooplankton, promoting colonization of new habitats and biological connectivity among communities. The theoretical frame of metacommunities provides an interesting conceptual tool for understanding ecological processes which govern the biological complexes inhabiting the floodplains of large rivers (Leibold et al., 2004). Biological connectivity of communities composed by organisms of passive dispersal strongly depends on the existence of dispersing vectors. In floodplains the main mechanism of dispersal for the zooplankton is hydrochory transporting of the active and passive individuals in the direction of flow, downstream and transversally. Any upstream dispersal is achieved by zoochory carried out mainly by migrating vertebrates which move in that direction, so endozoochory performed by an abundant and active migrating fish, constitutes an important factor in maintaining biological diversity and connectivity of the floodplain complex. Moreover, it have been shown that there is a close relationship between resilience and biological connectivity as well as the important role of some animals in ecosystem processes and organization as dispersal agents (Frank & Wissel, 1998; Montoya et al., 2008; Botsford et al., 2009).
Our experimental results demonstrate that an important number of zooplankton species, mostly rotifers, was able to hatch successfully after passage through the digestive tract of P. lineatus. Moreover, we found that incubation at 25°C, preceded by a cooling period, results in hatching of more species and individuals than storage at room temperature without subsequent cooling. Future experimental works in order to analyze the dispersal probabilities should include the assessment of the proportion of viability of the ingested eggs. This task would be particularly difficult, mainly for rotifers and copepods, because of the likelihood of erroneous inclusion of propagules of other organisms. Picking up of resting stages from sediments could be somewhat easier in the case of cladocerans which are, in general, larger and included in more visible structures (ephippium) which are relatively identifiable at level of families, genera, and even species in limited groups. Anyway, future advances in this type of research are largely conditioned by the development of taxonomical tools specifically designed to identify the resting stages of organisms belonging to the local fauna.
References
Alekseev, V. R., 2002. Copepoda. In Fernando, C. H. (Ed.), A Guide to Tropical Freshwater Zooplankton. Backhuys Publishers, Leiden: 123–188.
Allen, M. R., 2010. Genetic and environmental factors influence survival and hatching of diapausing eggs. Limnology and Oceanography 55: 549–559.
Angelescu, V. & F. S. Gneri, 1949. Adaptaciones del aparato digestivo al régimen alimenticio de algunos peces del rio Uruguay y rio de La Plata. Tipo omnívoro e iliófago en representantes de las familias “Loricaridae” y “Anostomatidae”. Revista Ciencias Zoológicas 1: 162–214.
Battauz, Y. S., S. B. José de Paggi & J. C. Paggi, 2014. Passive zooplankton community in dry littoral sediment: reservoir of diversity and potential source of dispersal in a subtropical floodplain lake of the Middle Paraná River (Santa Fe, Argentina). International Review of Hydrobiology 99: 277–286.
Battistoni, P., 1995. Copepoda. In Lopretto, E. & G. Tell (eds), Ecosistemas de aguas continentales. Metodologías para su estudio. Ediciones Sur, La Plata: 953–971.
Bayo, V. & E. Cordiviola de Yuan, 1996. Food assimilation of a neotropical riverine detritivorous fish, Prochilodus lineatus, studied by fatty acid composition (Pisces, Curimatidae). Hydrobiologia 330: 81–88.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Bonetto, A. & H. P. Castello, 1985. Pesca y piscicultura en aguas continentales de América Latina. OEA, Washington.
Bonetto, A. A., E. Cordiviola de Yuan, C. Pignalberi & O. Oliveros, 1969. Ciclos hidrológicos del río Paraná y las poblaciones de peces contenidas en las cuencas temporarias de su valle de inundación. Physis 29: 213–223.
Bonetto, A. A., M. Canon Veron & D. Roldan, 1981. Nuevos aportes al conocimiento de las migraciones de peces en el río Paraná. Ecosur 8: 29–40.
Bonetto, A. A., I. Waiss & H. Castello, 1989. The increasing damming of the Paraná basin and its effects on the lower reaches. Regulated Rivers 4: 333–346.
Botsford, L. W., J. W. White, M. A. Coffroth, C. B. Paris, S. Planes, T. L. Shearer, S. R. Thorrold & G. P. Jones, 2009. Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs 28: 327–337.
Bowen, S. H., 1983. Detritivory in neotropical fish communities. Environmental Biology of Fishes 9: 137–144.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Cáceres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408.
Chittapun, S., P. Pholpunthin & H. Segers, 2005. Restoration of tropical peat swamp rotifer communities after perturbation: an experimental study of recovery of rotifers from the resting egg bank. Hydrobiologia 546: 281–289.
Clarke, K. R. & R. M. Warwick, 1994. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRIMER-E, Plymouth.
Cook, C. D. K., 1988. Dispersion in aquatic and amphibious vascular plants. In Crawford, R. M. M. (Ed.), Plant Life in Aquatic and Amphibious Habitats. Blackwell Scientific Publications, Oxford: 179–190.
Correa, S. B., K. O. Winemiller, H. Lopez-Fernandez & M. Galett, 2007. Evolutionary perspectives on seed consumption and dispersal by fishes. Bio Science 57: 748–756.
Del Barco, D., D. Demonte, R. P. Sánchez & A. Espinach Ros, 2007. Antecedentes. In Espinach Ros, A. & R. P. Sanchez (eds), Proyecto de Evaluación del Recurso Sábalo en el Paraná. Informe de los resultados de la primera etapa 2005–2006 y medidas de manejo recomendadas. Serie Pesca y Acuicultura: Estudios e investigaciones aplicadas Buenos Aires. SAGPyA, Buenos Aires: 15–18.
Duffy, M. A., L. J. Perry, C. A. Kearns, L. J. Weider & N. G. Hairston, 2000. Paleogenetic evidence for a past invasion of Onondaga Lake, New York, by exotic Daphnia curvirostris using mtDNA from dormant eggs. Limnology and Oceanography 45: 1409–1414.
Edmondson, W. T., 1965. Reproductive rate of planktonic rotifers as related to food and temperature. Ecological Monographs 35: 61–111.
Espinach Ros, A. & C. M. Fuentes, 2000. Recursos pesqueros y pesquerías de la cuenca del plata. In Bezzi, S., R. Akselman & E. Boschi (eds), Sintesis del estado de las pesquerías marítimas Argentinas y de la Cuenca del Plata). Instituto Nacional de investigación y Desarrollo Pesquero, Mar del Plata: 353–388.
Frank, K. & C. Wissel, 1998. Spatial aspects of metapopulation survival – from model results to rules of thumb for landscape management. Landscape Ecology 13: 363–379.
Garcia-Roger, E. M., X. Armengol, M. J. Carmona & M. Serra, 2008. Assessing rotifer diapausing egg bank diversity and abundance in brackish temporary environments: an ex situ sediment incubation approach. Fundamental and Applied Limnology 173: 79–88.
Gyllström, M. & L. A. Hansson, 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquatic Science 66: 274–295.
Hairston, N. G., M. Kearns & D. R. Engstrom, 1995. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76: 1706–1711.
Hammer, Ø., D. A. T. Harper & P.D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia [available on internet at http://www.palaeo-electronica.org/2001_1/past/past.pdf].
Harrison, S. & A. D. Taylor, 1997. Empirical evidence for metapopulation dynamics. In Hanski, I. A. & M. E. Gilpin (eds), Metapopulation Biology. Academic Press, San Diego, CA: 27–42.
Havel, J. E. & J. B. Shurin, 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238.
Hutchinson, G. E., 1967. A Treatise on Limnology. I. Introduction to Lake Biology and Limnoplankton. Wiley, New York.
Iriondo, M., 1993. Geomorphology and late quaternary of the Chaco (South America). Geomorphology 7: 289–303.
Jarnagin, S. T., B. K. Swan & W. C. Kerfoot, 2000. Fish as vectors in the dispersal of Bythotrephes cederstroemi: diapausing eggs survive passage through the gut. Freshwater Biology 43: 579–589.
Jarnagin, S. T., W. C. Kerfoot & B. K. Swan, 2004. Zooplankton life cycles: direct documentation of pelagic births and deaths relative to diapausing egg production. Limnology and Oceanography 49: 1317–1332.
Jenkins, D. G. & M. O. Underwood, 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiology 387: 15–21.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Kořínek, V., 2002. Cladocera. In Fernando, C. H. (Ed.), A Guide to Tropical Freshwater Zooplankton. Backhuys Publishers, Leiden: 69–122.
Korovchinsky, N. M., 1992. Sididae and Holopediidae (Crustacea: Daphniiformes). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, Vol. III. SPB Academic Publishing, Amsterdam.
Koste, W., 1978. Die Rädertiere Mitteleuropas. Borntraeger, Berlin.
Leccia, M. F., 1972. Consideraciones sobre la sistemática de la familia Prochilodontidae (Osteichthyes, Cypriniformes), con una sinopsis de las especies de Venezuela. Acta Biologica Venezuelica 8: 35–96.
Leibold, M. A., M. Holyoak, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Magurran, A., 1988. Ecological Biodiversity and its Measurement. Princeton University Press, New York.
Maia-Barbosa, P. M., E. M. Eskinazi-Santanna, C. F. Valadares & G. C. D. Pessoa, 2003. The resting eggs of zooplankton from a tropical, eutrophic reservoir (Pampulha Reservoir, south-east Brazil). Lakes & Reservoirs: Research & Management 8: 269–275.
Mellors, W. K., 1975. Selective predation of ephippial Daphnia and the resistance of ephippial eggs to digestion. Ecology 56: 974–980.
Montoya, D., M. A. Zavala, M. A. Rodríguez & D. W. Purves, 2008. Animal versus wind dispersal and the robustness of tree species to deforestation. Science 320: 1502–1504.
Paggi, J. C., 2004. Importancia de la fauna de “Cladóceros” (Crustacea, Branchiopoda) del Litoral Fluvial Argentino. INSUGEO Miscelanea 12: 239–246.
Palazzo, F., C. Costa Bonecker & A. P. C. Fernandez, 2008. Resting cladoceran eggs and their contribution to zooplankton diversity in a lagoon of the Upper Paraná River floodplain. Lakes & Reservoirs: Research & Management 13: 207–214.
Pollux, B. J. A., 2011. The experimental study of seed dispersal by fish (Ichthyochory). Freshwater Biology 56: 197–212.
Proctor, V. W., 1964. Viability of crustacean eggs removed from ducks. Ecology 45: 656–658.
Proctor, V. W., C. R. Malone & V. L. De Vlaming, 1967. Dispersal of aquatic organisms: viability of disseminules recovered from the intestinal tracts of captive Killdeer. Ecology 48: 672–676.
Rossi, L. M., 1992. Evolución morfológica del aparato digestivo de postlarvas y prejuveniles de Prochilodus lineatus (Val., 1847) (Pisces, Curimatidae) y su relación con la dieta. Revista Hidrobiologia Tropical 25: 159–167.
Santangelo, J. M., L. Rabelo Araújo, F. A. Esteves & M. Manca, 2011. Method for hatching resting eggs from tropical zooplankton: effects of drying or exposing to low temperatures before incubation. Acta Limnologica Brasiliensia 23: 42–47.
Segers, H., 1995. Rotifera. In Dumont, H. J. (Ed.), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. Backhuys Publishers, Leiden: 1–226.
Segers, H. & D. Smet, 2008. Diversity and endemism in Rotifera: a review, and Keratella Bory de St Vincent. Biodiversity & Conservation 17: 303–316.
Simberloff, D. & M. Rejmanek (eds), 2010. Encyclopedia of Biological Invasions. University of California Press, Berkeley.
Sørensen, T. A., 1948. Method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of vegetation on Danish commons. Videnski Selskab Biologiske Skrifter 5: 1–34.
Shiel, R. J., K. F. Walker & W. D. Williams, 1982. Plankton of the lower River Murray South Australia. Australian Journal of Marine & Freshwater Research 33: 301–327.
Shurin, J. B. & J. E. Havel, 2003. Hydrologic connections as dispersal roues for the spread of the exotic cladoceran Daphnia lumholtzi. Biological Invasions 4: 431–439.
Tablado, A. & N. Oldani, 1984. Consideraciones generales sobre las migraciones de peces en el río Paraná. Boletín de la Asociación de Ciencias Naturales del Litoral 4: 31–34.
Torres, R. & E. Zoppi de Roa, 2010. Latencia en cladóceros y copépodos (Crustacea) de un humedal de la península de Paria, Venezuela. Metodos en Ecologia y Sistematica 5: 23–35.
Vandekerkhove, J., B. Niessen, S. Declerck, E. Jeppesen, J. M. C. Conde-Porcuna, L. Brendonck & L. De meester, 2004. Hatching rate and hatching success with and without isolation of zooplankton resting stages. Hydrobiologia 526: 235–241.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. C. Conde-Porcuna, E. Jeppesen, L. S. Johansson & L. De meester, 2005. Uncovering hidden species: hatching diapausing eggs for the analysis of cladoceran species richness. Limnology and Oceanography Methods 3: 399–407.
Wilson, D. S., 1992. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 73: 1984–2000.
Acknowledgments
This research was funded by Provincia de Santa Fe (SECTeI 2010-037-11). We thank Lic. Danilo Demonte and Dr. Pablo Scarabotti for their generous assistance to the collection of fish samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Rights and permissions
About this article
Cite this article
Battauz, Y.S., de Paggi, S.B.J. & Paggi, J.C. Endozoochory by an ilyophagous fish in the Paraná River floodplain: a window for zooplankton dispersal. Hydrobiologia 755, 161–171 (2015). https://doi.org/10.1007/s10750-015-2230-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2230-4