Abstract
We examined the potential importance of distance from the spring source (reach), season, and physicochemical variability on zoobenthic biodiversity, biomass, and trait-based metrics [functional feeding groups (FFG) and habit traits] in three karst spring systems in the Ozarks region of Missouri, USA. Density and biomass were measured during summer and winter in each spring source and three downstream reaches of each springbrook. Nearly 20,000 invertebrates in total were collected, identified, and counted. We measured biomass directly or by length–weight relationships. Overall downstream and seasonal patterns of diversity, biomass, and traits were similar among springs, but species dominance and composition varied significantly. Taxonomic richness increased downstream in all springs, but evenness decreased. Amphipods and isopods were the most abundant taxa at the spring source but were progressively replaced downstream by insects (with dominance varying among springs and seasons). Density and biomass for flatworms, snails, and insects increased downstream, while crustacean numbers diminished along this ecotone. FFG increased from 3 at the spring source (mostly shredders) to 4–7 downstream where scrapers, collectors, and/or predators predominated. Physicochemical factors proved minor at best as mechanistic explanations for ecotonal changes. We conclude that structural and functional community changes could be attributed to a matrix of interrelated factors including organic substrate, food availability, and biotic interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Explaining systematic patterns of change across space in communities is a major goal of community ecology. Understanding variation along gradients and linking community and ecosystem ecology together provide a better understanding of these patterns and a means of making predictions regarding the ecological impact of climate change. Springs are well suited for examining environmental gradients because of the constancy of abiotic conditions which reduces the number of variables to be considered in field investigations (Stern & Stern, 1969; Glazier, 1991). The level of scientific interest in springs and other headwater ecosystems has increased due to their high sensitivity and vulnerability to environmental change (Cantonati et al., 2006). Their importance is likely to increase in those areas where global climate change is predicted to enhance variability of precipitation, as in parts of the U.S. Great Plains (e.g., Dodds et al., 2004).
Springs are rich in unique species, though their ecological importance was rarely recognized before 1980. Their contribution to epigean systems represents as much as one third of the benthic species richness of a region’s freshwater biodiversity (Danks & Williams, 1991), leading to their recent designation as “hot spots” for aquatic biodiversity (e.g., Barquín & Death, 2006; Cantonati et al., 2006). Known as natural laboratories (Odum, 1957; Glazier, 2009), these first-order, headwater systems (Lindegaard et al., 1988) are three-way ecotones between groundwater, surface water, and terrestrial ecosystems (Cantonati et al., 2012) where discharge, temperature, and chemical conditions remain relatively constant (Death et al., 2004; Glazier, 2012). Their permanency of flow and relative constancy of other physicochemical conditions enable establishment of a disproportionately large number of rare, relict, and endemic species (Hynes, 1983; Smith et al., 2003, but see Glazier, 1991, 2009 for exceptions). They also contribute to the success of species that reproduce asynchronously all year (e.g., Gammarus minus), accentuate the dominance of non-insect taxa lacking a life cycle with an aerial dispersal phase, and function as refugia for downstream epigean communities during floods and droughts (Williams & Hogg, 1988; Erman & Erman, 1995). Despite their biological complexity and ecological value, springs are commonly neglected in freshwater ecological studies.
Understanding the mechanisms responsible for patterns of biological complexity across spatial gradients and through time has long been a dominant theme in aquatic ecological research (Ward & Dufford, 1979; Jacobsen et al., 2010), but these patterns can vary depending on the response metrics used to define them. Phylogenetic methods provide a detailed structural perspective upon which to view biological community patterns. Trait-based characterization of benthic communities has recently gained considerable notice as a means for providing a mechanistic way of linking biological community response patterns and environmental gradients (Finn & Poff, 2005). Trait-based approaches help increase our understanding of patterns associated with specific stressors and the relationship between variability in lotic biodiversity and ecosystem functioning (Heino, 2005; Pollard & Yuan, 2010). Others have found that functional attributes can enhance taxonomic methods for assessment of gradient shifts in community structure because of the commonality of species traits over large and small spatial scales (Dolédec et al., 2006).
The downstream pattern of invertebrates within carbonate rheocrenes transitions from non-insect taxa (primarily amphipods and isopods) at the spring source to an insect-dominated fauna downstream in the springbrook (Gooch & Glazier, 1991). Biotic diversity, therefore, is predicted to increase with distance from the spring source, but mechanisms causing such changes are not always evident, nor do we know how differences in habitat conditions, riparian characteristics, and season might alter this basic relationship.
We sampled the macroinvertebrate fauna and measured environmental parameters in four reaches along each of three similar, neighboring karst springs in the Ozarks region of Missouri, USA. Our main goals were to: (i) determine overall longitudinal patterns of biotic (richness, evenness, biomass, density) and community functional organization [functional feeding groups (FFG) and habit traits (HT)]; and (ii) examine the influence of reach (distance from the spring source), season, and habitat conditions (physicochemical) on these patterns. Beyond their physicochemical stability, limestone springs are noted for their unique biotic organizational patterns (Gooch & Glazier, 1991). Based on these factors, our initial hypotheses were: (i) each spring would be physicochemically stable; (ii) spring communities would conform to Glazier and Gooch’s general longitudinal model of species distribution and diversity; and (iii) the three springs would exhibit similar biotic and functional organizational patterns based on commonalities shared in their geographic location, subsurface geology, geomorphology, point of origin, flow magnitude and permanency, physicochemical stability, and inorganic substrate—each known to be mechanisms responsible for structural and functional characteristics of zoobenthic communities in springs (Smith et al., 2003; Glazier, 2009, 2012). We predicted that taxonomic classification, coupled with species trait attributes, would provide a mechanistic understanding about the relationships between ecotonal variability in structural and functional community–habitat relationships within and among springs and seasons.
Methods
Study area and site descriptions
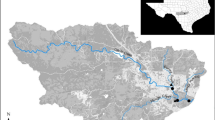
We conducted this study in three karst springs, Haseltine, Danforth, and Steury, each situated in the Ozark uplift region of Greene County, Missouri, USA (Fig. 1). All three springs lie in the Springfield Plateau physiographic region, a subdivision of the deeper Ozarks Plateau, and are, therefore, all underlain by the Burlington-Keokuk Limestone of Mississippian age and older, slightly deeper Ordovician dolomites (Thomson, 1986). The three study springs occur in adjoining watersheds; Haseltine in the Sac River Basin (altitude 350.52 m a.s.l.) and Danforth and Steury in the James River Basin (altitude 409.95 and 396.24 m a.s.l., respectively). Land cover in each basin is predominantly grassland and pasture (~60%), with forest and urban cover representing much smaller proportions (30 and 7%, respectively). Average annual precipitation in this region of the Ozarks is 116 cm (~45.5 in.), with monthly daily average air temperatures ranging from 0.3°C in January to 25.7°C in July.
The spring source for each of our springs was clearly defined, as all issue from caves. Haseltine (37°25′N, 93°43′W), lies just northwest of the city of Springfield, Missouri and issues from a small cave (area = ~39 m2). Danforth and Steury are located just east of Springfield. Danforth (37°23′N, 93°15′W) arises from a small, cave-like opening (area = ~12 m2) at the base of a limestone bluff, and Steury (37°20′N, 93°21′W) issues from a larger cave (area = ~64 m2). Each is defined as fourth magnitude or medium volume (0.01–0.5 m3/s−1), cool, freshwater, permanent rheocrenes (Danks & Williams, 1991).
While vastly similar in stream habitat characteristics, our three spring systems varied in their characteristic riparian cover. Haseltine Spring (HS) is located in open, mowed grassland with only one side of the springbrook lined with deciduous trees for a short distance adjacent to the cave (~30 m). Danforth Spring (DS) is located in a planted coniferous forest that supports 17 different conifer genera, while Steury resides in a deciduous forest which includes a mixture of hickory, oak, walnut, and cedar trees.
The predominant substrate-current combination of each springbrook is primarily a coarse gravel-riffle complex (25–64 mm). While substrate size at each spring source is in the upper fraction of this range, the average percent of the substrate composed of coarse gravel in each springbrook is 54, 50, and 45% in Haseltine, Danforth, and Steury, respectively. A qualitative assessment of organic substrate composition and distribution showed that each spring source and upper springbrook reaches were dominated by moss and water cress (Nasturtium officinale) (Table 2) which served as a major invertebrate habitat. The density of these mats decreased with distance from the source. Potamogeton amplifolius (a large-leaved pond weed), and Impatiens capensis (jewel weed) lined upper springbrooks with just a few small clumps in the active channel, but both died back during winter. Because of the temporal stability of each springbrook and their location within the spring channel, mosses and Nasturtium grew profusely year round. During summer months, however, water cress died back to minor clumps along the spring bank in HS due to the total absence of any riparian canopy, and consequent much greater incident light over the spring channel.
The distances from the spring source to the most downstream reach studied varied among springs from 101 to 145 m. While these springbrook lengths may seem short compared to those of a typical epigean stream study, they encompass the critical ecological transitional zone, comparable to other studies that have examined springbrooks of similar lengths (>100 m; Minshall, 1968; Ward & Dufford, 1979, but see Webb et al., 1988; Von Fumetti et al., 2006 for studies on springbrooks, typically <40 m).
For the purpose of this study, spring source (eucrenon) refers to the area of spring origin at the cave mouth in each of the springs, and springbrook (hypocrenon) designates downstream reaches below the source. A sharp delineation occurs between the end of the eucrenon and both the beginning and end of the hypocrenon. Caves from which all springs issue lie against a limestone bluff. The eucrenon is the area within and under the cave ceiling or overhang where water issues from the subterranean habitat. The hypocrenon, is the point below this, but before the downstream rheocrene is influenced by input from any tributaries. The end of the hypocrenon is the point prior to the confluence with another tributary (Steury and Danforth) or termination into a lake (Haseltine).
Biological and physicochemical sampling
We tested hypotheses with macroinvertebrate data from four sampling reaches in each spring; the cave source and three reaches downstream in the springbrook (henceforth referred to as R1–R4) during two seasons (early summer and winter). We collected three replicate zoobenthic samples from each reach (spaced throughout the reach from head to base) to produce 12 invertebrate samples/spring/season). Because these springs are small, benthic sampling represented a potentially substantial disturbance to these fragile systems and was, therefore, limited to two yearly sampling periods to avoid unnecessary destruction of biota in the eucrenal and hypocrenal zones (cf. Erman, 2002). Macroinvertebrates were collected using a Surber sampler (0.093 m2; 500 μm mesh), with the substrate being disturbed to a depth of ~15 cm for 1 min. Faunas were identified to species where possible, using Merritt et al. (2008) and Thorp & Covich (2010). However, chironomid midges and oligochaete worms were identified only to subfamily or family, respectively.
We measured several environmental parameters at each spring (Table 1). Canopy cover (incident light values as % transmittance) was measured using an AccuPAR Linear PAR/LAI ceptometer (Decagon Devices, Inc.). Mean light values (% transmittance) were generated from an average of 12 measures taken in full leaf-out conditions at noon along and over the length of each spring. Depth and current velocity (at 0.6× depth from the water surface) were measured at three random locations in cross-channel transects placed at the head, middle, and base of each of the four reaches and then averaged. Water velocity was measured using an electromagnetic flow meter (Marsh-McBirney, Inc. Flo-Mate™ Model 2000 portable flow meter). Mean spring channel width was also measured at each reach based on the three cross-channel transects. Discharge was then computed from these measures. Hydrochemical analyses were done with standard methods (APHA, 2000) for orthophosphate, nitrate (NO3), ammonia (NH3), total organic and inorganic carbon (TIC, TOC), calcium (Ca), and magnesium (Mg). Dissolved oxygen, pH, temperature, turbidity, and specific conductivity were measured in situ using a YSI 6920 Environmental Monitoring System Sonde and a YSI 650 Multi-parameter Display System (YSI Incorporated). All environmental variables were measured in triplicate along transects at the head, middle, and base of each reach (n = 3/reach) bimonthly for a year. Even though these systems are small, this level of replication was done in order to capture any potential spatial or temporal groundwater, longitudinal, or hyporheic effects, as physicochemical ranges have been shown to be highly variable along the length of some spring and spring-fed systems (Barquín & Death, 2011).
Biomass
We quantified invertebrate density and biomass along the longitudinal spring source–springbrook gradient as measures of relative importance of each invertebrate group. Biomass was estimated in two ways. For taxa having densities >100 specimens per Surber sample, we established taxon-specific length–dry weight relationships (cf. Benke et al., 1999). We measured the lengths and weights of 100 individuals and then developed length–weight equations to determine total biomass of each taxonomic group. Body lengths (exclusive of appendages) were measured to the nearest 0.05 mm using a dissecting microscope and ocular micrometer. Specimens were dried at 60°C for 48 h, and then individuals were weighed using a microbalance (sensitivity 0.002 mg) to determine dry mass. Dry-weight biomass measurements for taxonomic groups with <100 specimens per sample were determined directly (2,600 total organisms measured directly). Crayfish, sculpin, and perch were excluded from biomass comparisons because of their disproportionate weight compared to other zoobenthic organisms and their rarity in the springs.
Trait-based measures
We selected two trait-based metrics, functional feeding groups (FFG) and habit trait groups (HT). We defined these traits categorically and assigned them to invertebrate taxa based on Merritt et al. (2008) and Thorp & Covich (2010). Categories for FFG were: (1) shredder, collector (gatherer/filterer), scraper, and predator; and (2) burrowers, clingers, sprawlers, and swimmers for HT groups.
Indices and data analyses
We applied five different community structure (taxonomic) metrics to characterize the benthic fauna: density of individuals (#/m2), invertebrate biomass (mg m−2), numerical relative proportions of peracaridans (i.e., amphipods and isopods) and insects, Shannon’s (H′) index of diversity (Shannon & Weaver, 1949), and Simpson’s (E 1/D) measure of evenness (Krebs, 1999), which is not sensitive to species richness (Magurran, 2004). Relationships between physicochemical measures and these metrics were tested with Pearson product moment correlation analysis (cf. Jacobsen et al., 2010; Pollard & Yuan, 2010; Kuhn et al., 2011). Benthic metrics were also tested with 2-way analysis of variance (ANOVA) to analyze for differences among seasons and springs (fixed factors). Post hoc Fisher’s LSD Multiple-Comparison tests were performed to explore significant differences.
For trait-based measures, we aggregated invertebrate community information (taxonomic) with functional attributes and calculated relative proportion of taxon measures for each categorical trait. Within-spring longitudinal trends of density, diversity, biomass, and functional traits of the zoobenthic communities were explored graphically and then analyzed by means of linear regression, as detection of longitudinal increasing/decreasing trends in community measures can most efficiently be achieved using this method (Taniguchi & Tokeshi, 2004; Finn & Poff, 2005). Regressions, ANOVAS, and Pearson’s product moment correlation analysis between log (x + 1)-transformed environmental variables and faunal metrics were performed using NCSS (NCSS, Kaysville, Utah, 2004). Statistical significance was determined at α = 0.05.
Results
Physicochemical characteristics
Habitat size varied little among the three springs, as evident from the consistent width, velocity, and discharge measures (Table 1). Likewise, spring source and springbrook sites displayed little variability in chemical parameters measured. Ammonia and orthophosphate were rarely detectable (≤0.1 and ≤0.3 mg l−1, respectively) and were, therefore, not included in the table. While the amount of incident light reaching each springbrook varied among springs based on riparian cover (Haseltine = 0.72% T; Danforth = 0.41% T; Steury = 0.35% T), temperature regimes of the three springs were comparable regardless of season. Intra-and inter-spring temperature variability was never greater than 1.5 and 1.6°C, respectively. Intra-spring dissolved oxygen concentrations varied by ≤2.6 mg l−1, but differed more among springs (5.36–10.34 mg l−1). Alkalinity was relatively neutral with a typically narrow range as well. As spring waters in the Ozarks are known for their clarity, turbidity was low, and varied by ≤1.7 NTU among springs. Variability in nitrate concentrations were negligible within springs, but showed more variability among springs, ranging from 1.83 to 3.62 mg l−1. Conductivity, calcium, TOC, and TIC concentrations were substantially more variable than other physicochemical parameters, both within and among springs. Conductivity values ranged from 406 to 554 µS/cm among springs, and were, on average 20 μS cm−1 higher in Haseltine and Steury springs (SS). Likewise, calcium concentrations were 18–29 mg l−1 higher in these two springs. Total organic carbon, while relatively stable within springs in a given season, was 15–20 times higher in the winter. Total inorganic carbon concentrations showed this same pattern and were 3–4 times higher in winter in all springs (Table 1).
Fauna and environmental parameters
Neither temperatures nor physical habitat parameters (width, depth, velocity, as incorporated in discharge measures) correlated significantly with any of the faunal metrics included in Table 2. Similarly, no other abiotic environmental parameter significantly correlated with any faunal metrics. However, temperature, TIC and TOC were included in further analysis because the former consistently had the highest correlation coefficients (although not significant, P > 0.05), and TOC and TIC exhibited such high temporal variability. We did not run comparable statistics with light availability because photo-conditions were only evaluated in the summer sampling period (full leaf-out conditions) as part of a separate study of riparian effects.
Macroinvertebrate general composition and relative abundance patterns
A total of 19,593 invertebrates from 31 taxa (representing 26 families) were collected from the three springs across two seasons (Table 3). The most abundant taxonomic group (mean = 7710 ind. m−2) at the spring source in all three springs during both seasons was Peracarida. Insects dominated the springbrook fauna in all three springs, but relative abundance patterns varied by season within each spring. Unlike previous studies on carbonate springs (Williams & Hogg, 1988), chironomid midges (Diptera) were not the most abundant insect species in springbrook sampling stations. Midges were scarce in summer collections, representing only 1–3% of overall invertebrate abundance in each spring. Their numbers doubled in Haseltine and Danforth in winter collections, however, and increased 10-fold in Steury, representing 60% of its winter community. Midges were found only in springbrooks and consisted of two subfamilies (Chironominae and Tanypodinae).
Faunal composition at the spring source consisted of two species of Amphipoda (G. minus Say and Crangonyx forbesi Hubricht and Mackin) and one species of Isopoda (Lirceus hoppinae Faxon). Springbrook species composition varied by spring. Dominant in Haseltine was Optioservus sandersoni Collier (riffle beetle; mean = 8,096 m−2), while Argia sedula Hagan (damselflies) was dominant in Danforth and Steury (mean = 12,043 and 1,087 ind. m−2, respectively). Though relatively few in number, Trichoptera was the most species-rich taxonomic group in all three springbrooks.
Ecotonal shifts in macroinvertebrate communities; taxonomic gradient
Biocomplexity of zoobenthic communities increased substantially along the eucrenon–hypocrenon gradient, and longitudinal zonation patterns were pronounced in each spring during both seasons. Spring sources supported a depauperate diversity of fauna and were generally devoid of aquatic insects (Fig. 2). Amphipods and isopods (superorder Peracarida) were the characteristic fauna in each spring source. In contrast, each springbrook supported a moderate diversity of aquatic insect and non-insect macroinvertebrates. The number of taxa ranged from as low as three at the spring source to as high as 15 at reach 4 during the summer, and from 4 to 21 along the same longitudinal gradient during the winter. Peracaridan-to-insect proportions decreased substantially with distance from the spring source in all springs. Hence, the springs were similar in that species composition shifted along the spring source–springbrook ecotone from a community composed of non-insect taxa to one dominated by aquatic insects.
Pie charts illustrating longitudinal zonation patterns for Haseltine, Danforth & Steury Springs (HS, DS, SS). Full list of taxa in Table 3. Reach 1 in the cave mouth, Reach 4 farthest downstream. Charts available for all reaches in each spring upon request
Linear regression analysis showed that diversity (H′) significantly increased with increasing distance from the spring source (Fig. 3) during both seasons in all three springs (summer, r 2 = 0.50, P = 0.001; winter, r 2 = 0.29, P = 0.04). Shannon indices ranged from as low as 0.98 at the cave to as high as 1.87 by reach 4 in the summer, and winter values ranged from 0.84 to 1.95. Evenness (E 1/D) significantly decreased along this gradient (summer, r 2 = 0.59, P = 0.001; winter, r 2 = 0.49, P = 0.001) in each springbrook. This was an unexpected result as increased diversity is typically accompanied by equitability (Ward & Dufford, 1979). As spring source communities contained a minimal number of species (especially Danforth & Steury; Fig. 2), similarity in their relative abundance patterns provides a likely explanation for why evenness was greater there.
Relationships between longitudinal position from the spring source and Shannon’s (H′) index of diversity and Simpson’s (E 1/D) index of evenness for Haseltine, Danforth & Steury Springs (HS, DS, SS). Top row summer; bottom row (shaded) winter. Diversity: summer, y = (0.29) + (0.16)(distance); winter, y = (0.31) + (0.14)(distance). Evenness: summer, y = (1.20) + (−0.87)(distance); winter, y = (1.20) + (−0.95)(distance)
Density (#/m2) and biomass (mg/m−2) also showed distinct patterns in relation to longitudinal position from the eucrenon within each spring across both seasons (Fig. 4). Overall, density and biomass increased linearly from the spring source to reach 2 as insect species began to join peracaridans. By reach 4, these measures decreased as most amphipods and isopods disappeared. Because of opposite downstream trends in biomass and density for crustaceans, invertebrates were placed into four taxonomic groups for further density and biomass analysis: flatworms, crustaceans, gastropods, and insects. Summer patterns showed a significant downstream increase in flatworm, gastropod, and insect biomass and density (biomass: r 2 = 0.72, P = 0.001; density, r 2 = 0.92, P = 0.001). Crustacean biomass and density, conversely, decreased with distance from the spring source (biomass, r 2 = 0.56, P = 0.001; density, r 2 = 0.61, P = 0.001). During the winter, downstream trends in biomass and density for flatworms, gastropods, and insects remained similar (biomass: r 2 = 0.55, P = 0.001; density, r 2 = 0.55, P = 0.001). Decreasing longitudinal trends in crustacean biomass and density varied as crustacean abundance decreased in upper and increased in lower reaches during the winter sampling period (biomass, r 2 = 0.06, P = 0.29; density, r 2 = 0.27, P = 0.28). Nonetheless, peracaridan dominance significantly decreased while insect dominance increased with distance from the spring source during both summer (Peracarida, r 2 = 0.36, P = 0.001; insect, r 2 = 0.94, P = 0.001) and winter (Peracarida, r 2 = 0.29, P = 0.001; insects, r 2 = 0.40, P = 0.001) sampling seasons. Peracaridan-to-insect proportions among springs and seasons were as high as 99:0% in reach 1, decreased to an average of 40:51% and 23:64% in reaches 2 and 3, respectively, and by reach 4 were as low as 0:88%.
Relationships between longitudinal position from the spring source and biomass (mg/m2) and density (#/m2) of invertebrate groups. The vertical axis represents the proportional amount of each category within a functional group and ranges from 0 to 1. Taxonomic groupings are shown under each six-box matrix for Haseltine, Danforth & Steury Springs (HS, DS, SS) for summer and winter
Although temperature was not significantly correlated with any of the community structure metrics (Pearson; P > 0.05), the correlation coefficients for distance from the spring source (longitudinal), biomass, and density were ≥0.50. Further regression analysis to detect possible longitudinal relationships between temperature and these biotic metrics in each spring revealed no significant results.
Season and spring-specific effects
While overall linear trends detected in benthic faunal metrics were distinct and significant “within” each spring, the nature of those relationships varied among springs and seasons (Table 4). Diversity (H′) was significantly higher in Steury, but because there were significant interaction effects, this variability was not consistent between seasons. Variations in density and Peracarida proportions were significant and similar across springs. Interactive effects were, however, significant for Peracarida indicating that these patterns differed markedly between seasons.
Total organic and inorganic carbon concentrations, although not significantly correlated with community structure metrics (Pearson; P > 0.05), exhibited distinct patterns with regard to differences in springs and seasons (ANOVA, TIC: season factor; F (1,70) = 3501.29, P < 0.0001, spring factor; F (2,69) = 8.75, P < 0.0001. TOC: season factor; F (1,70) = 11039.86, P < 0.0001, spring factor; F (2,69) = 27.03., P < 0.0001). Post hoc tests revealed that TIC and TOC were both significantly higher in the winter, as well as significantly higher in SS. Interactive effects were significant for TIC (F (2,69) = 22.30, P = < 0.0001) indicating that the effect of season was not consistent among springs.
Ecotonal shifts in FFG and HT
Shifts in the relative importance of each trait group (FFG and HT) are illustrated and described below in terms of the composition and number of functional groups present along the spring source–springbrook gradient, along with the proportion of individuals represented among the groups. Shifts were similar in spatial pattern to those found for taxonomic diversity, density, and biomass.
During the summer, three trait groups were present at the spring source, but four to seven occurred by reach 4 (Fig. 5). Shredders (amphipods and isopods) dominated each spring source and significantly (r 2 = 0.59, P = <0.001) decreased in importance along the springbrook (reaches 2–3: Haseltine, 56–37%; Danforth, 32–4%; Steury, 47–4%). By reach 4 they were virtually absent. Two HT groups, swimmers and sprawlers (amphipods and isopods, respectively), were always associated with this FFG and consequently exhibited this same spatial pattern along the spring source–springbrook gradient (swimmers, r 2 = 0.15, P = 0.02; sprawlers, r 2 = 0.35, P = <0.001).
Composition and proportion of individuals represented among functional groups at the spring source (R1) and the most downstream springbrook station (R4) in Haseltine, Danforth & Steury Springs (HS, DS, SS). The vertical axis represents the proportional amount of each category within a group and ranges from 0 to 1
The relative abundance of scrapers in the Haseltine springbrook and predators in Danforth and Steury springbrooks, increased significantly with distance from the spring source (scrapers, r 2 = 0.49, P < 0.0001; predators, r 2 = 0.71, P < 0.0001). Scrapers (e.g., snails and riffle beetles), absent from all HS source samples, represented 44 and 59% of feeding groups in reaches 2 and 3, respectively; and by reach 4, it was clearly the dominant FFG. In the Danforth and Steury springbrooks, predator dominance (primarily damselflies) increased from 18–60 to 44–58% between reaches 2 and 3, respectively, and attained their maxima by reach 4 (73 and 86%, respectively). Collectors (primarily worms, caddisflies, midges, and craneflies) represented smaller proportions of springbrook FFGs, but were positively associated with distance from the spring source (r 2 = 0.31, P < 0.001). Worm and cranefly burrowers were so poorly represented in each spring they did not exhibit a strong spatial pattern (r 2 = 0.06, P = 0.14).
Collector and predator distinctions in Danforth and Steury were complicated as the dominant springbrook taxa were typically damselflies (A. sedula), known for their ontogenetic shift in food reliance. Young damselfly larvae are collectors of fine transported detrital matter, while adults are predators (Webster & Benfield, 1986). As we did not differentiate between the number of young and old instars, the proportional FFG relationships illustrated here may be somewhat obscured, because collectors could be more dominant than determined upon initial analysis. Nonetheless, as the clinger HT was associated with scrapers, damselflies (regardless of ontogenetic feeding pattern), and collectors, it represented the dominant HT group in all three springbrooks. They increased significantly between reach 2 and 4 from 44 to 87% in Haseltine, 51 to 99% in Danforth, and 50 to 100% in Steury (r 2 = 0.69, P = <0.0001).
Winter patterns of ecotonal shift in functional traits within springs were similar to those found in summer. The total number of functional groups present at the spring source, however, increased to between four and five, but they never exceeded three during summer. In contrast, springbrook numbers remained between six and seven. Additionally, seasonal shifts in the relative abundance of each functional trait occurred to varying degrees across all reaches and springs (Fig. 5). Shredders remained dominant at the spring source, and their decreasing longitudinal patterns were similar to those found in summer. However, as amphipods migrated downstream during the winter (e.g., shredders reach 4 Haseltine = 40% of the FFG’s winter vs. 7% summer), the pattern was no longer statistically significant (r 2 = 0.03, P = 0.33). This reversed the longitudinal trend for the swimmer HT group, from that found in summer, to one that increased along the eucrenon–hypocrenon gradient (r 2 = 0.49, P = <0.0001). Sprawlers, as in summer, significantly decreased along this gradient (r 2 = 0.54, P = <0.0001). Even with the migration of amphipods into lower reaches of Haseltine, scrapers remained positively associated with distance from the spring source (r 2 = 0.49, P = <0.0001). Collectors, and correspondingly clingers, represented a substantially larger proportion of springbrook FFG’s over that found in the summer with additions of soldierflies, mayflies, and collector midges (Chironominae). Each of these trait groups demonstrated a significant increase along the spring source–springbrook gradient (collectors, r 2 = 0.53, P = 0.02; clingers, r 2 = 0.23, P = 0.002) as they had during the summer. Predators remained significantly associated with position along the length of the springbrooks (r 2 = 0.57, P = <0.0001) especially as damselflies were now joined by predator midges (Tanypodinae). Additions of soldierflies (collectors) and predatory midges correlated with a substantial increase in the sprawler HT group, but they remained negatively associated with distance from the spring source (r 2 = 0.54, P = <0.0001). Burrowers, although few in number, were positively associated with distance from the spring source (r 2 = 0.24, P = 0.003).
Discussion
Initial hypotheses
Our first hypothesis was that each spring would be physicochemically stable along the eucrenon–hypocrenon gradient. This hypothesis was not rejected because all three spring sources, and associated rheocrene springbrooks exhibited stable thermal, chemical, and flow regimes. Of note is the fact that our designation of spring source (eucrenon) and springbrook (hypocrenon) differs from the commonly accepted criteria proposed by Erman & Erman (1995) which defines the springbrook as the point where water temperature differs by two or more degrees from that at the spring source (typically 5–10 m downstream). It is downstream of this point that they, and others, have shown marked changes in biotic community structure and composition (Smith et al., 2003; Barquín & Death, 2009). Additional studies have cited relationships between annual and diurnal temperature fluctuations, with distance from a spring source, and variability in macroinvertebrate community composition and diversity (Minckley, 1963; Minshall, 1968; Ward & Dufford, 1979). These findings, however, were based on springbrooks much longer than ours. Longitudinal studies in springbrooks more similar to ours (in length, lacking tributary input, and patterns of diversity) have shown little downstream increases in temperature variability (Stern & Stern, 1969; Williams & Hogg, 1988; Barquín & Death, 2011). We conclude, as have others, that thermal fluctuations did not appear to have an effect on downstream patterns of benthic assemblages in our springs (cf. Bottazzi et al., 2011; Kubíkoá et al., 2012; Dumnicka et al., 2013).
Of all the environmental variables measured, conductivity, calcium, TOC, and TIC showed the most variability. Variability in calcium concentrations is likely attributable to the fact that Haseltine and Steury Spring waters originate from the shallower limestone bedrock of the Springfield Plateau physiographic region, as their Ca to Mg ratios are substantially higher than found in Danforth, which originates from deeper dolomite bedrock. Higher Ca concentrations would also promote higher conductivity. However, none of these environmental variables were correlated with any of our biotic measures. Both TIC and TOC concentrations were higher in winter in all springs, and substantially higher in Steury. This probably resulted from seasonal leaf fall and decomposition, resulting from allochthonous input from Steury’s deciduous canopy.
The faunal composition in our springs is consistent with our assessment of physicochemical stability. Stable, cool hard-water springs commonly have over 10,000 individuals m−2 (as in our springs), an order of magnitude higher than in more environmentally variable epigean systems (Minshall, 1968). Additionally, the largest density of animals in these ecosystems (peracarid crustaceans), and the dominant organic substrate (moss) are indicators of spring permanence and long-term hydrogeological stability (Barquín & Death, 2006; Death & Barquín, 2012).
Our findings showed negligible physicochemical variability between the head of each spring source and the base of the springbrooks, and yet there were substantial changes in biotic and functional diversity along these lengths. Consequently, we concur with others who have found that physicochemical parameters had little influence on macroinvertebrate community composition in their springs (Smith et al., 2003; Von Fumetti et al., 2006; Staudacher & Füreder, 2007; Kubíkoá et al., 2012).
Our second hypothesis was that spring communities would fit the longitudinal pattern noted by Gooch & Glazier (1991) for hard water limestone springs. As expected, the taxonomic gradient was significantly associated with the eucrenon–hypocrenon gradient in all three springs during both seasons, as species composition shifted along this ecotone from a community composed predominately of peracaridan crustaceans to one dominated by aquatic insects. Our findings vary substantially from that of Cantonati et al. (2012), who argued that rheocrenes are the spring type in which the assemblage at the spring source most closely resembles the assemblage in the subsequent springbrook. Increases in biotic diversity found along the length of our systems, conversely, are consistent with other spring studies that found similar longitudinal patterns (Minshall, 1968; Ward & Dufford, 1979; Bonettini & Cantonati, 1996; Dumnicka et al., 2013; but see Resh, 1983 and Spitale et al., 2012 for exceptions).
Limitation of species, dominance by peracaridans, and the paucity of aquatic insects at the eucrenon, beyond being noted in previous studies (Minckley, 1963; Danks & Williams, 1991; Erman, 1995), was expected for a number of reasons. First, hard water limestone springs are typically characterized by a prevalence of amphipods and isopods, and carbonate waters, in particular, provide constituents of the crustacean exoskeleton (Glazier, 1991). Second, eucrenon habitats are characterized by the absence of transported detritus from upper reaches (Ward & Dufford, 1979), which explains why collectors were virtually absent in the eucrenon, because fine transported organic matter (FTOM) is mostly generated in situ (Glazier, 1991). High densities of peracaridans in our eucrenons may also be attributed to the rarity of large efficient predators (Minshall, 1968; Glazier, 1991). Aquatic vertebrate predators in our springs included sculpin, (Cottus bairdi), stippled darter (Etheostoma punctulatum), and salamanders (Eurycea multiplicata griseogaster). Fish were never found at the spring source and never numbered more than two in any given reach collection. Salamanders, while found only at the spring source, never numbered more than three. This reduced vertebrate predation is often why some species of Gammarus reach densities of thousands of individuals per square meter (Hobbs, 1992), as was the case in our springs.
Finally, our third hypothesis stated that the three springs would exhibit similar biotic and functional organizational patterns. This prediction was not supported. While spring source communities were taxonomically and functionally similar, there were substantial differences in diversity, species composition, and functional traits at similar points in the three springbrooks.
Mechanistic explanations
The distinct structural and functional longitudinal zonation patterns of macroinvertebrate communities found in our springs confirm studies that have identified longitudinal position as an important predictor of community structure and function in streams (Heino, 2005; Ilg & Castella, 2006; Jacobsen et al., 2010; Kuhn et al., 2011). As Spitale et al. (2012) reported, studies of benthic assemblage change along longitudinal gradients have long been described for rivers (Vannote et al., 1980) and streams (Hawkins & Sedell, 1981), but few studies have examined whether mechanisms important in these habitats are applicable in springs. Of the longitudinal studies done in springs and spring-related systems, mechanisms such as variation in substrate composition (both organic and inorganic) have been identified as more important than changes in discharge or chemistry in explaining zoobenthic community composition (Minckley, 1963; Ward & Dufford, 1979; Bonettini & Cantonati, 1996; Heino, 2005). Additionally, the relative abundance of food sources has been cited as important in shaping spring communities (Smith et al., 2003; Barquín & Death, 2006). Gooch & Glazier (1991) also hypothesized that in springs where non-insect fauna are replaced by insect fauna downstream (as in ours); species interactions may be critical organizational mechanisms.
Substrate
While bed form (substrate particle size) remained relatively consistent throughout the length of each springbrook, substrate at the spring source typically occupied the upper fraction of the rubble range (25–64 mm), while more distant reaches fell in the lower fraction (cf. Minshall, 1968). Moss and water cress were especially dense near spring sources, possibly associated with the larger, less alluvial, lithic substrates. Peracaridans were dominant here, as these substrates often serve as their major habitat (cf. Minckley, 1963; Stern & Stern, 1969; Glazier, 1991). Peracaridan-substrate associations provide another plausible explanation for the dominance of peracaridans in spring sources.
Likewise, decreases in the density of these vegetative mats has been correlated with decreases in these species and increases in insect and other non-insect invertebrates (similar pattern in the lower springbrook reaches of our springs). For example, removal of water cress from springs in Pennsylvania (Gooch & Glazier, 1991) and Virginia (Miller & Buikema, 1977) was followed by a dramatic decrease in density of Gammarus, but contrasting increases in ephemeropterans, dipterans, and plecopterans. Similarly, Gammarus density in Haseltine reach 4 shifted from 688 m−2 in the summer to winter values of 7,075 m−2 in correlation with winter increases in density of water cress mats.
These patterns support the notion that even slight changes in lithic substrate size could be associated with changes in the density of organic substrate coverage that affects the colonization of associated taxa (e.g., moss and amphipods). In turn, this association would result in subsequent shifts in species composition and diversity along substrate gradients. Substrate composition has been found to be the dominant mechanism controlling faunal composition in a number of both spring and epigean stream studies (Minshall, 1968; Finn & Poff, 2005; Ilg & Castella, 2006; Dumnicka et al., 2013). Substrate homogeneity (primarily moss) at the spring source and increased heterogeneity in springbrooks is indicative of a shift from reduced to increased habitat complexity along this longitudinal gradient. Many stream studies have shown that habitat complexity is important in sorting species based on their “fit” with prevailing habitat conditions (Poff, 1997; Taniguchi & Tokeshi, 2004; Heino, 2005).
Availability of food resources
Another mechanism which could account for observed longitudinal differences in community structure and function is the variation in ecotonal, inter-spring, and seasonal composition and availability of food sources. Qualitative visual assessment and taxonomic identification of organic substrate in this study serves as a partial estimate of availability of food sources. Temporal and spring-specific differences in TIC and TOC concentrations provide more insight regarding community–habitat relationships. For simplicity of this discussion only FFG designations are used. Gammarus (the dominant peracaridan in our spring sources), is known to feed on moss, water cress, and associated detritus, as the large surface area of these matted macrophytes provides large detrital traps (Minckley, 1963; Stern & Stern, 1969; Glazier, 1991). Use of these food sources may seem unlikely as herbivory on these macrophytes is widely considered minimal due to their relatively high indigestible fiber (cellulose) and low nitrogen content (Gregory, 1983; Mann, 1988). Others have found moss and water cress to be important sources of carbon for these aquatic consumers (Jones, 1949; Rosenfeld & Roff, 1992; Robinson et al, 2008). This could be because peracaridans generally have higher cellulase activities and, therefore, greater efficiencies in assimilating these plants (Kesler, 1983; Chamier, 1991; Glazier, 1991). Additionally, as watercress is known to produce defensive toxins, amphipods often prefer to eat detrital forms after toxins have been leached through decomposition (Newman, 1991).
Second, springbrook communities were dominated by insects, but the three springbrooks were functionally dissimilar. The amount of incident light reaching Haseltine springbrook was nearly twice that found in either of the other springbrooks. Variability in canopy cover would likely affect the amount of light available for autochthonous primary production and alter organic leaf litter input, and could be pivotal in defining taxonomic and functional assemblages. This is evidenced by the predominance of scrapers in Haseltine (more open canopy), while predators or collectors dominated in Danforth (coniferous canopy) and Steury (deciduous canopy).
Moreover, seasonal and spring-specific analyses indicated that diversity was highest in SS (deciduous canopy), but only during the winter with the seasonal appearance of midges, soldierflies, and mayfly species (absent from summer samples). The seasonal increase in biodiversity is consistent with the notion that several species synchronize their life cycles to coincide with litter fall (Lindegaard et al., 1988). These collectors were likely responding to seasonal shifts in the input and breakdown of allochthonous carbon as FTOM is mostly generated in situ (McCabe, 1998). A similar significant increase in winter TIC and TOC concentrations in all springs (but significantly higher in Steury) further supports this conclusion as litter fall from Steury’s deciduous canopy and subsequent decomposition would affect these measures.
The patterns noted here with regard to food availability are tightly linked with shifts in substrate composition and habitat complexity noted above. Substrate surface features are critical as they often affect food supplies (Dudley et al., 1986). Additionally, diversity and quantity of food resources, as well as the number of places they can be obtained, increase with habitat complexity which is often associated with substrate heterogeneity (Heino, 2005; Kuhn et al., 2011). This explains why foods like FTOM often increase with habitat complexity (Taniguchi & Tokeshi, 2004) and provides a possible mechanistic understanding for longitudinal increases in structural and functional diversity observed in our springs.
Of note is that our observed changes in FFG from the spring source to the downstream reach of each springbrook are consistent with the concept that the relative abundance of food sources shapes community composition, an idea championed for the main channel of long, hydrogeomorphically simple rivers by the river continuum concept (Vannote et al., 1980). The river continuum concept, however, makes these predictions based on systems that extend from headwaters (first-order streams) to large rivers, and is therefore limited in its application here.
Biotic interactions
Finally, biotic interactions may also be a powerful mechanism defining the structure and function of our benthic communities. First, in limestone springs, there is typically only one numerically dominant peracaridan (Glazier, 1991). As amphipods were dominant in our spring sources, it is likely that they may have been the first to colonize this microhabitat, as the first colonist associated with a particular substrate often maintains its dominance until a major change occurs (Minckley, 1963). Competitive interactions, then could explain their dominance, and also explain why isopods and amphipods rarely co-dominated our spring communities. Second, rarity of large predators in springs and environmental stability often result in high peracaridan population densities (e.g., Gammarus; Glazier, 1991; Hobbs, 1992). In turn, these highly crowded habitats promote powerful competition for resources. Selective forces would favor those species with superior efficiency in exploiting those resources (Glazier, 1991). Peracaridan efficiency in utilizing moss as a food source (higher cellulase activities) is the competitive advantage Glazier refers to, and adds further insight into patterns of peracaridan distribution in our springs that strongly correlate with substrate composition and availability of food resources. Additionally, the presence of amphipods has been shown to be correlated with lower invertebrate diversity, as they often prey on insect eggs, larvae, and pupae (Glazier, 1991; Death et al., 2004).
As Dumnicka et al. (2013) stated, few studies have compared zoobenthic communities in spring eucrenal zones with those in hypocrenonal zones unaffected by input from surface tributaries. Spitale et al. (2012) concurred, stating that hypotheses about the mechanisms responsible for longitudinal patterns in springs are rare. Our study is one of the few that has examined shifts in both structural and functional complexity from eucrenon to hypocrenon zones in springs unaffected by surface tributaries. This study provides insights into the effects of ecotonal changes in structural and functional traits (at the community level) on ecosystem functioning through analysis of biomass (an index of productivity; Hawkins & Sedell, 1981) and trophic transfer (cf. Heino, 2005; Ilg & Castella, 2006). As an extension of this first study of ours, the significant role of organic sources in controlling the species composition and density of invertebrates along the downstream paths of these three springs is being evaluated in a stable isotope based, food web study and a field experiment on food availability (Carroll & Thorp, in prep.). As organic substrate and food availability are so closely linked, a food web analysis could further elucidate mechanisms controlling the structure and function of these zoobenthic communities.
Future scenario
Springs have traditionally been seen as clean and pristine environments of high biological integrity, but they are also incredibly fragile and extremely sensitive to disturbances (e.g., water abstraction, sedimentation, excessive nutrients, removal of the surrounding vegetation). The biotic and functional ecotonal shifts in our three Ozark springs were stark and occurred along strikingly short distances devoid of changes in spring magnitude (e.g., stream order). Ecotonal shifts such as these will likely become even more dynamic with climactic changes and increases in anthropogenic modifications of headwater systems like springs. Ecologists are increasingly emphasizing the need to predict how communities and ecosystem functioning will respond to rapid environmental change. A mechanistic understanding of variation along gradients such as those exhibited in springs should improve our understanding of the link between community and ecosystem ecology and facilitate our ability to make predictions about effects of environmental change—both factors which are essential in achieving resource management solutions.
References
APHA (American Public Health Association), 2000. Standard Methods for the Examination of Water and Wastewater, 20th edn. American Public Health Association, American Water Works Association, and Water Environment Federation, Washington, DC.
Barquín, J. & R. G. Death, 2006. Spatial patterns of macroinvertebrate diversity in New Zealand springbrooks and rhithral streams. Journal of the North American Benthological Society 25: 768–786.
Barquín, J. & R. G. Death, 2009. Physical and chemical differences in karst springs of Cantabria, northern Spain: do invertebrate communities correspond? Aquatic Ecology 43: 445–455.
Barquín, J. & R. G. Death, 2011. Downstream changes in spring-fed stream invertebrate communities: the effect of increased temperature range? Journal of Limnology 70: 134–146.
Benke, A. C., A. D. Huryn, L. A. Smock & J. B. Wallace, 1999. Length–mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 18: 308–343.
Bonettini, A. M. & M. Cantonati, 1996. Macroinvertebrate assemblages of springs of the River Sarca catchment (Adamello-Brenta Natural Park, Trention, Italy). Crunoecia 5: 71–78.
Bottazzi, E., M. C. Bruno, V. Pieri, A. Di Sabatino, L. Silveri, M. Carolli & G. Rossetti, 2011. Spatial and seasonal distribution of invertebrates in Northern Apennine rheocrene springs. Journal of Limnology 70: 77–92.
Cantonati, M., R. Gerecke & E. Bertuzzi, 2006. Springs of the Alps—sensitive ecosystems to environmental change: from biodiversity assessments to long-term studies. Hydrobiologia 562: 59–96.
Cantonati, M., L. Füreder, R. Gerecke, I. Jüttner & E. J. Cox, 2012. Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshwater Science 31: 463–480.
Chamier, A. C., 1991. Cellulose digestion and metabolism in the freshwater amphipod Gammarus pseudolimnaeus Bousfield. Freshwater Biology 25: 33–40.
Danks, H. V. & D. D. Williams, 1991. Arthropods of springs, with particular reference to Canada. Memoirs of the Entomological Society of Canada 155: 203–217.
Death, R. G., J. Barquín & M. R. Scarsbrook, 2004. Coldwater and geothermal springs. In Harding, J. S., M. P. Mosley, C. Pearson & B. Sorrell (eds), Freshwaters of New Zealand. New Zealand Hydrological Society and New Zealand Limnological Society, Christchurch: 30.31–30.14.
Death, R. G. & J. Barquín, 2012. Geographic location alters the diversity – disturbance response. Freshwater Science 31: 636–646.
Dodds, W. K., K. Gido, M. R. Whiles, K. M. Fritz & W. J. Matthews, 2004. Life on the Edge: the ecology of Great Plains prairie streams. Bioscience 54: 205–216.
Dolédec, S., N. Phillips, M. Scarsbrook, R. H. Riley & C. R. Townsend, 2006. Comparison of structural and functional approaches to determining land use effects on grassland stream invertebrate communities. Journal of the North American Benthological Society 25: 44–60.
Dudley, T. L., S. D. Cooper & N. Hemphill, 1986. Effects of macroalgae on stream invertebrate community. Journal of the North American Benthological Society 5: 93–106.
Dumnicka, E., J. Galas, I. Jatulewicz, J. Karlikowska & B. Rzonca, 2013. From spring sources to springbrook: changes in environmental characteristics and benthic fauna. Biologia 68: 142–149.
Erman, N. A., 2002. Lessons from a long-term study of springs and spring invertebrates (Sierra Nevada, California, U.S.A.) and implications for conservation and management. In Conference Proceedings. Spring-Fed Wetlands: Important Scientific and Cultural Resources of the Intermountain Region.
Erman, N. A. & D. C. Erman, 1995. Spring permanence, species richness and the role of drought. In Ferrington, C. (ed.), Biodiversity of Aquatic Insects and Other Invertebrates in Springs. Journal of the Kansas Entomological Society Supplement, Special Publication No. 2: 50–64.
Finn, D. S. & N. L. Poff, 2005. Variability and convergence in benthic communities along the longitudinal gradients of four physically similar Rocky Mountain streams. Freshwater Biology 50: 243–261.
Glazier, D. S., 1991. The fauna of North American temperate cold springs: patterns and hypotheses. Freshwater Biology 26: 527–542.
Glazier, D. S., 2009. Springs. In Likens, G. E. (ed.), Encyclopedia of Inland Waters, Vol. 1. Academic Press Elsevier, Oxford, UK: 734–755.
Glazier, D. S., 2012. Temperature affects food-chain length and macroinvertebrate species richness in spring ecosystems. Freshwater Science 31: 575–585.
Gooch, J. L. & D. S. Glazier, 1991. Temporal and spatial patterns in mid-Appalachian springs. Memoirs of the Entomological Society of Canada 155: 29–49.
Gregory, S. V., 1983. Plant–herbivore interactions in stream systems. In Barnes, J. R. & G. W. Minshall (eds), Stream Ecology. Plenum Press, New York, NY: 157–190.
Hawkins, C. P. & H. J. Sedell, 1981. Longitudinal and seasonal changes in functional organization of macroinvertebrate communities in four Oregon streams. Ecology 62: 387–397.
Heino, J., 2005. Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshwater Biology 50: 1578–1587.
Hobbs, III, H. H., 1992. Caves and springs. In: Biodiversity of the Southeastern United States: Aquatic Communities. Wiley, New York: 59–131.
Hynes, H. B. N., 1983. Groundwater and stream ecology. Hydrobiologia 100: 93–99.
Ilg, C. & E. Castella, 2006. Patterns of macroinvertebrate traits along three glacial stream continuums. Freshwater Biology 51: 840–853.
Jacobsen, D., O. Dangles, P. Andino, E. Rodrigo, L. Hamerlik & E. Cadier, 2010. Longitudinal zonation of macroinvertebrates in an Ecuadorian glacier-fed stream: do tropical glacial systems fit the temperate model? Freshwater Biology 55: 1234–1248.
Jones, J. R. E., 1949. A further ecological study of calcareous streams in the Black Mountain district of South Wales. Journal of Animal Ecology 18: 41–57.
Kesler, D. H., 1983. Variation in cellulase activity in Physa heterostropha (Gastropoda) and other species of gastropods in a New England pond. American Midland Naturalist 109: 280–287.
Krebs, C. J., 1999. Ecological Methodology, 2nd ed. Harper & Row, New York.
Kubíkoá, L., O. Simon, K. Tichá, K. Douda, M. Maciak & M. Bíly, 2012. The influence of mesoscale habitat conditions on the macroinvertebrate composition of springs in a geologically homogeneous area. Freshwater Science 31: 668–679.
Kuhn, J., P. Andino, R. Calvez, R. Espinosa, L. Hamerlik, S. Vie, O. Dangles & D. Jacobsen, 2011. Spatial variability in macroinvertebrate assemblages along and among neighboring equatorial glacier-fed streams. Freshwater Biology 56: 2226–2244.
Lindegaard, C., K. P. Brodersen, P. Wiberg-Larsen & J. Skriver, 1988. Multivariate analyses of macrofaunal communities in Danish springs and springbrooks. In Botosaneanu, L. (ed.), Studies in Crenobiology: The Biology of Springs and Springbrooks. Backhuys Publishers, Leiden: 201–219.
Magurran, A. E., 2004. Measuring Biological Diversity. Blackwell Science Ltd., Malden, MA.
Mann, K. H., 1988. Production and use of detritus in various freshwater, estuarine, and coastal marine ecosystems. Limnological Oceanography 33: 910–930.
McCabe, D. J., 1998. Biological communities in springbrooks. In: Botosaneanu, L. (ed.), Studies in Crenobiology: The Biology of Springs and Springbrooks. Backhuys Publishers, Leiden: 221–228.
Merritt, R. W., J. B. Cummins & M. B. Berg (eds), 2008. An Introduction to the Aquatic Insects of North America, 4th ed. Kendall/Hunt, Dubuque, IA.
Miller, J. D. & A. L. Buikema Jr, 1977. The effect of substrate on the distribution of the spring form (Form III) of Gammarus minus Say. Crustaceana 4: 153–163.
Minckley, W. L., 1963. The ecology of a spring stream Doe Run, Meade County, Kentucky. Wildlife Monographs 11: 1–124.
Minshall, G. W., 1968. Community dynamics of the benthic fauna in a woodland spring brook. Hydrobiologia 32: 305–339.
Newman, R. M., 1991. Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. Journal of the North American Benthological Society 10: 89–114.
Odum, H. T., 1957. Trophic structure and productivity of Silver Springs, Florida. Ecological Monographs 27: 55–112.
Poff, N. L., 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16: 391–409.
Pollard, A. I. & L. L. Yuan, 2010. Assessing the consistency of response metrics of the invertebrate benthos: a comparison of trait- and identity-based measures. Freshwater Biology 55: 1420–1429.
Resh, V. H., 1983. Spatial differences in the distribution of benthic macroinvertebrates along a springbrook. Aquatic Insects 5(4): 193–200.
Robinson, C. T., D. Schmid, S. Matthias & S. M. Bernasconi, 2008. Functional measures and food webs of high elevation springs in the Swiss Alps. Aquatic Sciences 70: 432–445.
Rosenfeld, J. S. & J. C. Roff, 1992. Examination of the carbon base in southern Ontario streams using stable isotopes. Journal of the North American Benthological Society 11: 1–10.
Shannon, C. E. & W. Weaver, 1949. The Mathematical Theory of Communication. University of Illinois Press, Urbana, IL.
Smith, J., P. J. Wood & J. Gunn, 2003. The influence of habitat structure and flow permanence on invertebrate communities in karst springs systems. Hydrobiologia 510: 53–66.
Spitale, D., M. Leira, N. Angeli & M. Cantonati, 2012. Environmental classification of springs of the Italian Alps and its consistency across multiple taxonomic groups. Freshwater Science 31: 563–574.
Staudacher, K. & L. Füreder, 2007. Habitat complexity and invertebrates in selected Alpine springs (Schütt, Carinthia, Austria). International Review of Hydrobiologia 92: 465–479.
Stern, M. S. & D. H. Stern, 1969. A limnological study of a Tennessee cold springbrook. American Midland Naturalist 82: 62–82.
Taniguchi, H. & M. Tokeshi, 2004. Effects of habitat complexity on benthic assemblages in a variable environment. Freshwater Biology 49: 1164–1178.
Thomson, K. C., 1986. Geology of Greene County, Missouri. Watershed Management Coordinating Committee, Springfield, MO.
Thorp, J. H. & A. P. Covich (eds), 2010. Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego, CA.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Von Fumetti, S., P. Nagel, N. Scheifhacken & B. Baltes, 2006. Factors governing macrozoobenthic assemblages in perennial springs in north-western Switzerland. Hydrobiologia 568: 467–475.
Ward, J. V. & R. G. Dufford, 1979. Longitudinal and seasonal distribution of macroinvertebrates and epilithic algae in a Colorado springbrook-pond system. Archiv für Hydrobiologie 86: 284–321.
Webb, D. W., M. J. Wetzel, P. C. Reed, L. R. Phillippe & T. C. Young, 1988. The macroinvertebrate biodiversity, water quality, and hydrogeology of ten karst springs in the Salem Plateau Section of Illinois, U.S.A. In Botosaneanu, L. (ed.), Studies in Crenobiology: The Biology of Springs and Springbrooks. Backhuys Publishers, Leiden: 39–48.
Webster, J. R. & E. F. Benfield, 1986. Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17: 567–594.
Williams, D. D. & I. D. Hogg, 1988. Ecology and production of invertebrates in a Canadian coldwater spring-springbrook system. Holarctic Ecology 11: 41–54.
Acknowledgments
We thank R.T. Pavlowsky, Marc Owen, and staff at the Ozarks Environmental and Water Resources Institute (OEWRI) at Missouri State University for their support. Special thanks are owed to spring owners T. Lynch, R. Campbell, and R. Lovett. We appreciate the help of L. Bullard from the Watershed Committee of the Ozarks for his assistance with locating the spring sites and sharing his expertise on spring ecosystems. We thank L.A. Bennett, M.A. Blackwood, and C. Freeman from the University of Kansas and J. Shepard and C. Copper for lab and field assistance. Comments from anonymous reviewers helped improve this final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sonja Stendera
Rights and permissions
About this article
Cite this article
Carroll, T.M., Thorp, J.H. Ecotonal shifts in diversity and functional traits in zoobenthic communities of karst springs. Hydrobiologia 738, 1–20 (2014). https://doi.org/10.1007/s10750-014-1907-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1907-4