Abstract
Biofilms play a key role in self-depuration processes in rivers. Whilst meiofauna is known to be abundant within river phototrophic biofilms and to perform both grazing and bioturbation within these matrixes, it is still unknown whether the activity of biofilm-associated meiofauna can influence the ability of biofilms to improve river water quality. In this study, we explored the effects of nutrient enrichment on river biofilm N–NO3 − uptake rates and associated meiofauna in microcosms for 5 days under nutrient-enriched/non-enriched conditions. Short-time nutrient enrichment stimulated biofilm-associated bacterial and rotifer density, as well as the biofilm uptake rates of N–NO3 −, but not algal biomass. Under non-enriched conditions, N–NO3 − uptake rate tended to reach a plateau around 104.2 μg g−1 AFDM h−1. At higher N–NO3 − concentrations, realised under enrichment, N–NO3 − uptake rate seemed to increase linearly, reaching up to 439.2 μg g−1 AFDM h−1. Our results showed a rapid response of rotifers to nitrate enrichment and suggest a possible link between bacteria–meiofauna interactions and the short-term N uptake capacity of biofilms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well-established that excessive nutrients concentrations, e.g. of nitrate and phosphate passing through surface- and ground-waters, have harmful consequences on ecosystem structure and functioning (Vitousek et al., 1997; Carpenter et al., 1998; Smith et al., 1999). At a time of increasing concerns about the impact of water quality on aquatic life and on the sustainability of water resources, recent works support that biological remediation (i.e. pollutant removal by microbial metabolism) by benthic biofilms appears as a promising way to cope with eutrophication threats (Singh et al., 2006; Sun et al., 2009; Cao et al., 2012).
In running waters, biofilms growing on hard submerged substrate are complex assemblages of microalgae, protozoans, fungi, bacteria and small invertebrates clustered within a self-produced mucous matrix of exopolymeric substances (Lock et al., 1984; Costerton et al., 1995). Biofilms act as a sink for nutrients in the water column, strengthen vertical and horizontal connectivity of resources and play a role in the self-depuration processes of running waters (Pusch et al., 1998; Sabater et al., 2002; Battin et al., 2003; Teissier et al., 2007). Short-term retention of nutrients via assimilatory uptake (i.e. incorporation of compounds in the biomass) in biofilms can be very high as nutrients are intensively recycled within benthic communities (Bernot & Dodds, 2005). Furthermore, specific bacterial processes such as nitrification (i.e. oxidization of NH4 + to NO3 −) and denitrification (i.e. respiratory process reducing NO3 − to N2), contribute to apparent uptake of nutrients in streams (Bernot & Dodds, 2005; Ribot et al., 2013).
Meiofauna (i.e. benthic invertebrates passing through a 500 µm mesh sieve and retained on 50 µm meshes, Giere, 2009) are extremely abundant in epilithic river biofilms (Gaudes et al., 2006; Majdi et al., 2012a). Although their grazing pressure on biofilm microphytobenthos is rather low (Majdi et al., 2012b, c; Mialet et al., 2013), their activity within the biofilm can affect oxygen turnover (Mathieu et al., 2007) and seemingly other key processes such as biofilm detachment and the release of secondary metabolites in the water column (Sabater et al., 2003; Gaudes et al., 2006). Recently, Derlon et al. (2013) have shown that, in gravity-driven membrane filtration water depuration systems, the presence of nematodes and oligochaetes increases the heterogeneity and porosity of membrane-associated microbial biofilms, and consequently increases the efficacy of filtration process used to treat organically polluted waters. Riemann & Helmke (2002) report that locomotion of nematodes creates dense micro-burrows from agar plate matrixes. Studies examining the impact of meiofauna on the microarchitecture, porosity and biogeochemical activity of biofilm have shown that meiofauna can: (1) stimulate the growth of bacteria and associated mineralization processes e.g. through agglutination of detritus particles by mucus secretions or proteolytic capacity (e.g. Riemann & Helmke, 2002; Nascimento et al., 2012); (2) enhance the primary productivity and oxygen turnover of diatom biofilms (Mathieu et al., 2007) and (3) likely modify light penetration and increase solute transport rates in superficial sediments (e.g. Pinckney et al., 2003). Thus, it is likely that positive interactions between meiofauna and microorganisms occur in epilithic biofilms. Since microphytobenthos and bacteria are key organisms involved in organic and inorganic nutrient retention processes in biofilms (Sabater et al., 2002; Cardinale, 2011), it can be expected that the interactions between meiofauna and microorganisms stimulate the self-depuration processes associated with biofilms in natural running waters.
Human activities can modify nutrient concentrations in streams, sometimes on short timescales, for example pulses caused by agricultural runoff during high rainfall periods (Lassaletta et al., 2009). Stream biofilms can adapt their uptake rate of nutrients according to nutrient availability and speciation in the environment (e.g. Dodds, 2003; Bernot & Dodds, 2005; Ribot et al., 2013), generally following a Michaelis–Menten response (but see “Discussion”) (Payn et al., 2005; Earl et al., 2006; Covino et al., 2010; O’Brien & Dodds, 2010). Moreover, recent studies report that nutrient enrichment can induce increase in the density of marine and freshwater sediment-dwelling meiofauna, although the observed functional responses are slow and highly variable (e.g. Hillebrand et al., 2002; Posey et al., 2002; Mitwally & Fleeger, 2013; Ristau et al., 2013). As detailed above, since it has been shown that meiofauna can influence primary productivity and stimulate bacterial growth, we hypothesized that biofilm-dwelling meiofauna could indirectly influence biofilm functions related to nutrient uptake. As the first step, this study aims to examine the short-term response of biofilm-dwelling meiofauna and microbial communities to nitrate enrichment in relation with the dynamics of the biofilm uptake rates of nitrates.
Methods
In situ biofilm growth
We wedged a total of 36 rubber stoppers (upper surface area = 12.56 cm2) onto the river bed in the middle part of the Garonne River, 30 km upstream Toulouse (location: 01°17′50″E, 43°23′43″N; elevation: 175 m a.s.l.). The Garonne River catchment is the third largest in France (~57,000 km2). This catchment is mostly agricultural, containing only one major urban area: Toulouse (>1 million inhabitants). At this site, nutrient conditions are oligotrophic (Lyautey et al., 2003; Muylaert et al., 2009), and a shallow river bed together with a low shading by riparian vegetation usually allows a thick epilithic phototrophic biofilm, crowded with meiofauna, to coat any hard submerged substrates (Majdi et al., 2012a). Biofilm colonization of rubber stoppers was allowed for 56 days (20th September–15th November 2012), a period deemed long enough for the establishment of mature biofilm communities in temperate rivers (e.g. Norf et al., 2009). The ambient N–NO3 − concentration at the study site—measured on water river samples collected for non-nutrient-enriched microcosms (see below)—ranged between 0.48 and 0.55 mg l−1 during the biofilm incubation.
At the end of the colonization period, the rubber stoppers covered by biofilm were retrieved and immediately placed in polyethylene microcosms (Ø 52 mm, h 68 mm) filled with 100 ml river water. Meanwhile, 40 l of river water were sampled. Microcosms and water were transported to the laboratory in cool boxes within 2 h, with minimal disturbance.
Experimental design
The experimental design consisted in two biofilm conditions (with biofilm: BIOF and without biofilm: WAT) crossed with two nutrient availability conditions (NAT: non-enriched and NUT: nutrient-enriched) (Fig. 1). So, our experiment had four treatments: (1) clean rubber stopper incubated with non-enriched Garonne water (NAT-WAT, n = 6), (2) clean rubber stopper incubated with nutrient-enriched water (NUT-WAT, n = 6), (3) rubber stopper covered by biofilm incubated with non-enriched Garonne water (NAT-BIOF, n = 12), (4) rubber stopper covered by biofilm incubated with nutrient-enriched water (NUT-BIOF, n = 12). Considering high variability of biofilm samples, the numbers of BIOF treatments were doubled (12 instead of 6 for WAT treatments). All biofilm microcosms were incubated for 5 days under the same experimentally controlled conditions (see below).
The nutrient concentrations in the Garonne River generally range for a total of N concentrations from 0.7 to 32 mg l−1, for a total of P concentrations from 0.1 to 2.5 mg l−1, respectively (Ameziane et al., 2002; Leflaive et al., 2008; Muylaert et al., 2009; Sow, unpublished data). We used GF/C filtered water river as a non-nutrient-enriched treatment (NAT). For the nutrient-enriched treatment (NUT), we added KNO3 (10 mg l−1, i.e. NO3 −, 6.14 mg l−1), Na2HPO4 (1 mg l−1) and CH3COONa·3H2O (30 mg l−1) to GF/C filtered river water in order to mimic downstream eutrophic condition as indicated in previous study (DIN > 2 mg l−1 and SRP > 20 μg l−1, Muylaert et al., 2009) i.e. at the beginning of the experiment, in NAT treatment: N–NO3 −, 0.54 ± 0.002 mg l−1; P-PO4 3−, 14 ± 0.8 μg l−1, in NUT treatments: N–NO3 −, 2.03 ± 0.004 mg l−1; P-PO4 3−, 401 ± 3.4 μg l−1 (n = 6, SE). High phosphate and acetate (as carbon substrate) addition into the NUT was used to prevent microbial growth limitation in microcosms during the experiment.
In the laboratory, the bottom and sides of the rubber stoppers were carefully scrubbed (to restrain biofilm only to upper surfaces), and the stoppers were quickly transferred into new microcosms filled with 100 ml GF/C filtered Garonne water. Microcosms were acclimatized during 24 h to experimentally controlled conditions: i.e. 10°C; light:dark 12:12 h, 2,300 lm m−2. After the 24 h acclimatization, the water in the microcosms was carefully removed and replaced by non-enriched Garonne water (NAT treatment: 18 microcosms) or nutrient-enriched (NUT treatment: 18 microcosms).
Sample treatment
One ml of water was sampled daily (t = 4, 24, 48, 72, 96, 120 h) from each microcosm and filtered (0.22 µm PTFE syringe filter) prior to analysis of Cl− and N–NO3 − concentrations by high-performance ionic chromatography (Dionex DX-120, Thermo Fisher Scientific Inc., Waltham, MA, USA) following standard procedures (NF EN ISO 10304-1, 1995). For each biofilm treatment, meiofauna density and biomass as well as density of bacteria and pigment concentrations were determined at the end of the experimental period (t = 120 h). The biofilm covering stoppers was gathered from each microcosm and divided into 3 subsamples for the following measurements:
-
(1)
For meiofaunal density and biomass quantification, half of the total biofilm surface on stoppers (i.e. 6.28 cm2) was carefully scraped, and preserved in 10 ml formaldehyde solution (5% final concentration) with addition of 100 µl of 1% Rose Bengal stain. Meiofauna were counted in a Dolfuss cell (Elvetec Services, Clermont-Ferrand, France) under a stereomicroscope (9–90×). A number of individual nematodes (n = 21) and rotifers (n = 32) were photographed to measure their body dimensions using ImageJ software version 1.46r (Abramoff et al., 2004). Mean individual dry mass (DM) was assessed from standard biometric conversions of the organism’s body dimensions (Giere, 2009; Majdi et al., 2012a), and multiplied by their density in biofilm to estimate biomass data.
-
(2)
For bacterial density measurement, a 200-µl subsample of the previously described homogenized 10 ml formaldehyde-fixed sample (containing biofilm and associated meiofauna) was used following a standard DAPI-staining method (Porter & Feig, 1980). A gentle sonication step was carried out to maximize bacterial detachment from algal aggregates prior counting (Buesing & Gessner, 2002). Bacterial counting was performed under a Leitz Dialux microscope (1,250×) fitted for epifluorescence: HBO 100 W mercury light source (Osram, Winterthur, Switzerland), with an excitation filter for 270 and 450 nm, a barrier filter of 410 and a 515 nm cut-off filter. All density calculations of bacteria were referred to the corresponding scraped biofilm area.
-
(3)
For the assessment of the algal community composition and biomass, a quarter of the total biofilm surface on stoppers (i.e. 3.14 cm2) was scraped, pelletized (3,220×g, 20 min) and freeze-dried to remove excess water. Biofilm pellets were weighed, and algal pigments from each obtained pellet were extracted (15 min at −20°C) in a total of 5 ml 98% cold-buffered methanol with 2% of 1 M ammonium acetate (Buffan-Dubau & Carman, 2000). Algal pigment release was favoured by ultra-sonication (Sonifier 250A, Branson Ultrasonics corp., Danbury, CT, USA). One ml of the pigment extract so obtained was then filtered on 0.2 µm PTFE syringe filter and analysed using a high-performance liquid chromatograph (HPLC) consisting of a 100 µl loop auto-sampler and a quaternary solvent delivery system coupled to a diode array spectrophotometer (LC1200 series, Agilent Technologies inc., Santa Clara, CA, USA). The mobile phase was prepared and programmed according to the analytical gradient protocol described in Barlow et al. (1997). Pigment separation was performed through a C8, 5 µm column (MOS-2 HYPERSIL, Thermo Fisher Scientific Inc.). The diode array detector was set at 440 nm to detect carotenoids, and at 665 nm to detect chlorophylls and phaeopigments (Wright et al., 1991). Data analysis was performed using ChemStation software (version A.10.02, Agilent Technologies Inc.). Pigments were identified by comparing their retention time and absorption spectra with those of authentic standards (DHI LAB products, Hørsholm, Denmark). For pigment quantification, a response factor was calculated for each standard from the linear relationship between the concentration and the corresponding peak area on HPLC chromatograms.
-
(4)
For the assessment of biofilm biomass, the remaining quarter of the total biofilm surface on stoppers (i.e. 3.14 cm2) was scraped in aluminium cups, dried overnight at 55°C, weighted for its DM, then combusted during 8 h at 450°C to determine its ash-free dry mass (AFDM).
Data analysis and statistics
Chloride (Cl−) is recognized to be non-reactive in ecosystems (Schlesinger & Bernhardt, 2013). Thus, we used the changes in Cl− concentrations during the experiment to calibrate N–NO3 − concentrations against potential increase caused by both water evaporation and the previous samplings as Eq. 1:
where \(C_{0}^{{\prime }}\) and \(C_{\text{t}}^{{\prime }}\) (mg l−1) are the N–NO3 − concentrations before and after calibration at a given time (t in hours), respectively; \(C_{{0({\text{Cl}}^{ - } )}}\) is the initial Cl− concentration; \(C_{{{\text{t}}({\text{Cl}}^{ - } )}}\) is the Cl− concentration at a given time (t in hours).
We calculated the N–NO3 − uptake rates measured in the water phase of the microcosms as U (μg h−1) with Eq. 2:
where 0.1 is the volume of water in each microcosm in litre, ΔC t is N–NO3 − concentration difference (in µg l−1) between mean concentrations recorded in the water of the microcosms at 4 h (used as the concentration at the outset of the incubation) and the concentration recorded at a given time (t in hour). The N–NO3 − uptake rates calculated for the water of the microcosms with/without biofilm were called U BIOF and U WAT, respectively.
We calculated the N–NO3 − specific uptake rates realized by the biofilm as U biofilm (μg g−1 AFDM h−1) with Eq. 3:
where AFDMbiofilm is the ash-free dry mass of the biofilm in NAT/NUT-BIOF at the end of the experiment, which is assumed as a constant for the two treatments throughout the short-term experiment.
Differences in U biofilm of N–NO3 − and in meiofaunal density and biomass between treatments were analysed by t test. Assumption of homoscedasticity was tested with Levene’s test. Data failing to fulfil homoscedasticity were log-transformed. The correlations between the U biofilm of N–NO3 − in each treatment and time were tested with Spearman’s rank. All model-fitting calculations and statistical tests were performed using R software version 3.0.2 (R Core Team, 2013).
Results
Biofilm-associated meiofaunal, microalgal and bacterial communities
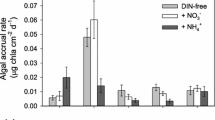
At the end of the experimental period (Fig. 2), nematodes, rotifers and chironomidae larvae were found in the biofilm and the first two dominated the meiofaunal community in terms of density (rotifers: 84.69%, nematodes: 10.35%, chironomidae larvae: 4.96%). The density and biomass of rotifers were significantly (i.e. twofold) higher under nutrient-enriched (NUT) versus non-enriched (NAT) conditions (P < 0.001). Though no significant trend was recorded for nematodes (P > 0.05), the higher density and biomass of rotifers under nutrient-enriched conditions resulted in a significant increase of total meiofauna density in NUT samples (P < 0.05). Chironomidae larvae were found in low densities: 0.74 ± 0.22 and 1.07 ± 0.74 ind. cm−2, respectively under NAT and NUT conditions between which no significant differences were found (P = 0.55). Bacterial density was higher under nutrient-enriched conditions (Fig. 3a; P < 0.05). However, this did not globally influence the total biofilm biomass (Fig. 3b; P > 0.05). The presence of typical biomarker pigments for diatoms (i.e. chlorophyll c, fucoxanthin and diadinoxanthin) indicated that biofilm algal communities were dominated by diatoms. Neither biomarker pigments nor chlorophyll a concentrations showed a significant change under nutrient-enriched conditions (Fig. 4).
N–NO3 − biofilm uptake rates and kinetics
The U biofilm of N–NO3 − was significantly higher in NUT-BIOF at all sampling occasions than in NAT-BIOF (Fig. 5, P < 0.001 for all dates). U biofilm decreased significantly in NUT-BIOF and NAT-BIOF with increasing time after the start of the experiment, though the decrease was steepest in NUT-BIOF (Spearman’s rank correlation; NAT-BIOF: r = −0.65, P < 0.001; NUT-BIOF: r = −0.80, P < 0.001).
The data from both treatments (NAT-BIOF and NUT-BIOF) were pooled in Fig. 6 and biofilm specific uptake rates values were related to N–NO3 − concentrations measured in the microcosm water. The biofilm uptake rate values from each treatment (NAT-BIOF and NUT-BIOF) were very similar for similar N–NO3 − concentrations (i.e. between 0.2 and 0.6 mg l−1) occurring in both the NAT-BIOF and NUT-BIOF in the course of the experiment. Our results suggest that uptake ability of the biofilm reached a plateau (around 104.2 μg g−1 AFDM h−1) under low nutrient concentrations, i.e. between 0.2 and 0.6 mg l−1 (Fig. 6). However, when exceeding this threshold, biofilm specific uptake rates seemed to increase linearly and consistently within the concentration range met in our experiment.
Discussion
Effects of nutrient enrichment on biofilm-dwelling meiofauna
The effects of nutrient enrichment on meiofauna are well-documented in both freshwater and marine sediments, but rarely in biofilms. Recently Kazemi-Dinan et al. (2014) compared the biofilm-dwelling meiofauna community across different lake trophic states and highlighted that nematode density and functional richness correlate positively with nutrient availability. This paper provided a rationale that changes in nutrient loads that primarily affect the composition of microbial communities and that bottom-up effect is differently transmitted to meiofaunal taxa, based on their feeding preferences. The results of the studies in sediments are somewhat divergent. Recently, Mitwally & Fleeger (2013) reported inconsistent and variable responses of densities of saltmarsh meiofauna to long-term (5 years) nutrient enrichment in marine muddy sediments. In contrast, nematodes responded to nutrient enrichment by increasing their density in marine sediments after 38–70 days (Wormald & Stirling, 1979) and in lake sediments during a 16-month experiment (Ristau et al., 2013). These findings suggest that meiofaunal responses are rather slow and can take months to years to develop (Hillebrand et al., 2002; Posey et al., 2002; Mitwally & Fleeger, 2013), and that long-term impacts of nutrient enrichment on density and/or biomass of meiobenthic invertebrates are context-dependant and comparatively weaker than the responses of meiofauna to other factors, such as temperature and biotic constraints (e.g. resource availability) (Hulings & Gray, 1976; Majdi et al., 2011; Ristau et al., 2013).
Studies of lacustrine meiofauna have shown that the density of lacustrine rotifers can increase with the increasing phosphorus concentrations in lake habitats (Särkkä, 1992; Wu et al., 2004; Ristau & Traunspurger, 2011) and in microcosm sediments (Ristau et al., 2012). Ristau et al. (2012) propose that the observed responses are indirectly linked to a nutrient-induced change in the availability of food (e.g. of unicellular diatoms and green algae) in experimental treatments. By comparison with our microcosms where bacterial density increased with nitrate enrichment but algal biomass did not, our results suggest that rotifer density and biomass indirectly responded to nitrate enrichment through consumption of bacteria. Indeed, benthic rotifers can consume a wide variety of preys such as algae, bacteria and yeast (e.g. Ricci & Balsamo, 2000; Duggan, 2001; Mialet et al., 2013). Moreover, previous studies report that the response of lacustrine meiofauna to nutrient addition differs amongst meiobenthic taxa (Särkkä, 1992; Wu et al., 2004; Ristau & Traunspurger, 2011; Ristau et al., 2012). Our results support these findings. Although in the Garonne River, meiofauna consist mainly of nematodes from the family Chromadoridae (Chromadorina bioculata and Chromadorina viridis) and bdelloidae rotifers (Majdi et al., 2012a), only rotifer density was increased by nutrient enrichment within the 5 days of our experiment. This was likely due to lower rates of population turnover of nematodes compared to rotifers which have parthenogenetic reproduction and short-time life cycles allowing them to show quick community responses to improving ambient conditions (Ricci & Balsamo, 2000; Majdi et al., 2012a). Considering a larger time-window would have been more appropriate to detect responses of nematode populations to nutrient enrichment.
Nevertheless, our study shows that in rivers, where rotifers are important contributors to the biofilm-dwelling meiofauna (Reiss & Schmid-Araya, 2008; Kathol et al., 2011; Majdi et al., 2012a), biofilm lotic meiofauna can potentially react rapidly to short-term nutrient enrichment (e.g. short-term nutrient pulses after rainfall-induced runoff from agricultural catchments). We suggest that the increase in the bacterial biomass enhanced food availability for biofilm-associated rotifers. The short-term response of meiofauna to nutrient enrichment has been previously overlooked and our results provide a first assessment of this response.
Nitrate uptake and kinetics
Our results support previous field enrichment experiments reporting that, in streams, nutrient uptake increases as environmental nutrient concentrations are increased (e.g. Dodds et al., 2002; Earl et al., 2006). Concerning uptake kinetics, when enrichment experiments use a given community, results often suggest that Michaelis–Menten model best fits DIN uptake kinetics (Payn et al., 2005; Earl et al., 2006; Covino et al., 2010; O’Brien & Dodds, 2010). Ribot et al. (2013), however, found that Michaelis–Menten model fits uptake of stream biofilms for NH4 + but not for NO3 − in a channel experiment. Michaelis–Menten kinetics is characterized by saturation of uptake meaning that availability exceeds biological demand (Earl et al., 2006). In our results, biofilm uptake rate of nitrogen seemed to reach a plateau under low nutrient concentrations but it tended to increase under higher N–NO3 − concentrations. Such differences in biofilm response (i.e. with or without saturation kinetics) have been previously reported. O’Brien & Dodds (2010) proposed that they were related to variations in biofilm biomass amongst the different streams considered in their study. However, this cannot explain our results since (1) we standardised N–NO3 − uptake rates for biofilm biomass and (2) we did not observe significant difference in biofilm biomass between treatments.
The lack of response of microalgal biomass to N–NO3 − enrichment could be due to the relatively high nitrate concentrations—ranging from 266 to 8,857 µg l−1 (i.e. from 60 to 2,000 µg l−1 of N–NO3 −)—which were above the growth-limiting level for freshwater benthic algae (i.e. >50–100 µg l−1, e.g. Stevenson et al., 1996) in both enriched and non-enriched treatments. Alternatively, since microalgal production was not measured, it can be envisaged that microalgal production might have been stimulated even though their biomass did not change. Considering that rotifers are effective grazers in river biofilms (Kathol et al., 2011; Mialet et al., 2013), the grazing of algae by increasing density of rotifer might on the one hand favour bacteria in the competition for N–NO3 − and on the other hand keep the algal population in an active growth phase and hence stimulate N–NO3 − uptake of the biofilms at high N–NO3 − concentration. We can, hence, not exclude that stimulated microalgal growth also participated in the increased N–NO3 − uptake in the enriched conditions.
Conversely, bacteria responded to nitrate enrichment by a density increase. Nitrate uptake results from assimilatory processes (i.e. incorporating nitrate into biofilm biomass). Furthermore, in streams, apparent nitrate uptake may include dissimilatory transformations (in which N is not incorporated into biomass) such as denitrification (i.e. the respiratory process by which bacteria reduce NO3 − to N2) (Bernot & Dodds, 2005; Ribot et al., 2013). Considering the observed increase in bacterial density between NUT-BIOF and NAT-BIOF microcosms in our experiment, it is possible that denitrification also contributed to N–NO3 − decrease in the water (Lyautey et al., 2003). The observed uptake kinetics may result from a saturation of photosynthetic incorporation of nitrate in biofilm biomass which was likely relayed by an increase of heterotrophic bacterial transformations of NO3 − (e.g. denitrification). This statement is supported by an experiment showing that uptake of nutrients in absence of leaf litter was longer relative to systems with a natural abundance litterfall (Webster et al., 2000) supporting the idea that short-term retention of dissolved N is increased by heterotrophic organisms associated with organic matter (Bernot & Dodds, 2005). Proia et al. (2012) also observed significant response of bacterial density but not of algal biomass in microcosm biofilms. Despite this lack of algal growth, their results suggest that microalgal–bacterial interactions were enhanced by nutrient enrichment, as suggested by our experiments.
The most remarkable result of our experiment was the important and rapid increase in rotifer density and biomass in the enriched microcosms. This is most likely a result of nutrient-stimulated resources for the rotifers (probably both algae and bacteria). On the other hand, rotifers might also themselves have contributed to the stimulation of bacterial growth, by their bioturbation activity which could favour oxygen turnover and solute exchanges, as it has been shown for nematodes (Traunspurger et al., 1997; Riemann & Helmke, 2002; Mathieu et al., 2007; Nascimento et al., 2012). This is supported by the concomitant increase of bacterial and meiofaunal densities. The stimulated development of meiofauna through nutrient enrichment could, through a feedback, enhance the microbial communities and hence nutrient uptake rates of biofilms. This requires further investigations to be confirmed. Previous studies of interactions between invertebrates and biofilms suggest that macrobenthos grazing indirectly reduces the relative nutrient uptake efficiency of biofilms, by simplifying the composition of the biofilm community and by decreasing its biomass (Sabater et al., 2002). Conversely, our results suggest that interactions between biofilm-associated meiofauna and microbial community could indirectly favour the performance of biofilms in the amelioration of the quality of river water. The effect of nutrient enrichment on both meiofauna and microbial communities has been relatively well studied, particularly in lentic ecosystems (Särkkä, 1992; Wu et al., 2004; Ristau & Traunspurger, 2011; Ristau et al., 2012). Nevertheless, to the best of our knowledge, in phototrophic biofilms, the present study is the first to provide results suggesting a possible link between bacteria–meiofauna interactions and short-term N uptake capacity.
References
Abramoff, M. D., P. J. Magalhães & S. J. Ram, 2004. Image processing with Image. The Journal of Biophotonics international 11: 36–42.
Ameziane, T., F. Garabetian, D. Dalger, S. Sauvage, A. Dauta & J. Capblancq, 2002. Epilithic biomass in a large gravel-bed river (the Garonne, France): a manifestation of eutrophication? River Research and Applications 18: 343–354.
Barlow, R. G., D. G. Cummings & S. W. Gibb, 1997. Improved resolution of mono- and divinyl chlorophylls a and b and zeaxanthin and lutein in phytoplankton extracts using reverse phase C-8 HPLC. Marine Ecology Progress Series 161: 303–307.
Battin, T. J., L. A. Kaplan, J. Denis Newbold & C. M. E. Hansen, 2003. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426: 439–442.
Bernot, M. J. & W. K. Dodds, 2005. Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems 8: 442–453.
Buesing, N. & M. O. Gessner, 2002. Comparison of detachment procedures for direct counts of bacteria associated with sediment particles, plant litter and epiphytic biofilms. Aquatic Microbial Ecology 27: 29–36.
Buffan-Dubau, E. & K. R. Carman, 2000. Extraction of benthic microalgal pigments for HPLC analyses. Marine Ecology Progress Series 204: 293–297.
Cao, W., H. Zhang, Y. Wang & J. Pan, 2012. Bioremediation of polluted surface water by using biofilms on filamentous bamboo. Ecological Engineering 42: 146–149.
Cardinale, B. J., 2011. Biodiversity improves water quality through niche partitioning. Nature 472: 86–89.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559–568.
Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber & H. M. Lappin-Scott, 1995. Microbial biofilms. Annual Review of Microbiology 49: 711–745.
Covino, T. P., B. L. McGlynn & R. A. McNamara, 2010. Tracer Additions for Spiraling Curve Characterization (TASCC): quantifying stream nutrient uptake kinetics from ambient to saturation. Limnology and Oceanography: Methods 8: 484–498.
Derlon, N., N. Koch, B. Eugster, T. Posch, J. Pernthaler, W. Pronk & E. Morgenroth, 2013. Activity of metazoa governs biofilm structure formation and enhances permeate flux during Gravity-Driven Membrane (GDM) filtration. Water Research 47: 2085–2095.
Dodds, W. K., 2003. The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. Journal of Phycology 39: 840–849.
Dodds, W. K., A. J. López, W. B. Bowden, S. Gregory, N. B. Grimm, S. K. Hamilton, A. E. Hershey, E. Martí, W. H. McDowell, J. L. Meyer, D. Morrall, P. J. Mulholland, B. J. Peterson, J. L. Tank, H. M. Valett, J. R. Webster & W. Wollheim, 2002. N uptake as a function of concentration in streams. Journal of the North American Benthological Society 21: 206–220.
Duggan, I. C., 2001. The ecology of periphytic rotifers. Hydrobiologia 446–447: 139–148.
Earl, S. R., H. M. Valett & J. R. Webster, 2006. Nitrogen saturation in stream ecosystems. Ecology 87: 3140–3151.
Gaudes, A., S. Sabater, E. Vilalta & I. Muñoz, 2006. The nematode community in cyanobacterial biofilms in the river Llobregat, Spain. Nematology 8: 909–919.
Giere, O., 2009. Meiobenthology – The Microscopic Motile Fauna of Aquatic Sediments. Springer, Berlin.
Hillebrand, H., M. Kahlert, A.-L. Haglund, U.-G. Berninger, S. Nagel & S. Wickham, 2002. Control of microbenthic communities by grazing and nutrient supply. Ecology 83: 2205–2219.
Hulings, N. C. & J. S. Gray, 1976. Physical factors controlling abundance of meiofauna on tidal and atidal beaches. Marine Biology 34: 77–83.
Kathol, M., H. Fischer & M. Weitere, 2011. Contribution of biofilm-dwelling consumers to pelagic–benthic coupling in a large river. Freshwater Biology 56: 1160–1172.
Kazemi-Dinan, A., F. Schroeder, L. Peters, N. Majdi & W. Traunspurger, 2014. The effect of trophic state and depth on periphytic nematode communities in lakes. Limnologica 44: 49–57.
Lassaletta, L., H. García-Gómez, B. S. Gimeno & J. V. Rovira, 2009. Agriculture-induced increase in nitrate concentrations in stream waters of a large Mediterranean catchment over 25 years (1981–2005). Science of the Total Environment 407: 6034–6043.
Leflaive, J., S. Boulêtreau, E. Buffan-Dubau & L. Ten-Hage, 2008. Temporal patterns in epilithic biofilm – relation with a putative allelopathic activity. Fundamental and Applied Limnology/Archiv für Hydrobiology 173: 121–134.
Lock, M. A., R. R. Wallace, J. W. Costerton, R. M. Ventullo & S. E. Charlton, 1984. River epilithon: toward a structural-functional model. Oikos 42: 10–22.
Lyautey, E., S. Teissier, J.-Y. Charcosset, J.-L. Rols & F. Garabetian, 2003. Bacterial diversity of epilithic biofilm assemblages of an anthropised river section, assessed by DGGE analysis of a 16S rDNA fragment. Aquatic Microbial Ecology 33: 217–224.
Majdi, N., W. Traunspurger, S. Boyer, B. Mialet, M. Tackx, R. Fernandez, S. Gehner, L. Ten-Hage & E. Buffan-Dubau, 2011. Response of biofilm-dwelling nematodes to habitat changes in the Garonne River, France: influence of hydrodynamics and microalgal availability. Hydrobiologia 673: 229–244.
Majdi, N., B. Mialet, S. Boyer, M. Tackx, J. Leflaive, S. Boulêtreau, L. Ten-Hage, F. Julien, R. Fernandez & E. Buffan-Dubau, 2012a. The relationship between epilithic biofilm stability and its associated meiofauna under two patterns of flood disturbance. Freshwater Science 31: 38–50.
Majdi, N., M. Tackx & E. Buffan-Dubau, 2012b. Trophic positioning and microphytobenthic carbon uptake of biofilm-dwelling meiofauna in a temperate river. Freshwater Biology 57: 1180–1190.
Majdi, N., M. Tackx, W. Traunspurger & E. Buffan-Dubau, 2012c. Feeding of biofilm-dwelling nematodes examined using HPLC-analysis of gut pigment contents. Hydrobiologia 680: 219–232.
Mathieu, M., J. Leflaive, L. Ten Hage, R. De Wit & E. Buffan-Dubau, 2007. Free-living nematodes affect oxygen turnover of artificial diatom biofilms. Aquatic Microbial Ecology 49(3): 281–291.
Mialet, B., N. Majdi, M. Tackx, F. Azémar & E. Buffan-Dubau, 2013. Selective feeding of bdelloid rotifers in river biofilms. PLoS ONE 8: e75352.
Mitwally, H. M. & J. W. Fleeger, 2013. Long-term nutrient enrichment elicits a weak density response by saltmarsh meiofauna. Hydrobiologia 713: 97–114.
Muylaert, K., J. M. Sanchez-Pérez, S. Teissier, S. Sauvage, A. Dauta & P. Vervier, 2009. Eutrophication and its effect on dissolved Si concentrations in the Garonne River (France). Journal of Limnology 68: 368–374.
Nascimento, F. J. A., J. Näslund & R. Elmgren, 2012. Meiofauna enhances organic matter mineralization in soft sediment ecosystems. Limnology and Oceanography 57: 338–346.
Norf, H., H. Arndt & M. Weitere, 2009. Responses of biofilm-dwelling ciliate communities to planktonic and benthic resource enrichment. Microbial Ecology 57: 687–700.
O’Brien, J. M. & W. K. Dodds, 2010. Saturation of NO3− uptake in prairie streams as a function of acute and chronic N exposure. Journal of the North American Benthological Society 29: 627–635.
Payn, R. A., J. R. Webster, P. J. Mulholland, Hm. Valett & W. K. Dodds, 2005. Estimation of stream nutrient uptake from nutrient addition experiments. Limnology and Oceanography: Methods 3: 174–182.
Pinckney, J. L., K. R. Carman, S. E. Lumsden & S. N. Hymel, 2003. Microalgal–meiofaunal trophic relationships in muddy intertidal estuarine sediments. Aquatic Microbial Ecology 31: 99–108.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography 25: 943–948.
Posey, M. H., T. D. Alphin, L. B. Cahoon, D. G. Lindquist, M. A. Mallin & M. B. Nevers, 2002. Top-down versus bottom-up limitation in benthic infaunal communities: direct and indirect effects. Estuaries 25: 999–1014.
Proia, L., A. M. Romaní & S. Sabater, 2012. Nutrients and light effects on stream biofilms: a combined assessment with CLSM, structural and functional parameters. Hydrobiologia 695: 281–291.
Pusch, M., D. Fiebig, I. Brettar, H. Eisenmann, B. K. Ellis, L. A. Kaplan, M. A. Lock, M. W. Naegeli & W. Traunspurger, 1998. The role of micro-organisms in the ecological connectivity of running waters. Freshwater Biology 40: 453–495.
R Core Team, 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Reiss, J. & J. M. Schmid-Araya, 2008. Existing in plenty: abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshwater Biology 53: 652–668.
Ribot, M., D. von Schiller, M. Peipoch, F. Sabater, N. B. Grimm & E. Martí, 2013. Influence of nitrate and ammonium availability on uptake kinetics of stream biofilms. Freshwater Science 32: 1155–1167.
Ricci, C. & M. Balsamo, 2000. The biology and ecology of lotic rotifers and gastrotrichs. Freshwater Biology 44: 15–28.
Riemann, F. & E. Helmke, 2002. Symbiotic relations of sediment-agglutinating nematodes and bacteria in detrital habitats: the enzyme-sharing concept. Marine Ecology 23: 93–113.
Ristau, K. & W. Traunspurger, 2011. Relation between nematode communities and trophic state in southern Swedish lakes. Hydrobiologia 663: 121–133.
Ristau, K., M. Faupel & W. Traunspurger, 2012. The effects of nutrient enrichment on a freshwater meiofaunal assemblage. Freshwater Biology 57: 824–834.
Ristau, K., M. Faupel & W. Traunspurger, 2013. Effects of nutrient enrichment on the trophic structure and species composition of freshwater nematodes – a microcosm study. Freshwater Science 32: 155–168.
Sabater, S., H. Guasch, A. Romaní & I. Muñoz, 2002. The effect of biological factors on the efficiency of river biofilms in improving water quality. Hydrobiologia 469: 149–156.
Sabater, S., E. Vilalta, A. Gaudes, H. Guasch, I. Munoz & A. Romani, 2003. Ecological implications of mass growth of benthic cyanobacteria in rivers. Aquatic Microbial Ecology 32: 175–184.
Särkkä, J., 1992. Lacustrine profundal meiobenthos as an environmental indicator. Hydrobiologia 243–244: 333–340.
Schlesinger, W. H. & E. S. Bernhardt, 2013. Biogeochemistry: An Analysis of Global Change. Academic Press, San Diego.
Singh, R., D. Paul & R. K. Jain, 2006. Biofilms: implications in bioremediation. Trends in Microbiology 14: 389–397.
Smith, V. H., G. D. Tilman & J. C. Nekola, 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100: 179–196.
Stevenson, R. J., M. L. Bothwell, R. L. Lowe & J. H. Thorp, 1996. Algal Ecology: Freshwater Benthic Ecosystem. Academic Press, San Diego.
Sun, L., Y. Liu & H. Jin, 2009. Nitrogen removal from polluted river by enhanced floating bed grown canna. Ecological Engineering 35: 135–140.
Teissier, S., M. Torre, F. Delmas & F. Garabétian, 2007. Detailing biogeochemical N budgets in riverine epilithic biofilms. Journal of the North American Benthological Society 26: 178–190.
Traunspurger, W., M. Bergtold & W. Goedkoop, 1997. The effects of nematodes on bacterial activity and abundance in a freshwater sediment. Oecologia 112: 118–122.
Vitousek, P. M., H. A. Mooney, J. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Webster, J. R., J. L. Tank, J. B. Wallace, J. L. Meyer, S. L. Eggert, T. P. Ehrman, B. R. Ward, B. L. Bennett, P. F. Wagner, M. E. McTammany, et al., 2000. Effects of litter exclusion and wood removal on phosphorus and nitrogen retention in a forest stream. Internationale Vereinigung fur Theoretische und Angewandte Limnologie Verhandlungen 27: 1337–1340.
Wormald, A. P. & H. P. Stirling, 1979. A preliminary investigation of nutrient enrichment in experimental sand columns and its effect on tropical intertidal bacteria and meiofauna. Estuarine and Coastal Marine Science 8: 441–453.
Wright, S. W., S. W. Jeffrey, R. F. C. Mantoura, C. A. Llewellyn, T. Bjornland, D. Repeta & N. Welschmeyer, 1991. Improved HPLC method for the analysis of chlorophylls and carotenoids in marine phytoplankton. Marine Ecology Progress Series 77: 183–196.
Wu, J., C. Fu, Y. Liang & J. Chen, 2004. Distribution of the meiofaunal community in a eutrophic shallow lake of China. Archiv für Hydrobiologie - Hauptbände 159: 555–575.
Acknowledgements
Many thanks to Didier Lambrigot for his help with HPLC pigment analyses. Yang Liu received a PhD grant by China Scholarship Council (CSC, Grant No. 201208320230). We acknowledge a financial contribution of the “Observatoire Midi-Pyrénées” (OMP, Toulouse).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
Liu, Y., Majdi, N., Tackx, M. et al. Short-term effects of nutrient enrichment on river biofilm: N–NO3 − uptake rate and response of meiofauna. Hydrobiologia 744, 165–175 (2015). https://doi.org/10.1007/s10750-014-2074-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2074-3