Abstract
Dreissena polymorpha (zebra mussel) is a freshwater bivalve mollusc and has been present for more than one decade in Spain. The zebra mussel causes serious ecological and socioeconomic impacts in areas where they settle. Our research aims to analyze its hydraulic habitat, developing physical models for this species, which indicates its preference and optimal microhabitat requirements. To get it, a survey was completed in a lotic reach in the lower Ebro River (Tarragona, Spain). Habitat suitability curves for the variables include water velocity, depth, Froude number, velocity/depth ratio, shear stress and shear stress ratio (RSS). In addition, interactive effects between hydraulic parameters on habitat selection and its use were studied and bivariate habitat models were developed. A close relationship was observed between the D. polymorpha presence and mean flow velocity combined with depth, Froude number and RSS. Suitable habitat requirements for Dreissena were detected in areas with stable river beds under high flows, and velocity below 1.2 m/s with a depth of less than 5 m under regular flows. Information about zebra mussel preferences may enhance the design of monitoring programmes and the integrated control management of this invasive mussel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The zebra mussel, Dreissena polymorpha (Pallas, 1771), is native to the Ponto-Caspian region and is considered to be one of the most invasive aquatic species in the world (Hallstan et al., 2010; Therriault & Orlova, 2010; Naddafi et al., 2011). However, its rapid dispersion is directly related to human activity (Bidwell, 2010). In Europe, the geographical dispersion of D. polymorpha started at the end of the eighteenth century, through the centre and north of this continent. Nowadays, it is present in many countries (Karatayev et al., 2006; Oscoz et al., 2010). The zebra mussel was detected in North America in 1986 in Lake Erie before quickly spreading to several more eastern regions (Hebert et al., 1989; Carlton, 2008).
In Spain, it was considered an invasive species since 2001 and has quickly invaded the Ebro river axis, starting in the lower reach (Palau et al., 2004; Durán et al., 2010). Today, it is present in other Spanish basins: Júcar, Segura and Guadalquivir (MAGRAMA, 2012), and it has become an important ecological and economic problem for Spanish rivers (Rajagopal et al., 2009).
In the environments where they establish, zebra mussels usually compete with native bivalves and other benthic invertebrates, they alter the nutrient cycle, considerably reducing the plankton supply and increasing water clarity (Casagrandi et al., 2007). All of this affects aquatic plant proliferation and also disturbs the benthic structure and fish community (Strayer & Malcom, 2006), although there is no consensus on the actual effects in new areas where this species has settled. In contrast, Dreissena also increases habitat complexity and benthic organic matter by biodeposition, which influence positively in some taxa (Gergs & Rothhaupt, 2008). Furthermore, it causes significant economic damage to water intakes and other structures (Rajagopal et al., 2009).
Abundance and distribution of bivalves is usually related to physicochemical (pH, dissolved oxygen, salinity, calcium concentration and temperature), hydrodynamic (depth, current velocity, substrate and suspended sediments) and biological parameters (predation or competition from other species, available food and host abundance) (Strayer, 1999; Morales et al., 2006; Navarro et al., 2006). Nevertheless, the most important variables in the presence of invertebrates in river systems are the hydraulic ones (Gore et al., 2001; Steuer et al., 2008; Allen & Vaugh, 2010). These also strongly influence larval mortality and their dispersal throughout the river (Horvath & Crane, 2010; Daraio et al., 2012). Consequently, bivalve habitats can be characterized by simple hydraulic indicators: flow velocity, depth, size and distribution of the substrate (Claudie & Mackie, 1994; Ackerman, 1999) or complex ones: a combination of the previous ones, such as shear stress, Reynolds number and Froude number (Hardison & Layzer, 2001; Zigler et al., 2008; Parasiewicz et al., 2012).
Scientific literature is focused on the invasive characteristics of the zebra mussel, its biology and the ecological and economic problems caused by its presence (Strayer & Malcom, 2006). However, there are few references to microhabitat requirements for this species (Palau et al., 2010) and virtually all of the requirements are based on the substrate (Claudie & Mackie, 1994; O’Neill, 1996) or refer to different species of mussels (Hastie et al., 2000; Howard & Cuffey, 2003; Gangloff & Femeniella, 2007). The cosmopolitan character of the zebra mussel and its little known requirements, possibly, determine this situation.
Nevertheless, knowing about the physical habitat preferences of bivalves can improve the programmes that monitor and manage these species (Hastie et al., 2000; Steuer et al., 2008; Zigler et al., 2008). Nowadays, the management of this invasive mussel in open environments is very complicated and requires thorough information about its habitat in order to attempt to eradicate it. In regulated rivers, the most common non-biological fight techniques focus on reducing water levels in reservoirs, causing controlled flood events for bed removal or displacing water volumes in reservoirs in order to reduce the recruitment during periods with high larvae density (O’Neill, 1996; Palau & Cía, 2006; Durán et al., 2010).
One of the most common methods for analyzing the physical habitat is based on the habitat suitability criteria (HSC) (Bovee et al., 1998; Gore et al., 2001). These biological models give uni- or multivariate preference curves which can be referred to a wide number of variables for each species and life stage considered. This information is incorporated into habitat simulation models (Bovee et al., 1998; Parasiewicz & Dunbar, 2001) in order to evaluate changes which occur in the physical habitat availability for the considered species, as a result of any disturbance in the hydraulic environment. Such disturbance could be caused by either a natural or artificial cause, as the application of controlled flood events (Batalla et al., 2008).
This paper analyses the influence of several hydraulic variables on zebra mussel distribution and develops physical habitat models for this species in lotic systems. These models might become a valid method to better evaluate the effect of certain control actions (i.e. flood events) and also to examine possible changes in the spatial distribution of the species and to see how hydraulic modification could affect the aquatic environment.
Materials and methods
Study area
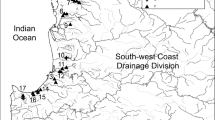
This research was carried out in the lower Ebro River (Fig. 1). The Ebro basin is located in the north-east of Spain and it drains a total area of 85,534 km2, the largest of the Iberian Peninsula. The selected area of study was 7 km long, between the Flix hydroelectric power plant and the Vinebre pier (Tarragona, Catalonia) (UTM-HUSO 31T; 294512X, 4566716Y; 298762 X, 4560742Y). This area is subject to a strict hydroelectric regulation by the nearby Mequinenza, Riba-roja and Flix reservoirs. In the middle of the study reach, the Ascó nuclear power plant and its small diversion 2-m-high dam are placed.
The flow regime is disturbed by hydroelectric production, which is why the water level manifests slight variations throughout the year. The minimum flow is around 126 m3/s (Q 5th), median flow (Q 50th) is 208 m3/s and the maximum annual flow is 1,000 m3/s (Q 99th), which practically corresponds to the dominant discharge. In this zone, the Ebro River has an average width of about 300 m, the slope is less than 0.001 m/m and maximum depth is around 9 m. The river bed substrate is mainly made of gravel, measuring 40–75 mm. In some areas, there are macrophytes close to both banks.
Selected variables for habitat models
Habitat models are usually based on physical parameters from hydraulic source (depth, flow velocity, substrate and their combinations: shear stress, Froude and others). These variables are directly related to the abundance and distribution of mussels (Steuer et al., 2008; Allen & Vaugh, 2010) and all of them are associated to discharge, so they can easily be incorporated to flow management tools (Parasiewicz & Dunbar, 2001).
In our case, the habitat description of the zebra mussel was made taking into account the aforementioned criteria. Thus, the selected variables were: mean column velocity v (m/s), depth d (m), velocity/depth ratio v/d (s−1), Froude number Fr (dimensionless), shear stress τ 0 (N/m2) and the ratio between the shear stress and the critical stress RSS (dimensionless). The substrate is undoubtedly an important variable in the physical habitat of the zebra mussel. However, for the dominant size range in the considered section (45–70 mm), it is not discriminatory according to research by Palau et al. (2010) in a nearby lentic zone. Therefore, the substrate has not been used directly as a test parameter.
v, d, v/d and Fr have been calculated for the median discharge (Q 50th = 208 m3/s), being the most habitual flow in the regulated reach. τ 0 and RSS evaluate the water tension that mussels have to resist and the substrate stability during flood events which is why they were estimated for Q 99th = 1,000 m3/s. Given that this is the maximum flow that the zebra mussel is exposed to once a year and it corresponds to the dominant discharge, so that this river area is very regulated.
The Froude number is established as: Fr = v (g d)0.5 where v and d are mean water velocity and depth (m) and g is the acceleration given the gravity (9.81 m2/s). The shear stress is defined as: τ 0 = γ d S, where γ is the specific mass of water (9,780 N/m3 at 20°C), d is the water depth (m) and S is the hydraulic slope (m/m). The shear stress ratio was calculated according to criteria by Morales et al. (2006) where RSS = τ 0/τ c, and τ c corresponds to critical shear stress which causes the movement of a particle. Moreover, τ c depends on the characteristics of the bed material, armouring and flow velocity. Its value was determined according to the Shields diagram (1936). When the RSS > 1 it means that substrate is moved at a given flow rate. However, these values can only be considered a point of reference since the accurate measurement of the hydraulic slope is very complex and small variations in its determination can significantly affect the final result.
Data collection
Physical habitat models to be developed need both hydraulic and biological information. These data were achieved by sampling the study area and using a hydraulic simulation model.
Field data were taken along 24 transects, with sampling points every 25–50 m. First, the study section was stratified in three lower reaches (Fig. 1) according to their hydrogeomorphological characteristics, to diversify sampling area. Transects were set up by alternating fast and slow mesohabitats. The presence/absence of the zebra mussel, the substrate type, depth and flow velocity were registered at each point. Samples were collected by divers during summer 2010. In order to carry out this complicated work, the flowing discharge was decreased to 158 m3/s at the Mequinenza–Ribarroja–Flix system of reservoirs.
The width was taken with a measuring tape (±10 cm): in which the depth and mean column velocity were measured with an OTT ADC flow meter (±0.01 m and ±0.01 m/s, respectively). Altogether, 112 sampling points and 65 zebra mussel colonies were detected. All of them were recorded by GPS (SETTOP AL 102 MOVIL model).
On the other hand, we used a hydraulic model to simulate the desired discharge conditions—median Q 50th and dominant Q 99th flow—to obtain the six selected variables. The software employed in modelling was River 2D (Steffler & Blackburn, 2002). It is a two-dimensional depth averaged finite element hydrodynamic model. To run this software, we implemented a digital terrain model made from a topographical study, performed by the Forest Sciences Center of Catalonia (Vericat et al., 2007). The model is based on bathymetry, which is conducted with a Lowrance echo sounder LCX-15ci (±0.1 m) and a GPS SETTOP AL102 MOVIL model. Previous field sample data provided a basis for adjusting bed roughness and to calibrate the model. The relation between simulated and measured velocity at 158 m3/s was high (r 2> 0.82; P value < 0.01) and even more in the case of depth (r 2 > 0.94; P value < 0.01). Other depth checks at 254 and 612 m3/s showed similar results.
Data analyses
Data have been processed in two different ways. First, the relationships between variables and their influence on mussel distribution were analyzed using statistical procedures: scatter plot, principal component analysis (PCA) and logistic regression. Second, physical habitat models (preference curves) for the zebra mussel were developed.
Physical habitat analyses were made by following the standard procedures established by Bovee et al. (1998) and applied to mussels by other scientists (Hastie et al., 2000; Palau et al., 2010). Hence, the preference index was calculated using the forage ratio (HSC category III): the ratio between habitat use and availability. Afterwards, a normalization process was applied, considering 0 as the minimum (null habitat) and 1 as the maximum value (optimal habitat). Before applying this kind of model, it is necessary to demonstrate that the distribution of the sampled variables (velocity and depth) be representative of the hydraulic variability in the complete section (Bovee et al., 1998).
Habitat availability for each hydraulic parameter was extracted from the hydrodynamic model using the given flows. Habitat use was determined in the same way, considering only the sampling points with the mussel presence. No data were weighed by density or number of individuals in the colony, since it is a species with a complex spatial distribution. Thus, given the sessile characteristic, its presence can be considered as an indicator of use.
In order to check the validity of the preference curves developed, it should be demonstrated that there is no relationship between habitat availability and use, which means that there is a predilection for a particular habitat and a selective use of resources (Bovee et al., 1998). Frequently, that is considered a secondary approach without discussion in many papers.
Results
Variables and mussel distribution relationships
The scatter-plot analysis (Fig. 2) described the values of dataset (the presence/absence of mussel) according to the hydraulic variables confronted in pairs. From this observation, there is a strong correlation between the variable Fr with v/d and v on one hand, and depth d with τ 0 and RSS, on the other (Table 1). The associated analysis (Table 2) showed that the mussel presence focuses on the value of the variables under the average. The v/d indicator did not present a significant relationship with the presence/absence of individuals.
Scatter plot of the zebra mussel presence or absence according to six variables: (a) mean water column velocity, (b) depth, (c) Froude number, (d) velocity/depth ratio at 208 m3/s, (e) shear stress and (f) shear stress ratio at 1000 m3/s. The cross symbol means the zebra mussel presence and the square represents the absence
The PCA showed two main axes which explained 94.86% of the variability of the dataset (Fig. 3; Table 2). The first factor (F1, eigenvalue 3.295) was related with the variables v, Fr and v/P. The second factor (F2, eigenvalue 2.397) included the parameters d, τ 0 and RSS. Based upon the sample values in the first two factorial axis, a class organization by Ward’s cluster procedure method and squared Euclidean distances were carried out. As a result, we distinguished three types of microhabitat use (Table 3). The first group (C1) was characterized by a high density of D. polymorpha (81.4% of the points of this class show its presence) and values of v and d under average. In the second group (C2) individual presence of this species was found, but in a lower quantity than the previous class (47.62%), according to areas with high velocities and low depths. Finally, the third group (C3) corresponds to locations with a low presence of the zebra mussel and it is associated to areas with high v and d. The weakest relationship between the factors and the presence of the zebra mussel correspond to the ratio v/d (similar values in C1 and C3).
The logistic regression was performed with all the hydraulic parameters, according to the variable selection criteria ‘stepwise’. Of these, velocity and depth were the best predictors to explain the presence of the zebra mussel. Thus, the logistic model built had a high significance: Wald (χ2) statistical value of 15.97 with two degrees of liberty and a P value of 0.0003 for testing the global null hypothesis (H 0): β = 0. The final result is:
where modelled probability is the presence of the zebra mussel (P = 1), v is the velocity (m/s) and d is the depth (m). The area under the ROC curve was 0.7395 and it indicated that the logistic regression model had a high level of discrimination capacity. These results also showed that velocity has a more significant influence than depth in the settlement of this species. High probability of the Dreissena presence (P > 0.85) was reached at depth values <4 m and velocities between 0.0 and 1.0 m/s. Any velocities more than 2.0 m/s predicted a nearly zero probability to find mussels.
Physical habitat models
Previously to develop HSC, a bivariate analysis of velocity and depth distributions using the Pearson Chi square test was performed to prove that these sample data were representative of the hydraulic variability in the whole section. The validity of the preceding assumption was proven, which allowed the mussel sample to be applied in habitat model development.
Another check was accomplished between habitat availability and use (KS test, P value < 0.05) for each study variable before elaborating univariate preference curves (Fig. 4). In all cases except depth (Fig. 4b), certain variable ranges were detected. This feature means that the zebra mussel could live at any depth of water, and this variable, by itself did not provide attractive qualities for the bivalve.
Univariate preference curves for Dreissena polymorpha according to velocity (m/s) (a), depth (m) (b), Froude number (dimensionless) (c), velocity/depth ratio (s−1) (d) at 208 m3/s and shear stress (N/m2) (e), shear stress ratio (dimensionless) (f) at 1,000 m3/s. The suitability index has a value of 1 for optimal habitat and 0 for unsuitable conditions
The velocity preference showed its maximum rate for values between 0.3 and 0.8 m/s for median flow (Fig. 4a). The predilection for velocity values above 1.2 m/s was virtually null. The habitat models for the variables velocity/depth ratio and Froude number also had a predilection to low intervals, <0.9 and <0.3 s−1, respectively (Fig. 4c, d). Something different was appreciated with shear stress and RSS for the high discharge (Fig. 4e, f), with a maximum preference to intermediate values, avoiding low water movement areas and unstable gravel beds.
Since the logistic regression indicated the important significance of the variables v and d in mussel distribution, it was performed a joint analysis of preference, after proving the independent relationship between habitat use and availability for both variables (χ2 of Pearson test), which validates its applicability. The bivariate physical habitat model (Fig. 5), suggested no preference for any range of depth values when velocity is above 1.4 m/s. Suitable requirements for Dreissena were presented when the velocity was <1.2 m/s and with <5 m of water. Any velocities oscillating between 0.2 and 0.6 m/s and depths of 2–4 m showed maximum preference.
Discussion
The parameters to determine the mussel presence are related to interactions between velocity, depth and substrate (Hastie et al., 2000; Gangloff & Femeniella, 2007). Numerous studies have currently demonstrated that the presence of mussels in lotic systems is more related to complex hydraulic variables, based on a combination of the previous parameters (Hardison & Layzer, 2001; Howard & Cuffey, 2003; Allen & Vaugh, 2010). The results obtained in this research corroborate that trend, although velocity, by itself, is an important parameter. In general, v, Fr, τ 0 and RSS predict the zebra mussel distribution in a successful and independent way. Dreissena is selective to different values of the previous variables, whereas d and v/d are indicators of lower quality. However, the combination of two simple variables (v and d) most precisely explains the distribution of Dreissena. The optimal values for median discharge focus on velocities of 0.2–0.6 m/s and depths ranging from 2 to 4 m. Almost all the specimens live in areas with <1.2 m/s and <5 m. This numerical information is conditioned by flow and it should be considered in management decisions. Some recent models analyze the temporal variability of complex hydraulics as an interesting predictor of the mussel presence (Parasiewicz et al., 2012).
Velocity is generally considered one of the most relevant variables in any physical habitat study for aquatic organisms. For mussels, low velocities can cause problems for feeding because of their filtering condition (Ackerman, 1999). The same author also established that high velocity values could interfere in the mussel’s active filtration. Thus, our univariate model reflects the maximum preference for values under 0.4 m/s according to Palau et al. (2010). Values higher than 1.2 m/s show zero preference of Dreissena. Claudie & Mackie (1994) and O’Neill (1996) also established practically no possibilities of settlement for values above 1.5 m/s.
The univariate physical habitat model relates to the depth which does not solely constitute a limiting factor for the Dreissena distribution. The zebra mussel is able to occupy a wide range of depth values in the study area. Similar features were observed in a nearby lentic ecosystem by Palau et al. (2010). Depth, amongst other parameters (velocity and shear stress), has a higher incidence in the presence of D. polymorpha. Palau et al. (2004) and Navarro et al. (2006) also explain that factors such as temperature, oxygen and food availability have a direct influence on depth and settlement of this species in the lower Ebro basin reservoirs.
Some of the preceding variables are associated with mussel distribution for low flows (Fr, v), so they ensure the nutrient transport, oxygen and waste products. Under high flows, other parameters (τ 0, RSS) influence on the mussel’s presence, which can affect bed stability and hydraulic refuge (Steuer et al., 2008; Zigler et al., 2008). D. polymorpha showed a clear preference for all these variables and is present in areas where there are low values of them.
Complex hydraulic variables should be easily measurable and they also must have a high hydraulic and biological coherence. The v/d ratio achieves the majority of the previous criterions, but it provides lower quality information than Fr, very similar to it but with a greater robustness. Given that, v/d could be considered as a dispensable parameter in future research. On the other hand, shear stress can be evaluated by different procedures (Morales et al., 2006). All of them are based on difficult field measurement parameters: roughness, particle size and slope; and submerged vegetation and armouring are rarely considered in bed mobility. Therefore, the numerical values provided by shear stress must be considered as indicative features and one should be cautious when comparing results.
Strayer (1999), Morales et al. (2006) and Allen & Vaugh (2010) establish that bed stability at flood events is the most important factor in mussel distribution. This feature is quantified by RSS. In our case, all mussel colonies were located in stable substrate areas for annual floods (RSS < 1). On the other hand, when shear stress values (τ 0) are increased or reduced, within a range (5–60 N/m2), the presence of mussels decline. This behaviour was previously described by other authors (e.g.: Strayer, 1999; Gangloff & Femeniella, 2007). Therefore, zebra mussel colonies are related to stable substrates during flood events and to a minimum velocity (τ 0 > 0) which ensures its position maintenance and the development of biological functions in regular flows. Steuer et al. (2008) found similar relationships with unionids.
One of the most decisive steps in the life cycle of the zebra mussel is the pelagic larval stage, before the pediveligers stage, when they settle and attach to the substrate using their byssal threads to metamorphose into the adult form. Hence, an important consideration to complement habitat models and to better know the Dreissena distribution is to understand how hydrodynamic forces affect larval mortality (Horvath & Crane, 2010) and dispersal along the river (Daraio et al., 2012).
Physical habitat preference data of any species are essential information in order to perform considerations on its management, recovery and control. In the case of endangered mussels, habitat knowledge is required to enhance and restore many river systems (Hastie et al., 2000; Steuer et al., 2008; Zigler et al., 2008; Parasiewicz et al., 2012). For the zebra mussel, as it is considered to be an invasive species, habitat requirements are necessary to conduct control actions (i.e. flood events and flow regulation) and also to evaluate possible changes in the spatial distribution of this bivalve. The physical habitat models developed help to do it and they are easily included in simulations models, used in the management of regulated rivers and environmental assessment of any hydraulic project (Bovee et al., 1998; Parasiewicz & Dunbar 2001).
References
Ackerman, J. D., 1999. The effect of velocity on the filter feeding of zebra mussels (Dreissena polymorpha and D. bugensis): implications for trophic dynamics. Canadian Journal of Fisheries and Aquatic Sciences 56: 1551–1561.
Allen, D. C. & C. C. Vaugh, 2010. Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. Journal of North American Benthological Society 29: 383–394.
Batalla, R. J., D. Vericat & A. Palau, 2008. Efectos de las presas en la dinámica geomorfológica del tramo bajo del Ebro. Crecidas controladas. Ingeniería del Agua 15: 243–255.
Bidwell, J. R., 2010. Range expansion of Dreissena polymorpha: a review of major dispersal vectors in Europe and North America, Chap. 6. In Van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden/Margraf Publishers, Weikersheim: 69–70.
Bovee, K. D., B. L. Lamb, J. M. Bartholow, C. B. Stalnaker, J. Taylor & J. Henriksen, 1998. Stream Habitat Analysis Using the Instream Flow Incremental Methodology. U.S. Geological Survey, Biological Resources Division Information and Technology Report USGS/BRD-98/04, Fort Collins: 131 pp.
Carlton, J. T., 2008. The zebra mussel Dreissena polymorpha found in North America 1986 and 1987. Journal of Great Lakes Research 34: 770–773.
Casagrandi, R., L. Mari & M. Gatto, 2007. Modelling the local dynamics of the zebra mussel (Dreissena polymorpha). Freshwater Biology 52: 1223–1238.
Claudie, R. & G. L. Mackie, 1994. Practical Manual for Zebra Mussel Monitoring and Control. Lewis Publishers, London: 227 pp.
Daraio, J. A., L. J. Weber, S. J. Zigler, T. J. Newton & J. M. Nestler, 2012. Simulated effects of host fish distribution on juvenile unionid mussel dispersal in a large river. River Research and Applications 28(5): 594–608.
Durán, C., M. Lanao, A. Anadón & V. Touyá, 2010. Management strategies for the zebra mussel invasion in the Ebro River basin. Aquatic Invasions 5: 309–316.
Gangloff, M. M. & J. W. Femeniella, 2007. Stream channel geomorphology influences mussel abundance in southern Appalachian streams, U.S.A. Freshwater Biology 52: 64–74.
Gergs, R. & K. O. Rothhaupt, 2008. Effects of zebra mussels on a native amphipod and the invasive Dikerogammarus villosus: the influence of biodeposition and structural complexity. Journal of the North American Benthological Society 27(3): 541–548.
Gore, J. A., J. B. Layzer & J. Mead, 2001. Macroinvertebrate instream flow studies after 20 years: a role in stream management and restoration. Regulated Rivers: Research & Management 17: 527–542.
Hallstan, S., U. Grandin & W. Goedkoop, 2010. Current and modeled potential distribution of the zebra mussel (Dreissena polymorpha) in Sweden. Biological Invasions 12: 285–296.
Hardison, B. S. & J. B. Layzer, 2001. Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Regulated Rivers: Research & Management 17: 77–84.
Hastie, L. C., P. J. Boon & M. R. Young, 2000. Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 429: 59–71.
Hebert, P. D. N., B. W. Muncaster & G. L. Mackie, 1989. Ecological and genetic studies on Dreissena polymorpha (Pallas): a new mollusc in the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 46: 1587–1591.
Horvath, T. G. & L. Crane, 2010. Hydrodynamic forces affect larval zebra mussel (Dreissena polymorpha) mortality in a laboratory setting. Aquatic Invasions 5(4): 379–385.
Howard, J. K. & K. M. Cuffey, 2003. Freshwater mussels in a California North Coast Range river: occurrence, distribution, and controls. Journal of the North American Benthological Society 22: 63–77.
Karatayev, A. Y., D. K. Padilla, D. Minchin, D. Boltovskoy & L. E. Burlakova, 2006. Changes in global economies and trade: the potential spread of exotic freshwater. Biological Invasions 9: 161–180.
MAGRAMA, 2012. Ministerio de Agricultura, Alimentación y Medio Ambiente. Catálogo español de Especies Exóticas Invasoras, Dreissena polymorpha. Madrid: 9 pp.
Morales, Y., L. J. Weber, A. E. Mynett & T. J. Newton, 2006. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. Journal of North American Benthological Society 25: 664–676.
Naddafi, R., T. Blenckner, P. Eklöv & K. Pettersson, 2011. Physical and chemical properties determine zebra mussel invasion success in lakes. Hydrobiologia 669: 227–236.
Navarro, E., M. Bacardit, L. Caputo, T. Palau & J. Armengol, 2006. Limnological characterization and flow patterns of a three-coupled reservoir system and their influence on Dreissena polymorpha populations and settlement during the stratification period. Lake and Reservoir Management 22: 293–302.
O’Neill, C. R., 1996. The zebra mussel: impacts and control. New York Sea Grant. Cornell University. State University of New York. Cornell Cooperative Extension, Information Bulletin 328: 1–62.
Oscoz, J., P. Tomás & C. Durán, 2010. Review and new records of non-indigenous freshwater invertebrates in the Ebro River basin (northeast Spain). Aquatic Invasions 5: 263–284.
Palau, A., I. Cía., D. Fargas., M. Bardina, & S. Massuti, 2004. Resultados preliminares sobre ecología básica y distribución del mejillón cebra en el embalse de Riba – Roja (río Ebro). Monografía de ENDESA, Lérida: 43 pp.
Palau, A. & I. Cía, 2006. Métodos de control y erradicación del mejillón cebra (Dreissena polymorpha). Monografía de ENDESA, Lérida: 71 pp.
Palau Ibars A., I. Cia Abaurre, R. Casas Mulet & E. Rosico Ramón, 2010. Zebra mussel distribution and habitat preference at the Ebro basin (north east Spain), Chap. 10. In Van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden/Margraf Publishers, Weikersheim: 113–118.
Parasiewicz P. & M. J. Dunbar, 2001. Physical habitat modelling for fish – a developing approach. Archiv für Hydrobiologie 135/2–4: 239–268 (Suppl. Large Rivers Vol. 12).
Parasiewicz, P., E. Castelli, J. N. Rogers & E. Plunkett, 2012. Multiplex modeling of physical habitat for endangered freshwater mussels. Ecological Modelling 228: 66–75.
Rajagopal, S., B. J. A. Pollux, J. L. Peters, G. Cremers, S. Moon-van der Staay, T. Van Alen, J. Eygensteyn, A. Van Hoek, A. Palau, A. Bij de Vaate & G. Van der Velde, 2009. Origin of Spanish invasion by the zebra mussel, Dreissena polymorpha (Pallas, 1771) revealed by amplified fragment length polymorphism (AFLP) fingerprinting. Biological Invasions 11: 2147–2159.
Shields, A., 1936. Änwendung der Aenlichkeitsmechanik und der Turbulenzforschung auf die Geschiebebewegung. Mitteilungen der Prevssische Versuchsanstalt für Wasserbau und Schiffbau, 26th ed. Heft, Berlin: 43 pp. (in German).
Steffler, P. & J. Blackburn, 2002. Two-Dimensional Depth Averaged Model of River Hydrodinamics and Fish Habitat. Introduction to Depth Averaged Modeling and User’s Manual. University of Alberta, Alberta: 119 pp.
Steuer, J. J., T. J. Newton & S. J. Zigler, 2008. Use of complex hydraulic variables to predict the distribution and density of unionids in a side cannel of the upper Mississippi River. Hydrobiologia 610: 67–82.
Strayer, D. L., 1999. Use of flow refuges by unionid mussels in rivers. Journal of the North American Benthological Society 18: 468–476.
Strayer, D. L. & H. M. Malcom, 2006. Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshwater Biology 51: 117–130.
Therriault, T. W. & M. I. Orlova, 2010. Invasion success within the familiy Dreissenidae: prerequisites, mechanisms and perspectives, Chap. 5. In Van der Velde, G., S. Rajagopal & A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys Publishers, Leiden/Margraf Publishers, Weikersheim: 59–68.
Vericat, D., R. Batalla & A. Palau, 2007. Análisis batimétrico del tramo bajo del río Ebro. Grupo de Geomorfologia Fluvial de la Universidad de Lleida – Centro Tecnológico Forestal de Catalunya. ENDESA Technical Report: 46 pp.
Zigler, S. T., T. J. Newton, J. J. Steuer, M. R. Bartsch & J. S. Sauer, 2008. Importance of physical and hydraulic characteristics to unionid mussels: a retrospective analysis in a reach of large river. Hydrobiologia 598: 343–360.
Acknowledgments
This research has been supported by the project No. 010/SGTB/2007/1.1: ‘Hydraulic management of reservoirs applied to the zebra mussel control’ and by the ‘Biodiversity Conservation Program’ of ENDESA. The zebra mussel sampling was carried out by the Subaquatic Activities Special Group (Guardia Civil corps). Nines Marín and Luis Adel (ENDESA) coordinated the field sampling and Damià Vericat (FSCC) provided us with the information relating to the bathymetry. We specifically thank Juan Francisco Fuentes Pérez (GEA-ecohidraulica) for his help with the illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Manuel P. M. Lopes-Lima, Ronaldo G. Sousa, Simone G. P. Varandas, Elsa M. B. Froufe & Amílcar A. T. Teixeira / Biology and Conservation of Freshwater Bivalves
Rights and permissions
About this article
Cite this article
Sanz-Ronda, F.J., López-Sáenz, S., San-Martín, R. et al. Physical habitat of zebra mussel (Dreissena polymorpha) in the lower Ebro River (Northeastern Spain): influence of hydraulic parameters in their distribution. Hydrobiologia 735, 137–147 (2014). https://doi.org/10.1007/s10750-013-1638-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1638-y