Abstract
This study aimed to evaluate whether hatchery-reared Siniperca chuatsi (HSC) competes significantly with wild Siniperca populations in a shallow lake. Two wild Siniperca fishes, S. chuatsi and Siniperca kneri, along with HSC, were collected in the shallow Biandantang Lake, and their diets were determined using both stomach contents and stable isotope analyses. The stomach contents showed that shrimps and fish were the major food items of HSC and wild Siniperca fishes, but their dietary composition and percentage contribution revealed significant differences. Isotopic mixing model analysis suggested similar conclusions that contribution proportions of end members were significantly different. The niche overlap index on the basis of prey importance from stomach content analysis indicated that there was not significant diet overlap between hatchery-reared and wild Siniperca populations in winter and summer. Similar results were found in winter based on the prey contributions from isotopic mixing model outputs, but meaningful diet overlap was observed in summer. Our findings demonstrated that HSC did not have apparent feeding competition with wild Siniperca fishes during the critical periods of the early stocking stages, suggesting that moderate stocking of HSC may not have a negative effect on the variability of wild Siniperca populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stock enhancement is a fisheries management approach involving the release of cultured organisms to increase abundance and yield of natural fish or invertebrate stocks (Lorenzen, 2005). Stocking of hatchery fish has been practised on a large scale since the mid-nineteenth century. By 2010, there are 94 countries around the world to develop stock enhancement, involving more than 180 different species (FAO, 2010). In general, successful stock enhancement can improve socioeconomic outcomes by (1) creating new economic opportunities for fisheries-related livelihoods, (2) increasing skills of local communities, and (3) providing high-quality food fish and protein (Pinkerton, 1994; Lorenzen & Garaway, 1998). However, stocked species may have ecological impacts as well as social and economic outcomes (Arthur et al., 2010; Lorenzen et al., 2012). Aquatic ecosystems may be affected by the stocked species through predation, competition, mixing of exotic genes, habitat modification, and the introduction of pathogens (Youngson & Verspoor, 1998; Weber & Fausch, 2003). As a result, stock enhancement has been a subject of controversy regarding its effectiveness and possible adverse impacts on wild stocks, and possible challenges to aquatic ecosystems (Lorenzen, 2005; Jonsson & Jonsson, 2006; Araki & Schmid, 2010).
Competition occurs when multiple organisms exploit a common resource and the fitness of at least one is reduced, because either the resource is in short supply or other organisms interfere with its use (Birch, 1957). When hatchery-reared fishes are released into the wild, they compete with wild fish for food, space, and breeding partners (Jonsson & Jonsson, 2006). This is the most obvious impact of hatchery-reared fishes on wild species and can lead to a decline in the wild species (Moyle, 1976; Moyle & Light, 1996; Becker et al., 2005). Therefore, understanding the food and habitat competition between hatchery-reared and wild fish is essential for evaluating the ecological effects of stocking hatchery fish on their wild counterparts.
Siniperca chuatsi (Basilewsky) and Siniperca kneri (Garman) are two important perciform species with a wide distribution from the southern Zhujiang River system to the north in the Huaihe River system in China (Han et al., 1996; Liang et al., 2001). They are two of the most valuable food fish and two important piscivorous species, having specialized feeding habits of only feeding on live fish or shrimp throughout their life (Liang et al., 1998; Li et al., 2005, 2013). In the past decades, a major conventional fishery practice in Yangtze lakes has been to overfish piscivorous fish and stock herbivorous species (e.g., grass carp Ctenopharyngodon idella) and planktivorous species (e.g., bighead carp Aristichthys nobilis Richardson and silver carp Hypophthalmichthys molitrix Cuvier et Valenciennes). This has caused wild Siniperca populations to decline sharply and has subsequently induced a series of ecological problems (e.g., reduction or elimination of submerged macrophytes, algae blooms, and flourishing of small-sized fishes; Chen, 1989; Cao et al., 1991). In recent years, there has been a shift in fish stocking from common carps to piscivorous fishes (especially Siniperca chuatsi) to recover populations of piscivores, to utilize the abundant small-sized fish resources (Xie et al., 2000; Cui & Li, 2005), and to ease conflicts between fishery development and water quality conservation based on the principle of trophic-cascading effects (Carpenter & Kitchell, 1988; Liere & Gulati, 1992).
Hatchery-reared S. chuatsi (HSC) has been stocked in Yangtze lakes since 1994, and economic and social benefits have accrued in many lakes (Cui & Li, 2005; Zhang, 2007). Previous studies mainly focused on the survival, growth, economic benefits, and foraging success of stocking S. chuatsi, but less is known about the possible ecological effects of stocking on the wild Siniperca fishes.
In this study, we focus on food competition between HSC and two wild Siniperca fishes, S. chuatsi (WSC) and S. kneri (WSK), because competition has frequently been cited as an important negative ecological interaction. We collected specimens of hatchery-reared and two wild Siniperca fishes in a shallow Yangtze lake, in order to compare their diet composition and evaluate whether HSC negatively affect wild Siniperca fishes. Their stomach contents were examined, and stable carbon and nitrogen isotopes were measured in fish muscle tissues and in potential food items collected from their habitats. By comparing the two methods of dietary analysis we attempted to determine whether competitive interaction existed between hatchery-reared and two wild Siniperca fishes. These results may be useful in understanding feeding interactions, and thus help managers determine the impact of HSC on wild Siniperca populations in shallow lake systems.
Materials and methods
Study area
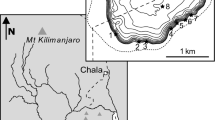
Biandantang Lake (30°15′N, 114°43′E) is located on the south bank of the middle reach of the Yangtze River, Hubei Province, central China. The lake, with an area of 3.333 km2 and depths ranging from 1.2 to 3.2 m (mean 2.0 m), has been separated from the larger Baoan Lake by a dyke (Fig. 1). 10 years ago, Biandantang Lake was heavily covered with submerged macrophytes, with Vallisneria spiralis L., Myriophyllum spicatum L., and Nelunbo nucefera G. the dominant species (Xie et al., 2000). For 6 years prior to this study, the major form of fishery had been the stocking of the Eriocheir sinensis, and subsequently submerged macrophytes as one of the most important food resources for E. sinensis gradually decreased due to overstocking of E. sinensis in past several years. In 2010, the lake was only sparsely vegetated with M. spicatum and Trapa bispinosa Roxb. in a small area of the littoral zone. The lake was slightly eutrophic at the time of sampling. A total of 47 fish species belonging to 14 families was recorded in the investigation of 2009–2010, of which A. nobilis, Cyprinus carpio L., Culter dabryi Bleeker, H. molitrix, Carassius auratus L., and S. chuatsi were the most important commercial fish. In June 2009, 4,800 juveniles of HSC (mean total length = 69.3 mm), marked with coded wire tags (tags with 0.25 mm diameter and 1 mm length, Northwest Marine Technology (NMT), Shaw Island, Washington) were stocked in the lake.
Sample collection
Siniperca chuatsi and S. kneri were sampled by electrofishing in January and June, 2010. Electrofishing was conducted in the night (from 20:00 h to 23:00 h) along littoral zones, with a generator-powered machine (DC, 4 kW, 220–380 V, 50 Hz). The cathode, a 2-m long copper cable, was put at the rear of boat. The hand-held anode (3.5-m long pole with a net of 40-cm diameter, mesh size 5 mm) was used for catching the fish. All specimens were placed in ice boxes after capture and transported immediately to the laboratory, and then the total length (TL, to the nearest mm) and weight (BW, to the nearest 0.1 g) were individually measured. A NMT hand-held tester was used to detect the coded wire tags to distinguish the hatchery-reared from wild S. chuatsi. Stomachs were removed and preserved in 10% buffered formalin solution for contents analysis. For specimens with stomachs that contained food, a small portion of white dorsal muscle tissue was removed and frozen for later stable isotope analysis.
The main food items (i.e., fish and shrimps) consumed by Siniperca fishes were known from previous feeding studies (Han et al., 1996; Li et al., 2013). Potential food items were sampled from littoral zone to pelagic zone using three trap-nets in January and June, 2010. Each set of trap-net had two cod-ends, with 2-m depth and 6-mm nylon mesh. Twelve trap-nets sets were completed in January and June, respectively. Potential prey fish were also collected when sampling for piscivorous fishes. The specimens were identified and individually measured for TL and standard length (SL) to the nearest mm, and body weight (BW) to the nearest 0.1 g. And then, white dorsal muscle tissue was taken and frozen for later stable isotope analysis. The main bones (cleithrum, opercular, preopercular, pharyngeal, and dentary bones) of potential prey fish were collected and their morphologies were observed.
Stomach content analysis
Stomach contents were analyzed by counting and measuring prey organisms under a stereo binocular microscope. Prey fish in stomach contents were identified to species or genus, and other prey was identified to genus or order. The lengths of intact prey fish were directly measured. Slightly digested prey fish could be identified to species by external morphology and their size was back-calculated by means of the linear equations between their SL and TL. Heavily digested prey fish were identified to species based on the morphology of intact species-specific bones of the prey fish remained in stomachs, and TL and BW of the digested prey fish were back-calculated theoretically according to the regression equations of Zhang (2005). The index of relative importance (% IRI) was used to describe the importance of all possible prey taxa in the diet (Pinkas et al., 1971), which provided the optimal balancing of frequency of occurrence, numerical abundance, and abundance by weight of taxa in fish diets (Liao et al., 2001).
Stable isotope analysis
All fish samples were oven dried at 60°C for at least 48 h to a constant weight and then ground into fine powder using a mortar and pestle. C/N ratios revealed that all tissues had values below 3.2, which is considered to be the threshold beyond which lipid extraction before stable isotope processing is suggested (Kiljunen et al., 2006; Hadwen et al., 2007). Therefore, we did not subject fish muscle tissues to lipid extraction before stable isotope analyses.
Approximately 3 mg of ground and dried samples was weighed into a tin cup and combusted in an elemental analyzer (Flash EA-1112 HT, Thermo Fisher Scientific, Inc., USA) to produce CO2 and N2, which flowed through a GC column for separation and into an isotope ratio mass spectrometer (MAT-253, Thermo Fisher Scientific, Inc., USA) for separately determining isotopic compositions. The isotope ratios were expressed as the difference in parts per thousand from a standard reference material:
where X is 13C or 15N, R is the corresponding ratio of 13C:12C or 15N:14N, and δ is a measure of the heavy to light isotopes in the sample. The reference materials used for δ13C and δ15N were the Vienna Pee Dee Belemnite (PDB) and atmospheric N2, respectively. Approximately, 20% of the samples were analyzed using two or more replicates. Two standards were also run after every eight samples to compensate for the drift over time. The deviations in the analysis of δ13C and δ15N replicates were both within 0.2‰.
The IsoSource mixing model was used to determine the feasible range of dietary contributions for each food source (Phillips & Gregg, 2003). All possible combinations of each source contribution (0–100%) were examined in 1% increments. Combinations that added to the observed stable isotopic signatures of the consumer within a tolerance of 0.1 were considered to be feasible solutions (Hicks et al., 2005). Trophic fractionations of carbon are generally low (0–1‰), and we assumed a trophic enrichment of +0.4‰ for δ13C, which were the increments suggested for either poikilothermic, ammonotelic, or aquatic animals (McCutchan et al., 2003). Nitrogen isotope signatures were not included in these analyses owing to difficulties associated with determining fractionation increments in the study organisms (Hadwen et al., 2007).
A modified approach was adopted to facilitate comparisons between stomach contents and stable isotope analyses. Specifically, end members were selected for use in the IsoSource mixing model on the basis of the results from our stomach contents analyses and published data on the diets of each species (Hadwen et al., 2007). Given that δ15N isotope signatures were not used in our mixing models, this approach avoids the need to account for significant trophic fractionation between prey items and consumers, and represents a more appropriate analysis of diets of carnivorous species (Hadwen et al., 2007). The end members chosen for HSC, WSC, and WSK were C. auratus, C. dabryi, Hemiculter leucisculus, Rhinogobius giurinus, Macrobrachium, and Caridina.
Data analysis
The % IRI was used to estimate the dietary importance of each food category from stomach content analysis and the isotopic mixing model outputs, respectively. Due to the TL range of HSC (100–300 mm) was smaller than that of WSC and WSK, specimen of WSC and WSK were divided into two different size classes (small: 100–300, large: >300 mm) to rule out the possible bias induced by length. The differences in the diet composition from stomach contents and stable isotope analysis with respect to species, season, and size groups were assessed by a Chi square test (χ 2) of the frequency of a given prey (Sley et al., 2009). The dietary overlap between fish species was determined using Schoener’s index, which expresses diet similarity between two species on a scale from 0, representing no overlap, to 1, representing complete overlap between species (Schoener, 1970). Biologically meaningful diet overlap is indicated by index values exceeding 0.60 (Martin, 1984). Differences in δ13C and δ15N values in different size classes in each season were tested by analysis of variance (ANOVA), and significant results (P < 0.05) were subsequently analyzed with a LSD test. All analyses were performed using SPSS 13.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Catch data
A total of 47 HSC, 50 WSC, and 30 WSK were collected from Biandantang Lake in two seasons (Table 1). 27 and 20 HSC individuals were, respectively, collected in winter and summer, and their TL was smaller than 300 mm. 30 and 20 WSC were, respectively, collected in winter and summer, and their TL ranged from 196 to 634 and 153 to 457 mm. The mean TL of two size classes in winter was smaller than those of in summer. 12 and 18 WSK were, respectively, collected in winter and summer, and their TL ranged from 236 to 346 and 171 to 310 mm. The mean TL of two size classes in winter was also smaller than those of in summer (Table 1). All stomach contents were analyzed, of which 28 HSC, 34 WSC, 17 WSK individuals, respectively, contained food.
Stomach content analyses
The diet of Siniperca fishes in Biangdantang Lake included 15 recognizable prey taxa, with 10 taxa of fish (C. auratus, C. dabryi, H. leucisculus, Cultrichthys erythropterus, R. giurinus, Odontobutis obscurus, Pseudorasbora parva, C. carpio, Rhodeinae, and unidentified fish). In addition, stomachs contained four invertebrate taxa (Caridina, Macrobrachium, Procambarus clarkia, Viviparidae) and plant detritus (Table 2). Fish and shrimp were the dominant prey taxa for the three piscivores, representing over 97% of the prey items (Table 2). However, diet composition among HSC, WSC, and WSK showed significant difference (χ 2 = 124.5, df = 28, P < 0.001). HSC diets were dominated by Caridina and Macrobrachium, averaging about 49.4 and 25.4% in importance, respectively. In contrast, WSC diets were dominated by C. auratus and C. dabryi, averaging about 57.3 and 20.09% in importance, respectively. Caridina, Macrobrachium, C. auratus, and C. dabryi were also the food items of WSK, but the most important food item was Macrobrachium (34.6%), followed by C. auratus (27.2%) and Caridina (23.8%) (Table 2).
Dietary composition among HSC, WSC, and WSK showed significant differences in winter and summer (winter: χ 2 = 125.2, df = 26, P < 0.001; summer: χ 2 = 167.4, df = 14, P < 0.001). In winter, HSC diets were dominated by Caridina and Macrobrachium, averaging about 59.4 and 20.2% in importance, respectively. In contrast, WSC diets were dominated by C. auratus and C. dabryi, averaging about 72.3 and 13.4% in importance, respectively. WSK also consumed Caridina, Macrobrachium, C. auratus, and C. dabryi, but the most important food item was C. auratus (42.3%), followed by Caridina (26.3%) and Macrobrachium (24.5%) (Table 2). In summer, HSC diets were dominated by H. leucisculus and Macrobrachium, averaging about 59.4 and 32.3% in importance, respectively. In contrast, WSC diets were dominated by C. dabryi and R. giurinus, averaging about 37.0 and 30.8% in importance, respectively. The most important food items of WSK were Macrobrachium (40.1%), followed by R. giurinus (25.0%) and C. dabryi (19.6%) (Table 2). Within species, there were significant seasonal variations in the diet composition for HSC, WSC, and WSK (HSC: χ 2 = 130.6, df = 8, P < 0.001; WSC: χ 2 = 134.4, df = 13, P < 0.001; WSK: χ 2 = 97.4, df = 8, P < 0.001) (Table 2).
Within seasons, the dietary composition among HSC, WSC, and WSK differed considerably at different size classes. In winter, the diet was significantly different between HSC and two wild Siniperca fishes with the same size class (χ 2 test, all P < 0.001). Caridina was the most important food item in the stomachs of HSC while Macrobrachium was the most important in the stomachs of WSC and WSK with same size class (Fig. 2). The significant differences in diet were found between HSC and two large size wild Siniperca fishes (length >300 mm) (χ 2 test, all P < 0.001). Compared with HSC, large size wild Siniperca fishes consumed more C. auratus (Fig. 2). In summer, the dietary composition was also significantly different between HSC and two wild Siniperca fishes with the same size class (χ 2 test, all P < 0.001). H. leucisculus was the most important food item in the stomachs of HSC while R. giurinus and Macrobrachium were the most important in the stomachs of WSC and WSK with same size class, respectively (Fig. 2). The significant differences in diet were found between HSC and two large size wild Siniperca fishes (length >300 mm) (χ 2 test, all P < 0.001). Compared with HSC, C. dabryi dominated the diet of large size wild Siniperca fishes, with 93.2 and 74.7% in importance, respectively (Fig. 2).
Stable isotope analysis
Stable carbon and nitrogen isotope signatures of the three target fish species were different between seasons, with fish (except large size WSK) from summer consistently 13C- and 15N-enriched relative to their conspecifics from winter (Fig. 4). These differences were also found for all mixing model end members (C. auratus, C. dabryi, H. leucisculus, R. giurinus, Macrobrachium, Caridina) (Fig. 4), suggesting that patterns of carbon and nitrogen assimilation, fractionation, and trophic transfer were different in two seasons in Biandantang Lake.
Within seasons, the δ13C and δ15N values in different size classes of the three species showed significant differences, respectively (winter, δ13C: F 4,50 = 6.19, P < 0.001; δ15N: F 4,50 = 13.58, P = 0.001; summer, δ13C: F 4,37 = 4.92, P = 0.003; δ15N: F 4,37 = 4.42, P = 0.005) (Table 2). In winter, both δ13C and δ15N values of HSC were significantly lower than those of WSC and WSK with the same size classes (100–300 mm) (LSD test, all P < 0.05) and the large size classes (>300 mm) (LSD test, all P < 0.05), respectively (Fig. 4). Mixing model results indicated that contribution proportions of end members for HSC were significantly different with those of WSC and WSK with the same size classes (χ 2 test, all P < 0.05) and the large size classes (χ 2 test, all P < 0.05). Specifically, H. leucisculus, Macrobrachium, and Caridina contributed 31.6, 25.9, and 14.6% of the carbon to HSC, respectively. In contrast, over 86% of the dietary carbon of the two Siniperca fishes (each two size classes) was derived from H. leucisculus, with less than 10% coming from shrimps (Fig. 3).
In summer, δ13C values of HSC were significantly lower than those of two sizes WSC and the same size WSK (LSD test, all P < 0.05), respectively (Fig. 4), and no significant difference was observed between HSC and large size WSK. Mixing model results indicated that contribution proportions of end members for HSC were significantly different to the size classes of WSC (χ 2 test, all P < 0.05) and the same size WSK (χ 2 test, P < 0.05). Specifically, R. giurinus contributed nearly 70% of the carbon to HSC. In contrast, about 30–40% of the dietary carbon of the two sizes WSC and the same size WSK was derived from R. giurinus, with over 17 and 15% coming from C. dabryi and H. leucisculus, respectively (Fig. 3). For large size WSK, the contribution proportions of end members were similar with HSC (χ 2 = 2.21, df = 5, P = 0.819), with nearly 60% of the carbon derived from R. giurinus and about 10% coming from C. dabryi and H. leucisculus, respectively.
Diet overlap
The diet overlaps varied with season and species (Table 3). The diet overlap index on the basis of prey importance from stomach content analysis between HSC and WSC, HSC and WSK, WSC and WSK was higher in winter than those of in summer, respectively. The index between WSC and WSK, HSC and WSK was, respectively, highest in winter and summer, and lowest between HSC and WSC in two seasons (Table 3). The diet overlap indexes among three species were much less than the threshold 0.6 required to be biologically meaningful (Martin, 1984). However, dietary overlap on the basis of prey contributions from the mixing model derived IRI was inconsistent with the results from the stomach content data. In winter, the diet overlap index between HSC and the two Siniperca fishes was less than the threshold 0.6, but the index between WSC and WSK was much higher than 0.6. In summer, the diet overlap indexes among three species were higher than the threshold 0.6.
Discussion
Diet composition of Siniperca fishes
Combined stomach contents and stable isotope analyses can provide useful insights into the trophic ecology of hatchery-reared and wild fish. In our study, there were considerable disagreement in diet reconstructions using stable isotope mixing model and gut content analyses for the three species (Figs. 2, 3). These results are consistent with many previous studies (Renones et al., 2002; Ho et al., 2007; Hadwen et al., 2007). In general, these discrepancies suggest that although individuals can focus their foraging on particularly abundant prey items on a day-to-day basis, they feed on a wider range of taxa over longer periods (Hadwen et al., 2007). Gut contents represent only recently acquired food and may include indigestible material that will not be incorporated into muscle tissue. In contrast, mixing model estimates reflect the proportions of stable isotopes from food assimilated rather than consumed (Renones et al., 2002). So, conventional stomach content analysis might have underestimated the nutritive contributions of fish in contrast to shrimps in our study. However, stomach contents and stable isotope mixing model analysis showed that the diet composition and contribution proportion existed significantly different between HSC and two wild Siniperca fishes, suggesting that HSC did not have apparent feeding competition with two wild Siniperca fishes during the critical periods of the early stocking stages. The difference of diet between HSC and two wild Siniperca fishes was presumably induced by their different foraging strategy and habitat selection. First, for HSC, it is easily to capture prey in hatchery. However, the prey abundance and availability in lake was much less than in hatchery, so they may change foraging strategy and focus more on that comparatively easily captured resource, such as shrimps. Second, there are different abundance and availability of preys in different habitats, which caused them feeding the prey with high availability.
There is limited quantitative information on Siniperca fishes diets despite the fact that these species are highly valued fisheries species and important for regulating ecosystems and sustaining biodiversity (Yang et al., 2002; Li et al., 2013). Nevertheless, Siniperca fishes can feed on a wide range of prey items and are assumed to have a broad diet (Han et al., 1996; Yang et al., 2002; Li et al., 2013). In our study, both stomach contents and stable isotope data from Biandantang Lake supported the suggestion of considerable dietary breadth for Siniperca fishes. The diet of S. chuatsi in Biangdantang Lake included 14 recognizable prey taxa, and fish and shrimp were their dominant food items, representing over 97% (% IRI) in importance. The results generally concur with previous study conducted in Lake Xiaosihai (Li et al., 2013). However, our study found that C. auratus was the most important prey fish for wild S. chuatsi in Biandantang Lake, whereas P. parva was the most important prey of this fish in Xiaosihai Lake (Li et al., 2013). Previous study indicated that P. parva was the most abundant prey fish in Xiaosihai Lake (Li et al., 2010) as well as C. auratus in Biandantang Lake (unpublished data). Thus, the difference in dominant prey fish consumed by S. chuatsi between the two lakes may result from their differences in availability and abundance. An ontogenetic diet shift in wild S. chuatsi was observed in Biandantang Lake. On basis of the stomach content analyses, small size wild S. chuatsi fed primarily on shrimp, but large individuals switched to feeding on large-sized prey such as C. auratus and C. dabryi. Similar cases were found by Mittelbach and Persson (1998), based on data of 27 piscivorous fish species.
The breadth of diet for HSC (nine species of fish, invertebrates, plant detritus) was large, but not as large as that observed for wild S. chuatsi. Meanwhile the diet composition between HSC and WSC showed significant difference, with Caridina and Macrobrachium dominated HSC diets and C. auratus and C. dabryi dominated WSC diets. These discrepancies are probably the dominant reasons that HSC can survive and coexist with WSC in Biandantang Lake. Stomach content analysis of HSC indicated that Caridina and Macrobrachium were the dominant food items in winter while H. leucisculus and Macrobrachium were the dominant food items in summer, which was considerable disagreement with stable isotope analysis that H. leucisculus and R. giurinus contributed significantly to the nutrition of HSC in winter and summer, respectively. These finding suggests that temporal variability in prey abundance might be detected when using paired stomach content and stable isotope methods, owing to their temporal resolutions (Creach et al., 1997; Hadwen et al., 2007).
The present research is the first time that the diet of Siniperca kneri has been quantitatively studied. Shrimps and fish were their dominant food resources, which were consistent with a previous report (Han et al., 1996) that the frequency of occurrence of shrimps and fish in stomach content of S. kneri was 81.03 and 31.23%, respectively. However, size-related changes in diet of S. kneri were found from stomach contents analyses in winter and summer (Fig. 2). Among shrimps were preferred by small size WSK in each season, representing over 50% of the prey items of this group. In stomachs of large size WSK, shrimps became less important and C. auratus and C. dabryi were more prevalent in winter and summer, respectively. In contrast, the stable isotope data indicated that the ontogenetic diet shift was not apparent, with H. leucisculus was the most important food resource for two sizes WSK in winter and R. giurinus was the most important food resource in summer.
Within species, stomach contents analysis showed that there were significant seasonal variations in the diet composition for HSC, WSC, and WSK. These differences are a reflection of the changes in the abundance and availability of various prey species in Lake Biandantang in different seasons (unpublished data). Although the most important food resource obtained from stable isotope mixing model differed with those from stomach contents analyses for the three species in each season, respectively, these results also revealed significant seasonal difference for them. The three species and all mixing model end members from summer consistently 13C- and 15N-enriched relative to their conspecifics from winter, which are presumably a result of the lower water exchange frequency and the higher water residence times from January to June in Biandantang Lake and the associated system carbon and nitrogen recycling.
δ15N values have been shown to be indicators of trophic level for marine and freshwater fish species (Minagawa & Wada, 1984; Owens, 1987; Post, 2002; Morrissey et al., 2013), and appropriate values for Δδ15N and Δδ13C are of central importance to estimates of the relative contribution of different food sources, and underpins estimates of trophic level (Wayland & Hobson, 2001; Hicks et al., 2005). Wada et al. (1991) and Post (2002) presented a mean fractionation in δ15N of 3.4‰ for various consumers, but variation in fractionation rates among species is large. In our study, the increase in δ15N ranged from 0.04 to 2.52‰ between the Siniperca fishes and their potential food items. The result indicated that the Δδ15N between Siniperca fishes and their prey might be lower than 3.4‰. The Δδ15N value of 2.3‰ suggested by McCutchan et al. (2003) for poikilothermic, ammonotelic, and aquatic consumers seems appropriate for our Siniperca fishes, but actually, the Δδ15N value of 2.3‰ is also too high to insure that the IsoSource software run normally. In general, controlled feeding experiments are the only way to accurately measure trophic fractionation rates, but these types of studies are lacking for Siniperca fishes. Some recent studies have found more extreme values for fish than those summarized in McCutchan et al. (2003) (Elsdon et al., 2010). Further research on trophic fractionation Siniperca fishes and their food in controlled feeding experiments is needed to understand their trophic position.
Implications for fisheries management
Hatchery-reared fish can contribute to the loss or depletion of wild populations through predation and ecological competition for food, space, and breeding opportunities (Fleming et al., 2000; Armstrong et al., 2008; Sweeting & Beamish, 2009), the spread of parasites and diseases (Johnsen & Jensen, 1994; Krkosek et al., 2005), and interbreeding with wild fish (Clifford et al., 1998; Jonsson & Jonsson, 2006). Competition between hatchery-reared and wild fish has frequently been described as an important negative ecological interaction (Weber & Fausch, 2003; Goodman, 2005). In China, stocking of HSC in lakes has been practised for more than 10 years, but its impacts on conspecifics and other populations in the wild have been rarely evaluated. This study is the first to compare the diet of HSC and the two wild Siniperca fishes during the critical periods of the early stocking stages (first year). Although fish and shrimp were the dominant prey taxa for HSC and the two wild Siniperca fishes, significant differences in the percentage contribution of the major food groups to the three diets were observed. These results suggested that HSC and the two wild Siniperca fishes might have avoided exploitative competition by this dietary separation. Similar conclusion was obtained from the stable isotope analyses. The significant differences of δ13C and δ15N values of HSC and the two wild Siniperca fishes indicated that HSC have very little food resource overlap with two wild Siniperca fishes. The dietary overlap index on the basis of prey importance from stomach content analysis between HSC and the two wild Siniperca fishes was much less than 0.6 in both winter and summer, suggesting that no significant diet overlap was existed between them. However, the index on the basis of prey contributions from the mixing model derived IRI was higher than the threshold 0.6 between HSC and the two wild Siniperca fishes in summer, which probably indicated that HSC has well suited to the natural environment after about 1 year growing in lake and subsequently utilize prey resources together with wild individuals.
In conclusion, our results demonstrated that HSC did not have apparent feeding competition with two wild Siniperca fishes during the critical periods of the early stocking stages, suggesting that moderate stocking of HSC may not have a negative effect on the variability of wild Siniperca populations. However, hatchery-reared and wild fish always interact at all stages of the life cycle and across the range of natural environments inhabited, and life history and fitness-related traits of the wild populations may be influenced (Jonsson et al., 1991; McGinnity et al., 2003). More detailed and long-term studies of the consequences of interactions between hatchery-reared and wild fishes are required in future.
References
Araki, H. & C. Schmid, 2010. Is hatchery stocking a help or a harm? Evidence, limitations and future directions in ecological and genetic surveys. Aquaculture 308(Supplement 1): 1–11.
Armstrong, J. L., K. W. Myers, D. A. Beauchamp, N. D. Davis, R. V. Walker, J. L. Boldt, J. J. Piccolo, L. J. Haldorson & J. H. Moss, 2008. Interannual and spatial feeding patterns of hatchery and wild juvenile Pink salmon in the Gulf of Alaska in years of low and high Survival. Transactions of the American Fisheries Society 137: 1299–1316.
Arthur, R. I., K. Lorenzen, P. Homekingkeo, K. Sidavong, B. Sanvilaikham & C. J. Garaway, 2010. Assessing impacts of introduced aquaculture species on native fish communities: Nile tilapia and major carps in SE Asian freshwaters. Aquaculture 299: 81–88.
Becker, A., L. J. B. Laurenson, P. L. Jones & D. M. Newman, 2005. Competitive interactions between the Australian native fish Galaxias maculatus and the exotic mosquitofish Gambusia holbrooki in a series of laboratory experiments. Hydrobiologia 549: 187–196.
Birch, L. C., 1957. The meanings of competition. American Naturalist 91: 5–18.
Cao, W., G. Zhang, J. Ma & H. Yu, 1991. Preliminary studies on the phenomenon of size diminution of the fish resources in Lake Honghu. In: Honghu Research Group, Institute of Hydrobiology, Chinese Academy of Sciences (eds), Studies on Comprehensive Exploitation of Aquatic Biological Productivity and Improvement of Ecological environment in Lake Honghu. China Ocean Press, Beijing: 148–152 (in Chinese).
Carpenter, S. R. & J. F. Kitchell, 1988. Consumer control of lake productivity. BioScience 38: 764–769.
Chen, H., 1989. Impact of aquaculture on the ecosystem of the Donghu Lake, Wuhan. Acta Hydrobiologica Sinica 13: 359–368. (in Chinese with English abstract).
Clifford, S. L., P. McGinnity & A. Ferguson, 1998. Genetic changes in an Atlantic salmon population resulting from escaped juvenile farm salmon. Journal of Fish Biology 52: 118–127.
Creach, V., M. T. Schricke, G. Bertru & A. Mariotti, 1997. Stable isotopes and gut analyses to determine feeding relationships in saltmarsh macroconsumers. Estuarine, Coastal and Shelf Science 44: 599–611.
Cui, Y. & Z. Li, 2005. Fishery Resources and Conservation of Environment in Lakes of the Yangtze River Basin. Science Press, Beijing. (in Chinese).
Elsdon, T. S., S. Ayvazian, K. W. McMahon & S. R. Thorrold, 2010. Experimental evaluation of stable isotope fractionation in fish muscle and otoliths. Marine Ecology Progress Series 408: 195–205.
FAO, 2010. The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome.
Fleming, I. A., K. Hindar, I. B. Mjølnerød, B. Jonsson, T. Balstad & A. Lamberg, 2000. Lifetime success and interactions of farm salmon invading a native population. Proceedings of the Royal Society B 267: 1517–1523.
Goodman, D., 2005. Selection equilibrium for hatchery and wild spawning fitness in integrated breeding programs. Canadian Journal of Fisheries and Aquatic Sciences 62: 374–389.
Hadwen, W. L., G. L. Russel & A. H. Arthington, 2007. Gut content- and stable isotope-derived diets of four commercially and recreationally important fish species in two intermittently open estuaries. Marine and Freshwater Research 58: 363–375.
Han, D., J. Hu & F. Hong, 1996. Comparative studies of the feeding habit and digestive organs of Siniperca fishes of Lushui Reservoir. Journal of Fisheries of China 20: 97–102. (in Chinese with English abstract).
Hicks, B. J., M. S. Wipfli, D. W. Lang & M. E. Lang, 2005. Marine-derived nitrogen and carbon in freshwater-riparian food webs of the Copper River Delta, southcentral Alaska. Oecologia 144: 558–569.
Ho, C., S. Kao, C. Dai, H. Hsieh, F. Shiah & R. Jan, 2007. Dietary separation between two blennies and the Pacific Gregory in northern Taiwan: evidence from stomach content and stable isotope analyses. Marine Biology 151: 729–736.
Johnsen, B. O. & A. J. Jensen, 1994. The spread of furunculosis in salmonids in Norwegian rivers. Journal of Fish Biology 45: 47–55.
Jonsson, B. & N. Jonsson, 2006. Cultured Atlantic salmon in nature: a review of their ecology and interaction with wild fish. ICES Journal of Marine Science 63: 1162–1181.
Jonsson, B., N. Jonsson & L. P. Hansen, 1991. Differences in life history and migratory behaviour between wild and hatchery-reared Atlantic salmon in nature. Aquaculture 98: 69–78.
Kiljunen, M., J. Grey, T. Sinisalo, C. Harrod, H. Immonen & R. I. Jones, 2006. A revised model for lipid-normalizing δ13C values for aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology 43: 1213–1222.
Krkosek, K., M. Lewis & J. Volpe, 2005. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proceedings of the Royal Society of London Series B 272: 689–696.
Li, X., D. Huang & H. Yang, 2005. Effect of light on young Siniperca kneri Garman’s ingested amount. Journal of Hunan Agricultural University 31: 187–190. (in Chinese with English abstract).
Li, W., T. Zhang & Z. Li, 2010. Spatial distribution and abundance of small fishes in Xiaosihai Lake, a shallow lake along the Changjiang (Yangtze) River, China. Chinese Journal of Oceanology and Limnology 28: 470–477.
Li, W., T. Zhang, S. Ye, J. Liu & Z. Li, 2013. Feeding habits and predator–prey size relationships of mandarin fish Siniperca chuatsi (Basilewsky) in a shallow lake, central China. Journal of Applied Ichthyology 29: 56–63.
Liang, X., J. Liu & B. Huang, 1998. The role of sense organs in the feeding behaviour of Chinese perch. Journal Fish Biology 52: 1058–1067.
Liang, X., H. Oku, H. Y. Ogata, J. Liu & X. He, 2001. Weaning Chinese perch Siniperca chuatsi (Basilewsky) onto artificial diets based upon its specific sensory modality in feeding. Aquaculture Research 32(Supplement 1): 76–82.
Liao, H., C. L. Pierce & J. G. Larscheid, 2001. Empirical assessment of indices of prey importance in the diets of predacious fish. Transactions of the American Fisheries Society 130: 583–591.
Liere, L. V. & R. D. Gulati, 1992. Restoration and recovery of shallow eutrophic lake ecosystems in the Netherlands: epilogue. Hydrobiologia 233: 283–287.
Lorenzen, K., 2005. Population dynamics and potential of fisheries stock enhancement: practical theory for assessment and policy analysis. Philosophical Transactions of the Royal Society 360: 171–189.
Lorenzen, K. & C. J. Garaway, 1998. How predictable is the outcome of stocking? FAO Fisheries Technical Paper 374: 133–152.
Lorenzen, K., M. C. M. Beveridge & M. Mangel, 2012. Cultured fish: integrative biology and management of domestication and interactions with wild fish. Biological Reviews 87: 639–660.
Martin, F. D., 1984. Diet of four sympatric species of Etheostoma (Pisces: Percidae) from southern Indiana: interspecific and intraspecific multiple comparisons. Environmental Biology of Fishes 11: 113–120.
McCutchan, J. H., W. M. Lewis Jr, C. Kendall & C. C. McGrath, 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390.
McGinnity, P., P. Prodohl, A. Ferguson, R. Hynes, N. O’Maoileidigh, N. Baker, D. Cotter, B. O’Hea, D. Cooke, G. Rogan, J. Taggart & T. Cross, 2003. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as result of interactions with escaped farmed salmon. Proceedings of the Royal Society of London Series B 270: 2443–2450.
Minagawa, M. & E. Wada, 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48: 1135–1140.
Mittelbach, G. G. & L. Persson, 1998. The ontogeny of piscivory and its ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences 55: 1454–1465.
Morrissey, C. A., A. Boldt, A. Mapstone, J. Newton & S. J. Ormerod, 2013. Stable isotopes as indicators of wastewater effects on the macroinvertebrates of urban rivers. Hydrobiologia 700: 231–244.
Moyle, P. B., 1976. Fish introductions in California: history and impact on native fishes. Biological Conservation 9: 101–118.
Moyle, P. B. & T. Light, 1996. Biological invasions of fresh water: empirical rules and assembly theory. Biological Conservation 78: 149–162.
Owens, N. J. P., 1987. Natural variation in 15N in the marine environment. Advances in Marine Biology 24: 389–451.
Phillips, D. L. & J. W. Gregg, 2003. Source partitioning using stable isotopes: coping with too many sources. Oecologia 136: 261–269.
Pinkas, L., M. S. Oliphant & I. L. K. Iverson, 1971. Food habits of albacore, bluefin tuna, and bonito in California waters. State of California, The Resources Agency Department of Fish and Game, Fish Bulletin 152: 1–105.
Pinkerton, E., 1994. Economic and management benefits from the coordination of capture and culture fisheries: the case of Prince William Sound pink salmon. North American Journal of Fisheries Management 14: 262–277.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Renones, O., N. V. C. Polunin & R. Goni, 2002. Size related dietary shifts of Epinephelus marginatus in a western Mediterranean littoral ecosystem: an isotope and stomach content analysis. Journal of Fish Biology 61: 122–137.
Schoener, T. W., 1970. Non-synchronous spatial overlap of lizards in patchy habitats. Ecology 51: 408–418.
Sley, A., O. Jarbou, M. Ghorbel & A. Bouain, 2009. Food and feeding habits of Caranx crysos from the Gulf of Gabes (Tunisia). Journal of the Marine Biological Association of the United Kingdom 89: 1375–1380.
Sweeting, R. M. & R. J. Beamish, 2009. A comparison of the diets of hatchery and wild Coho salmon (Oncorhynchus kisutch) in the Strait of Georgia from 1997 to 2007. North Pacific Anadromous Fish Commission Bulletin No. 5: 255–264.
Wada, E., H. Mizutani & M. Minagawa, 1991. The use of stable isotopes for food web analysis. Critical Reviews in Food Science and Nutrition 30: 361–371.
Wayland, M. & K. A. Hobson, 2001. Stable carbon, nitrogen, and sulfur isotope ratios in riparian food webs on rivers receiving sewage and pulp-mill effluents. Canadian Journal of Zoology 79: 5–15.
Weber, E. D. & K. D. Fausch, 2003. Interactions between hatchery and wild salmonids in streams: differences in biology and evidence for competition. Canadian Journal of Fisheries and Aquatic Sciences 60: 1018–1036.
Xie, S., Y. Cui & Z. Li, 2000. Ecological studies on lake fisheries on piscivorous fishes: theory and methods. Acta Hydrobiologica Sinica 24: 72–81. (in Chinese with Chinese abstract).
Yang, R., C. Xie & X. Yang, 2002. Food composition of six piscivorous fishes in Liangzi Lake. Reservoir Fishery 22: 1–3. (in Chinese with English abstract).
Youngson, A. F. & E. Verspoor, 1998. Interactions between wild and introduced Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55(Supplement 1): 153–160.
Zhang, T., 2005. Life-history strategies, trophic patterns and community structure in the fishes of Lake Biandantang. Doctoral dissertation. Graduate School of Chinese Academy of Sciences, Beijing (in Chinese with English abstract).
Zhang, B., 2007. Effects of stocking enhancement and genetic diversity of mandarin fish (Siniperca chuatsi) in shallow lakes along the middle and lower reaches of Yangtze River. Doctoral dissertation. Graduate School of Chinese Academy of Sciences, Beijing (in Chinese with English abstract).
Acknowledgments
The authors would like to thank Prof. Sovan Lek, Dr. Songguang Xie, and three anonymous reviewers for their constructive comments and critically reading the manuscript. This study was financially supported by the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201303056), the Projects of the National Natural Science Foundation of China (Grant Nos. 31201994 and 30830025), and the R and D Project of Ministry of Science and Technology of China (Grant No. 2012BAD25B06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Jiang-Shiou Hwang & Koen Martens / Challenges in Aquatic Sciences
Rights and permissions
About this article
Cite this article
Li, W., Hicks, B.J., Guo, C. et al. Does hatchery-reared Siniperca chuatsi (Actinopterygii, Perciformes) compete significantly with two wild Siniperca populations for diets in a shallow lake?. Hydrobiologia 741, 125–138 (2014). https://doi.org/10.1007/s10750-014-1866-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1866-9