Abstract
Global warming may affect snail–periphyton–macrophyte relationships in lakes with implications also for water clarity. We conducted a 40-day aquaria experiment to elucidate the response of submerged macrophytes and periphyton on real and artificial plants to elevated temperatures (3°C) under eutrophic conditions, with and without snails present. With snails, the biomass and length of Vallisneria spinulosa leaves increased more at the high temperature, and at both temperatures growth was higher than in absence of snails. The biomass of periphyton on V. spinulosa as well as on artificial plants was higher at the highest temperature in the absence but not in the presence of snails. The biomass of Potamogeton crispus (in a decaying state) declined in all treatments and was not affected by temperature or snails. While total snail biomass did not differ between temperatures, lower abundance of adults (size >1 cm) was observed at the high temperatures. We conclude that the effect of elevated temperature on the snail–periphyton–macrophyte relationship in summer differs among macrophyte species in active growth or senescent species in subtropical lakes and that snails, when abundant, improve the chances of maintaining actively growing macrophytes under eutrophic conditions, and more so in a warmer future with potentially denser growth of periphyton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
By the end of the twenty-first century, the global average surface air temperature is projected to increase between 1.1 and 6.4°C relative to 1980–1990 temperatures (Solomon et al., 2007), with implications also for lake ecosystems. The effect of warming on the relationship between zooplankton, phytoplankton, and submerged macrophytes has been intensively studied (McKee et al., 2002; Meerhoff et al., 2007; Kosten et al., 2011; Nicolle et al., 2012), and warming has been shown to enhance the biomass and distribution of submerged macrophytes in clear water lakes (Rooney & Kalff, 2000) but to intensify eutrophication and the disappearance of submerged macrophytes in eutrophic lakes (Moss et al., 2011). Macrophytes are important structuring components in freshwater ecosystems as they contribute to establish and maintain clear water conditions, for example by providing refuge for zooplankton, thereby enhancing the grazing pressure on phytoplankton, and by reducing resuspension of the sediment (Moss, 1990; Scheffer et al., 1993; Schriver et al., 1995; Jeppesen et al., 1998).
Macrophyte growth may be hampered by periphyton growth on their surfaces as this affects light conditions and nutrient availability for the host plants, not least in eutrophic lakes (Phillips et al., 1978); however, high grazing by invertebrates, not least snails, potentially has a modulating effect on this (Jones & Sayer, 2003). The relationship between snails and the periphyton–macrophyte complex is particularly well studied in temperate lakes (Brönmark, 1989; Underwood et al., 1992; Brönmark & Vermaat, 1998). While there is evidence of direct herbivory by snails on aquatic plants (Lodge, 1991; Li et al., 2009), the relationship between snails and macrophytes is most often mutualistic (Thomas, 1990; Underwood et al., 1992)—by reducing periphyton biomass, snails promote macrophyte growth by decreasing shading (Brönmark, 1985; Cattaneo & Kalff, 1986; Brönmark, 1994; Strand & Weisner, 2001; Roberts et al., 2003). Little is, however, known about the effect of warming on snail–periphyton–macrophyte interactions and the results that are available are ambiguous. An experimental pond study conducted in Canada showed that warming reduced the effects of eutrophication on the periphyton of artificial plants due to higher invertebrate grazing (Shurin et al., 2012). Likewise, a mesocosm study conducted by McKee et al. (2003) in the U.K. indicated that invertebrate biomass (mainly snails) will increase with higher temperatures and thus augment the chances of macrophyte presence at high nutrient levels. However, a recent mesocosm experiment run at low nutrient levels in Denmark revealed no change in periphyton on plants (Potamogeton crispus and Elodea canadensis) in response to heating (Y, Cao, unpublished data), while other mesocosm experiments have shown enhanced periphyton growth with increasing temperature (Patrick et al., 2012) as well as higher periphyton productivity (Kishi et al., 2005).
We conducted a microcosm study of the response of two submerged macrophytes and periphyton to elevated temperatures in early summer under eutrophic conditions, with and without snails. One plant species (Vallisneria spinulosa, Hydrocharitaceae) was in the active growth phase, while the other (Potamogeton crispus, Potamogetonaceae), being a spring species at our locality, was in a senescent state. We hypothesized that warming would stimulate periphyton growth under eutrophic conditions, with potentially adverse effects on the host plant unless snail grazing is high. We also expected stronger effects of warming on snail–periphyton–macrophyte interactions of plants in the active growth phase than for plants that have passed this phase or for artificial plants as the host plants through their growth may in part compensate for a potentially enhanced shading effect by the periphyton.
Materials and methods
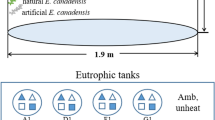
The experiment was conducted from May to June 2012. V. spinulosa was collected from Wuhan Botanical Garden, China, where it grew in a concrete pond (length × width × depth: 30 × 15 × 1.5 m) exclusively dominated by this species, and P. crispus was collected from Houhu Lake, China, where it dominates the macrophyte community from fall to early summer. For the experiment, we selected approx. 10-cm-long V. spinulosa with three leaves and approx. 10-cm-long P. crispus with no branches. We also used an artificial plant consisting of a 20 cm metal stick with five long oval-shaped leaves to measure periphyton development without plant interaction and growth. The natural plants were washed carefully to remove snail eggs and periphyton and then cultivated in plastic pots (top diameter 10.6 cm, bottom diameter 9 cm, height 8.5 cm) filled with washed river sand. Five individuals of each plant were chosen to mimic a high coverage (80–100%) plant dominated state in a natural lake and placed in a glass aquarium (length × width × height: 40 × 30 × 60 cm). Two unconnected concrete pools (length × width × depth: 4 × 4 × 1 m) were both half filled with lake water and acted as temperature buffer. The heating system consisted of a control unit, two temperature sensors, one in each of the two concrete pools, and a spiral heater (diameter 9 cm, power 800 w) in one of the pools, and four water pumps (800 L h−1), two in each pool. The sensors measured the temperature simultaneously in the two pools, and the heater was switched on/off at 3°C temperature elevation. The recording frequency was 10 ± 0.1 min.
We had four treatments, each in 4 replicates: high temperature and snail presence (HS), high temperature and snail absence (HN), low (ambient) temperature and snail presence (LS), low temperature and snail absence (LN). Five individuals of Radix swinhoei (size: around 1 cm), a dominant epiphytic gastropod in the Yangtze lakes (Wang et al., 2006), were used as periphyton grazers. The plants were placed in the aquaria a week before initiating the experiment. After this, the pesticide decamethrin (effective concentration: 2.5%) was added to remove the snails in half of the aquaria prior to initiation of the experiment. For each aquarium, 10 ml of the pesticide solution was diluted with water in a small bucket and subsequently transferred into the aquarium. Dead snails were removed from the pesticide-treated tanks. At the end of the experiment, the water in the aquaria was poured through a 500 µm net to collect the snails after which snails remaining in the aquaria were collected to determine snail biomass.
Every 10 days, one individual of each macrophyte (or artificial plant) was randomly chosen and harvested to determine macrophyte biomass. Macrophytes were separated into leaf and root (or stem) and were subsequently dried at 80°C for 48 h to determine dry weight. The third leaf counting from the shoot apex was extracted by ethanol to measure the chlorophyll a (chla) and chlorophyll b (chlb) content after cleaning the surface (Wang et al., 2008). The periphyton on real plants was carefully sampled before cleaning the plant by adding water to a sealed plastic bag followed by thorough shaking after which the washed-off water was filtered on GF/C filters. After extraction by acetone, the solution was analyzed spectrophotometrically. If the periphyton was too dense to allow determination, a subsample from the washed-off water was used. The periphyton on artificial plants was sampled in a similar manner. Periphyton biomass was calculated according to sampled leaf surface area with the unit of µg chla cm−2.
A 500 ml water sample was gathered to determine water chemistry. For determination of total phosphorus (TP) and total nitrogen (TN), water samples were first digested with K2S2O8. TN was then determined using a spectrophotometric method after the addition of hydrochloric acid, and TP and soluble reactive phosphorus (SRP) were spectrophotometrically determined as molybdate-reactive phosphorus (Huang et al., 1999). NH4–N was determined with the Nessler’s reagent colorimetric method and NO3–N by using the spectrophotometric method with phenol disulphonic acid (Huang et al., 1999). For determination of chla, 200–500 ml of water was filtered through Whatman GF/C filters and extracted using the acetone method (Huang et al., 1999).
The day before the heating was switched on (Day0), water and plants were sampled, and soluble phosphorus KH2PO4, nitrogen NH4Cl, and KNO3 with a 1:2 nitrogen ratio were added to obtain an initial nutrient level of 0.15 mg l−1 TP and 3 mg l−1 TN, typical nutrient levels of eutrophic lakes in the middle–low reaches of the Yangtze River and within the range of regime shifts between macrophyte-dominated clear water lakes and phytoplankton-dominated turbid lakes in this area (Wu et al., 2006; Wang et al., 2014). After Day0, water was sampled every 10 days for nutrient and phytoplankton analysis. Efforts were made to maintain similar nutrient levels as at the start of the experiment by adding nutrients after each sampling event. TN and TP loadings needed to maintain nutrient concentrations were calculated from the data obtained in the previous sampling. Total nutrient loadings were calculated by summing up the additions during the experiment. The ratio of periphyton biomass to nutrient loading was obtained by dividing periphyton biomass at the end of the experiment by total nutrient loadings, the ratio being used as an indicator to show the direct effects of nutrient loading on periphyton biomass.
One-way ANOVA was used to analyze nutrient conditions on Day0 and to determine the ratio of periphyton biomass and total nutrient loading. A t test was performed to analyze for differences in snail biomass. The data gathered after initiation of the experiment were analyzed by RM-ANOVA (shown in Table 1), and the Student–Newman–Keuls (SNK) method was chosen for the post hoc test. Data were SQRT-transformed, when needed, to satisfy the assumptions in the Mauchly’s test of sphericity; otherwise, the Greenhouse-Geisser value was used for the modification. The related statistical results are included in Table 1 if not explicitly shown in the text. For the statistical analyses, we used SPSS16.0. The data presented are mean ± SD.
Results
Temperature
The average ambient temperature was 25.4 ± 1.4°C (Fig. 1) and was elevated by 2.8 ± 0.5°C during the experiment.
Macrophytes
The maximum length of V. spinulosa did not differ significantly between treatments on Day0 (ANOVA, F 3,12 = 0.056, P > 0.05), indicating similar initial conditions (Fig. 2). During the experiment, the maximum length of V. spinulosa in the HS treatment was higher than in the remaining treatments, and it changed with time (Table 1). The root biomass of V. spinulosa was not significantly affected by the treatments but changed with time. The leaf biomass of V. spinulosa was significantly higher in the HS treatment than in the remaining treatments. The leaf chlorophyll content was higher in the snail treatments than in those without (Fig. 3). Both changed with time and showed significant chlorophyll–time interactions.
The maximum length of P. crispus decreased with time but did not differ significantly between the treatments (Fig. 2; Table 1). Leaf and stem biomass of P. crispus as well as the leaf chlorophyll content (chla and chlb) did not differ between treatments, but leaf chla and chlb decreased with time (Fig. 3; Table 1).
Periphyton
The periphyton biomass on V. spinulosa was significantly higher in the HN treatment than in the remaining treatments (Fig. 4; Table 1), being up to 1.35 ± 0.33 µg cm−2 by the end of the experiment. For P. crispus, the periphyton biomass was higher in the two treatments without snails than in those with snails (but the time or interaction effects were not significant). The periphyton biomass on the artificial plants was highest in the HN treatment, while the biomass was higher in the LN treatment than in the HS and LS treatments. The periphyton biomass on V. spinulosa and artificial plants increased differently during the experiment as indicated by significant time and treatment interaction, while no such difference was found for P. crispus. During the experiment, the periphyton biomass in the HN treatment did not differ among the three plant types (two natural and one artificial) (F 2,9 = 2.947, P > 0.05, RM-ANOVA), but it increased with time (F 2,9 = 4.598, P < 0.05, RM-ANOVA). In the remaining three treatments, the periphyton biomass on P. crispus was higher than on V. spinulosa and artificial plants, whereas no significant difference was traced for the periphyton biomass on V. spinulosa and on the artificial plants (F 2,9 = 7.979, P < 0.05 for LN, F 2,9 = 27.436, P < 0.001 for HS, F 2,9 = 9.599, P < 0.01 for LS, RM-ANOVA), but it did not increase with time (F 2,9 = 2.724 for LN, F 2,9 = 2.881 for HS, F 2,9 = 0.589 for LS, P > 0.05, RM-ANOVA).
Phytoplankton and water chemistry
Phytoplankton biomass did not differ between treatments (Fig. 4; Table 1). In all treatments, TN fluctuated near the initial concentration of 3 mg l−1, and no significant difference appeared among the treatments (Fig. 5; Table 1). The ammonia concentrations were lower in the two snail treatments than in those without snails. In contrast, the concentration of nitrate was lowest in the HN treatment, while nitrate in the LS treatment was lower than in the HS and LN treatments. Even though efforts were made to maintain similar TP concentrations, TP was still marginally higher in the LN treatment than in the HN treatment. SRP in the HN treatment was lower than in the other three treatments.
Nutrient loading
By the end of the experiment, the total TN loading did not differ between treatments (F 3,12 = 1.16, P > 0.05, ANOVA), while total TP loading was marginally higher in the HN than in the LS and LN treatments (F 3,12 = 3.977, P < 0.05, ANOVA). The ratio of periphyton biomass to nutrient loading was calculated in order to show the nutrient dependency of the periphyton increase (Fig. 6). The periphyton biomass on V. spinulosa and the total TN loading ratio were higher in the HN treatment than in the other three treatments (F 3,12 = 61.026, P < 0.001, ANOVA), while the periphyton biomass on artificial plants and the total TN loading ratio were highest in the HN treatment and lowest in the LS treatment (F 3,12 = 26.623, P < 0.001, ANOVA). The periphyton biomass and total TP loading ratio were highest in the HN treatment for V. spinulosa and higher in the HN treatment than in the with-snail treatments for artificial plants (F 3,12 = 61.026, P < 0.001 for V. spinulosa and F 3,12 = 9.706, P < 0.01 for artificial plants, ANOVA).
Snails
After the experiment, total snail biomass did not differ between the two snail treatments (P > 0.05, t test). However, the biomass of the larger snails (size >1 cm) was significantly lower in the HS treatment than in the LS treatment (P < 0.05, t test), while no difference was found for the biomass of small snails (size <1 cm) (P > 0.05, t test).
Discussion
For the actively growing V. spinulosa, leaf biomass and leaf length were higher at the higher temperature when snails were present and higher than in the absence of snails at both temperatures. The optimum temperature for carbon uptake by V. americana is around 32.6°C (Titus & Adams, 1979), but is as yet undetermined for V. spinulosa. The significant interaction between time and treatment for the leaf biomass and chlorophyll content of V. spinulosa suggests, in agreement with our expectations, more pronounced differences by the end of the experiment as has been demonstrated in a warming experiment in the UK (McKee et al., 2002). The abundance of P. crispus declined in all treatments, which is in line with summer observations made in other subtropical freshwaters (Rogers & Breen, 1980), and this apparently also affected the chlorophyll content of leaves, which declined during the course of the experiment independent of treatment. P. crispus has a lower optimum for photosynthesis (30°C) (Saitoh et al., 1970). In a mesocosm experiment, McKee et al. (2002) observed that warming enhanced the growth of exotic Lagarosiphon major but not of two other submerged macrophyte species present (Elodea nuttallii and P. natans).
Shading by phytoplankton is often a key factor for a decline of macrophytes in eutrophic lakes (Phillips et al., 1978; Hough et al., 1989). In our experiment, however, the phytoplankton biomass was overall low and did not differ among the treatments. The aquaria were transparent to the bottom, indicating low shading effects of phytoplankton. By contrast, the periphyton formed an intact layer on the leaves of the macrophytes and on the artificial plants in the treatments without snails (personal observation), and the biomass ranged from 0.07 to 2.08 µg cm−2. Earlier studies have revealed that a periphyton biomass of 1 µg cm−2 induces severe light limitation of the growth of the submerged macrophyte P. pectinatus (Roberts et al., 2003). Köhler et al. (2010) found that two-week-old periphyton on Sagittaria sagittifolia reduced the light supply by 28%; in older mats the reduction may be as much as 85% (van Dijk, 1993), demonstrating the potential strong shading effect of periphyton. We found that the periphyton biomass on V. spinulosa and artificial plants was higher at higher temperatures than at ambient temperatures in the absence of snails, obviously suggesting a temperature effect. Also other mesocosm studies have revealed enhanced periphyton growth with increasing temperature (Patrick et al., 2012) and higher periphyton productivity (Kishi et al., 2005). By contrast, the periphyton biomass on P. crispus was not affected by temperature in the treatments without snails (Fig. 4), perhaps reflecting decay of the host plants, causing nutrient release (Guariento et al., 2009; Tarkowska-Kukuryk & Mieczan, 2012) that overrules the positive effects of higher temperatures. Indeed, we found a higher periphyton biomass on P. crispus in the without-snail treatment than on V. spinulosa and the artificial plants.
We regulated the nutrient loading during the course of the experiment to obtain approximately similar nutrient concentrations in all treatments. In this way, the microcosm with the higher temperatures and absence of snails received a significantly higher phosphorus loading than the other treatments, which potentially may have influenced the response of the periphyton. However, even when correcting for higher loading by using the ratio between the periphyton biomass on V. spinulosa and total TP loading, the difference remained significant.
The periphyton concentrations on the two submerged plants and the artificial plants were low in the snail-presence treatments compared to the without-snail treatments, indicating high snail grazing as seen in other studies with natural plants (Brönmark et al., 1992; Underwood et al., 1992; Dillon, 2000; Wojdak, 2005). A temperature effect on periphyton could not be discerned in the with-snail treatments, possibly due to a high grazing capacity of the snails. It has earlier been reported that periphyton declines with elevated temperatures (3°C above ambient) in the presence of invertebrates or fish (Shurin et al., 2012). Moreover, temperature affects the size structure, metabolism, growth, and reproduction of snails (Dillon, 2000). By the end of the experiment, we found that the biomass of large (size >1 cm) snails was higher at the lower temperature. The temperature-size rule predicts that snails mature at a smaller size in warmer environments (Atkinson, 1994) and that small-sized specimens are more efficient at meeting the metabolic demands (Sheridan & Bickford, 2011), leading to higher grazing on periphyton. Laboratory studies have shown, however, that R. swinhoei via grazing can negatively affect the growth of submerged macrophytes, such as P. crispus and V. spiralis (Xiong et al., 2008; Li et al., 2009) when periphyton is removed. However, in our outdoor study we did not find any evidence of a negative effect of R. swinhoei on macrophyte growth or decay. Periphyton can, however, also be controlled by fish, and the effect of fish is likely disproportionally higher in warm lakes due to a higher degree of herbivory and dominance of small-sized fish (Meerhoff et al., 2007; González-Bergonzoni et al., 2012). Fish grazing on periphyton may therefore potentially benefit the plant growth in warm lakes if not fully outweighed by an indirect effect of fish predation on snails that may lead to higher growth of periphyton. Dominance of small fish in warm lakes may benefit large-bodied snails such as golden apple snails that may cause high loss of aquatic plants (Carlsson et al., 2004). Our experiment was run at relatively high nutrient concentrations allowing extensive growth of periphyton in the absence of snail grazing. The outcome of warming on periphyton growth might be different at low nutrient concentrations. Trochine et al. (2014) found longer duration of nitrogen limitation of periphyton growth at higher temperatures in mesocosm experiments in Denmark during a 1-year study run at contrasting temperatures as well as a shift from overall single-nutrient limitation to co-limitation of nitrogen and phosphorus.
In conclusion, our results indicate that the response of snail–periphyton–macrophyte interactions to elevated temperatures differs among plants in active growth stages and under decay and that the chances of macrophyte dominance under eutrophic conditions in a warmer future climate rise at high snail abundance. Our results confirm that snails have a vital effect on periphyton under eutrophic conditions via their grazing on the periphyton, and they also suggest that temperature affects the size of snails in a warming world, leading to more efficient grazing provided that the snail abundance is not kept low by fish predation. More studies are, however, needed on fish–snail–periphyton–nutrient interactions in warm lakes to allow general conclusions about the response of periphyton to warming.
References
Atkinson, D., 1994. Temperature and organism size – a biological law for ectotherms? Advances in Ecological Research 25: 1–58.
Brönmark, C., 1985. Interactions between macrophytes, epiphytes and herbivores: an experimental approach. Oikos 45: 26–30.
Brönmark, C., 1989. Interactions between epiphytes, macrophytes and freshwater snails: a review. Journal of Molluscan Studies 55: 299–311.
Brönmark, C., 1994. Effects of tench and perch on interactions in a freshwater, benthic food chain. Ecology 75: 1818–1828.
Brönmark, C. & J. E. Vermaat, 1998. Complex Fish–Snail–Epiphyton Interactions and Their Effects on Submerged Freshwater Macrophytes. The Structuring Role of Submerged Macrophytes in Lakes. Springer, New York: 47–68.
Brönmark, C., S. P. Klosiewski & R. A. Stein, 1992. Indirect effects of predation in a freshwater, benthic food chain. Ecology 73: 1662–1674.
Carlsson, N. O., C. Brönmark & L. A. Hansson, 2004. Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85: 1575–1580.
Cattaneo, A. & J. Kalff, 1986. The effect of grazer size manipulation on periphyton communities. Oecologia 69: 612–617.
Dillon, R. T., 2000. The Ecology of Freshwater Molluscs. Cambridge University Press, Cambridge.
González-Bergonzoni, I., M. Meerhoff, T. A. Davidson, F. Teixeira-de Mello, A. Baattrup-Pedersen & E. Jeppesen, 2012. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems 15: 492–503.
Guariento, R. D., A. Caliman, F. A. Esteves, R. L. Bozelli, A. Enrich-Prast & V. F. Farjalla, 2009. Substrate influence and temporal changes on periphytic biomass accrual and metabolism in a tropical humic lagoon. Limnologica 39: 209–218.
Hough, R. A., M. D. Fornwall, B. J. Negele, R. L. Thompson & D. A. Putt, 1989. Plant community dynamics in a chain of lakes: principal factors in the decline of rooted macrophytes with eutrophication. Hydrobiologia 173: 199–217.
Huang, X. F., W. M. Chen & Q. M. Cai, 1999. Survey, observation and analysis of lake ecology. Standard Methods for Observation and Analysis in Chinese Ecosystem Research Network, Series V. Standards Press of China, Beijing (in Chinese).
Jeppesen, E., T. L. Lauridsen, T. Kairesalo & M. R. Perrow, 1998. Impact of submerged macrophytes on fish–zooplankton interactions in lakes. The Structuring Role of Submerged Macrophytes in Lakes. Springer, New York: 91–114.
Jones, J. I. & C. D. Sayer, 2003. Does the fish–invertebrate–periphyton cascade precipitate plant loss in shallow lakes? Ecology 84: 2155–2167.
Kishi, D., M. Murakami, S. Nakano & K. Maekawa, 2005. Water temperature determines strength of top–down control in a stream food web. Freshwater Biology 50: 1315–1322.
Kosten, S., E. Jeppesen, V. L. M. Huszar, N. Mazzeo, E. H. Van Nes, E. T. H. M. Peeters & M. Scheffer, 2011. Ambiguous climate impacts on competition between submerged macrophytes and phytoplankton in shallow lakes. Freshwater Biology 56: 1540–1553.
Köhler, J., J. Hachoł & S. Hilt, 2010. Regulation of submersed macrophyte biomass in a temperate lowland river: interactions between shading by bank vegetation, epiphyton and water turbidity. Aquatic Botany 92: 129–136.
Li, K. Y., Z. W. Liu, Y. H. Hu & H. W. Yang, 2009. Snail herbivory on submerged macrophytes and nutrient release: implications for macrophyte management. Ecological Engineering 35: 1664–1667.
Lodge, D. M., 1991. Herbivory on freshwater macrophytes. Aquatic Botany 41: 195–224.
McKee, D., K. Hatton, J. W. Eaton, D. Atkinson, A. Atherton, I. Harvey & B. Moss, 2002. Effects of simulated climate warming on macrophytes in freshwater microcosm communities. Aquatic Botany 74: 71–83.
Meerhoff, M., C. Iglesias, F. T. De Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshwater Biology 52: 1009–1021.
Moss, B., 1990. Engineering and biological approaches to the restoration from eutrophication of shallow lakes in which aquatic plant communities are important components. Biomanipulation Tool for Water Management, Springer: 367–377.
Moss, B., S. Kosten, M. Meerhoff, R. W. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. W. Liu, L. De Meester, H. Paerl & M. Scheffer, 2011. Allied attack: climate change and eutrophication. Inland Waters 1: 101–105.
Nicolle, A., P. Hallgren, J. Von Einem, E. S. Kritzberg, W. Graneli, A. Persson, C. Brönmark & L. A. Hansson, 2012. Predicted warming and browning affect timing and magnitude of plankton phenological events in lakes: a mesocosm study. Freshwater Biology 57: 684–695.
Patrick, D. A., N. Boudreau, Z. Bozic, G. S. Carpenter, D. M. Langdon, S. R. LeMay, S. M. Martin, R. M. Mourse, S. L. Prince & K. M. Quinn, 2012. Effects of climate change on late-season growth and survival of native and non-native species of watermilfoil (Myriophyllum spp.): implications for invasive potential and ecosystem change. Aquatic Botany 103: 83–88.
Phillips, G., D. Eminson & B. Moss, 1978. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany 4: 103–126.
Roberts, E., J. Kroker, S. Körner & A. Nicklisch, 2003. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes. Hydrobiologia 506: 525–530.
Rogers, K. & C. Breen, 1980. Growth and reproduction of Potamogeton crispus in a South African lake. Journal of Ecology 68: 561–571.
Rooney, N. & J. Kalff, 2000. Inter-annual variation in submerged macrophyte community biomass and distribution: the influence of temperature and lake morphometry. Aquatic Botany 68: 321–335.
Saitoh, M., K. Narita & S. Isikawa, 1970. Photosynthetic nature of some aquatic plants in relation to temperature. Botanical Magazine Tokyo 83: 10–12.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Schriver, P., J. Bøgestrand, E. Jeppesen & M. Søndergaard, 1995. Impact of submerged macrophytes on fish–zooplanl phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33: 255–270.
Sheridan, J. A. & D. Bickford, 2011. Shrinking body size as an ecological response to climate change. Nature Climate Change 1: 401–406.
Shurin, J. B., J. L. Clasen, H. S. Greig, P. Kratina & P. L. Thompson, 2012. Warming shifts top–down and bottom–up control of pond food web structure and function. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 3008–3017.
Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller, 2007. Climate Change 2007: The Physical Science Basis, Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
Strand, J. A. & S. E. Weisner, 2001. Morphological plastic responses to water depth and wave exposure in an aquatic plant (Myriophyllum spicatum). Journal of Ecology 89: 166–175.
Tarkowska-Kukuryk, M. & T. Mieczan, 2012. Effect of substrate on periphyton communities and relationships among food web components in shallow hypertrophic lake. Journal of Limnology 71: 279–290.
Thomas, J. D., 1990. Mutualistic interactions in freshwater modular systems with molluscan components. Advances in Ecological Research 20: 125–178.
Titus, J. E. & M. S. Adams, 1979. Coexistence and the comparative light relations of the submersed macrophytes Myriophyllum spicatum L. and Vallisneria americana Michx. Oecologia 40: 273–286.
Trochine, C., M. E. Guerrieri, L. Liboriussen, T. L. Lauridsen & E. Jeppesen, 2014. Effects of nutrient loading, temperature regime and grazing pressure on nutrient limitation of periphyton in experimental ponds. Freshwater Biology 59: 905–917.
Underwood, G. J. C., J. D. Thomas & J. H. Baker, 1992. An experimental investigation of interactions in snail–macrophyte–epiphyte systems. Oecologia 91: 587–595.
Van Dijk, G. M., 1993. Dynamics and attenuation characteristics of periphyton upon artificial substratum under various light conditions and some additional observations on periphyton upon Potamogeton pectinatus L. Hydrobiologia 252: 143–161.
Wang, C., S. H. Zhang, P. F. Wang, J. Hou, W. Li & W. J. Zhang, 2008. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquatic Toxicology 87: 88–98.
Wang, H. J., B. Z. Pan, X. M. Liang & H. Z. Wang, 2006. Gastropods on submersed macrophytes in Yangtze lakes: community characteristics and empirical modelling. International Review of Hydrobiology 91: 521–538.
Wang, H. J., H. Z. Wang, X. M. Liang & S. K. Wu, 2014. Total phosphorus thresholds for regime shifts are nearly equal in subtropical and temperate shallow lakes with moderate depths and areas. Freshwater Biology. doi:10.1111/fwb.12372.
Wojdak, J. M., 2005. Relative strength of top–down, bottom–up, and consumer species richness effects on pond ecosystems. Ecological Monographs 75: 489–504.
Wu, S. K., P. Xie, G. D. Liang, S. B. Wang & X. M. Liang, 2006. Relationships between microcystins and environmental parameters in 30 subtropical shallow lakes along the Yangtze River, China. Freshwater Biology 51: 2309–2319.
Xiong, W., D. Yu, Q. Wang, C. Liu & L. Wang, 2008. A snail prefers native over exotic freshwater plants: implications for the enemy release hypotheses. Freshwater Biology 53: 2256–2263.
Acknowledgments
This study was supported by Wuhan Academic Leader Program (201051730562), CSC (The China Scholarship Council), CRES (Danish Strategic Research Council), CLEAR (a Villum Kann Rasmussen Centre of Excellence project), the EU-FP7 project REFRESH (Adaptive strategies to Mitigate the Impacts of Climate Change on European Freshwater Ecosystems, Contract No.: 244121) and the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), Contract No.: 603378 (http://www.mars-project.eu). We thank both reviewers and the associate editor for their insightful comments on the paper. We thank Anne Mette Poulsen for valuable editorial comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei Magela Thomaz
Rights and permissions
About this article
Cite this article
Cao, Y., Li, W. & Jeppesen, E. The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: a microcosm approach. Hydrobiologia 738, 49–59 (2014). https://doi.org/10.1007/s10750-014-1914-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1914-5