Abstract
While numerous reports exist on the results of lake restoration initiatives in temperate regions, only a few exist from subtropical lakes. We present results of the lake restoration of shallow, subtropical Lake Wuli, China, conducted between 1999 and 2010. After restoration, annual average concentrations of total nitrogen, total phosphorus (TP), and chlorophyll a and the chemical oxygen demand declined significantly, though summer TP remained high. Suspended solids increased significantly over the years, whereas transparency decreased, though not significantly so. The contribution of cryptophytes to total phytoplankton biomass decreased, while the proportion of cyanobacteria, especially potentially N2-fixing species, increased. Rotifers were superseded by crustaceans as the dominant taxon of the zooplankton community. Enhanced abundance of Daphnia spp., appearance of Leptodora kindti, and increased biomass ratios of zooplankton to phytoplankton, calanoids to cyclopoids, and nauplii to copepods in the post-restoration period indicate reduced fish predation and stronger top-down control of phytoplankton. However, the increase in non-algal turbidity, probably caused by the higher biomass of benthivorous fish, apparently prevented the re-establishment of submerged macrophyte communities. We conclude that removal of fish, particularly benthivorous species, will further improve water quality in this and other subtropical shallow lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past 40 years, much effort has been devoted to reduce the eutrophication of lakes in Europe and North America (Sas, 1989; Jeppesen et al., 2005b). In shallow lakes, increases in nutrient input have often been accompanied by reduced transparency, disappearance of submerged macrophytes, and repeated cyanobacterial blooms, leading to deterioration in water quality and ecological degradation. In temperate zones, such lakes have often been found to respond to reductions in nutrient loading, and they typically approach a new, low-nutrient equilibrium in <5 and <10–15 years for nitrogen (N) and phosphorus (P), respectively (Jeppesen et al., 2005b), but the recovery period may, in some cases, be much longer (Søndergaard et al., 2002). In hypereutrophic shallow lakes with high TN and TP, sometimes associated with high organic loading, phytoplankton are often dominated by green algae, cryptophytes, or euglenophytes (Leah et al., 1980; Jensen et al., 1994; Mataloni et al., 2000), and several of these lakes have shown a decline in such algae groups and an increase of cyanobacteria in the early phase of recovery from loading reduction (Krienitz et al., 1996; Jeppesen et al., 2005a, b, 2007). Numerous methods have been employed to reinforce recovery in shallow temperate lakes (Cooke et al., 2005; Jeppesen et al., 2007) after nutrient loading reduction, including sediment capping to reduce internal P loading, manipulation of the fish community and the food web, and restoration of submerged macrophyte communities (Gulati et al., 2008; Jeppesen et al., 2012).
A prerequisite for successful restoration of shallow lakes after a decrease of the external nutrient loadings is a reduction of the biomass of benthivorous and planktivorous fish, either naturally or, when needed, by targeted fish removal, as frequently undertaken in temperate lakes (Hansson et al., 1998; Jeppesen et al., 2005a, b, 2012; Søndergaard et al., 2008). A high fish biomass can negatively affect water quality in a variety of ways. Apart from directly influencing nutrient levels in the water and underlying sediments by excretion (Attayde & Hansson, 2001; Jeppesen et al., 2007; Bajer et al., 2009), fish may also influence the balance of biological communities by feeding selectively on large-bodied cladocerans. Heavy predation reduces the ratio of zooplankton to phytoplankton, thereby restricting the potential impact of zooplankton grazing and reducing the top-down control of phytoplankton. Meanwhile, disturbance and resuspension of sediments by benthivorous fishes in particular are likely to elevate levels of suspended solids (SS) and decrease the sedimentation rate (Jeppesen et al., 2007; Søndergaard et al., 2008). High concentrations of SS are an important cause of water quality deterioration, which may affect the composition of zooplankton communities and inhibit the rehabilitation of macrophytes (Bilotta & Brazier, 2008; Søndergaard et al., 2010).
Contrary to the relatively well-documented results of restoration attempts in temperate lakes, the responses of subtropical and tropical lakes to restoration measures are less well known (Jeppesen et al., 2007, 2012). In warm lakes, the greater abundance and frequent spawning of small fish tend to prevent large-bodied zooplankton from achieving dominance, which naturally limits the potential effect of enhanced grazing control on phytoplankton (Lazzaro, 1997; Havens & Beaver, 2011; Iglesias et al., 2011).
In China, the environmental state of many lakes has rapidly deteriorated to eutrophic conditions due to excessive external nutrient loading. A recent investigation concluded that 85.4% of 138 Chinese lakes with an area >10 km2 were eutrophic, while 40.1% were hypereutrophic (Yang et al., 2010). In recent years, national and regional governments have made efforts to restore some of these lakes, including urban lakes, by adopting various management strategies (Li et al., 2005; Chen et al., 2006). However, the negative effects of high nutrient levels and high levels of fish disturbance and predation are often so strong that attempts to improve water quality by rehabilitating aquatic plants have often been unsuccessful (van de Bund & van Donk, 2002; Jeppesen et al., 2005a; Søndergaard et al., 2010).
Lake Wuli, a subtropical shallow lake located close to Wuxi City on the northern end of Lake Taihu, changed from a mesotrophic state with abundant macrophytes during the 1950–1970s to a eutrophic state with few macrophytes and frequent cyanobacterial blooms during the early 1980s. The increased productivity encouraged stocking of commercial fish, mainly bighead carp Aristichthys nobilis, silver carp Hypophthalmichthys molitrix, common carp Cyprinus carpio, Crucian carp Carassius auratus, and grass carp Ctenopharyngodon idellus. The annual fish yield increased to about 650–880 t in total, equivalent to 714–967 kg ha−1 (Chen, 2007). From 2002 to 2005, various restoration measures were introduced by the local government of Wuxi City in order to improve lake water quality. These included reduction of external nutrient loading and sediment dredging, but not fish removal. In the present study, we hypothesized that the reduction of nutrient loading would potentially result in a fast reduction in lake nutrient concentration, but that improvements in lake water transparency may be hampered by no control of benthivorous fish in subtropical shallow lakes. To test these hypotheses, we followed the effect of restoration on the water quality, zooplankton, and phytoplankton in Lake Wuli and compared the results with studies from temperate and other subtropical lakes. Possible methods for further improvements of the water quality of the lake and other subtropical hallow lakes were also discussed.
Methods and materials
Study site
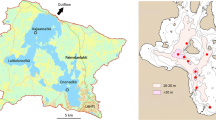
Lake Wuli is located south of Wuxi City in Jiangsu, China (Fig. 1). Prior to sediment dredging and removal of in-lake ponds between 2002 and 2005, its area was 5.8 km2 and its average depth 1.8 m. Following restoration, the lake area and average depth increased to 8.6 km2 and 2.25 m (Zhu & Zhang, 2009), respectively. Annual average precipitation and air temperature in Wuxi City are about 1,100 mm and 15.4°C, respectively. Average wind speeds between 1961 and 2000 were 3.1 m s−1 in winter and 3.0 m s−1 in summer (Chen, 2007).
Location of Lake Wuli. Sites 1 and 2 are sampling sites. Site 3 is not a sampling site. Baojie Bridge and an embankment divide Lake Wuli into three sections—East Lake Wuli (Site 1), West Lake Wuli (Site 2), and an area formerly occupied by fish ponds (Site 3). Two small bridges along the embankment permit water exchange between West Lake Wuli and Site 3. A sluice gate prevents exchange of water between Lake Wuli and Meiliang Bay in Lake Taihu
Since the 1950s, Lake Wuli has undergone significant changes in its ecological state (Table 1).
-
(1)
In 1951, the annual averages of phosphate phosphorus (PO4 3−-P), ammonium nitrogen (NH4 +-N), and Secchi depth (SD) were 0.0097 mg l−1, 0.12 mg l−1, and 1.44 m, respectively. Macrophytes, including Vallisneria natans, Potamogeton crispus, Trapa incise, Myriophyllum sp., and Phragmites communis among others, were abundant (Wu, 1962). This state persisted until the beginning of the 1960s (NIGLAS, 1965)
-
(2)
At the end of the 1960s, fish stocking in Lake Wuli and external nutrient loading from Wuxi City led to eutrophication (IEQLT, 1983; Sun & Huang, 1993). Macrophytes, especially submerged species, gradually disappeared. By 1980–1981, the average total nitrogen (TN), TP, and NH4 +-N concentrations had increased to 0.85 mg l−1, 0.016 mg l−1, and 0.21 mg l−1, respectively, and SD decreased to 0.45 m (IEQLT, 1983; Li et al., 1994).
-
(3)
From the end of the 1980s to the beginning of the 1990s, cyanobacterial blooms occurred, mainly of Chroococcus spp. and Microcystis spp. (Li et al., 1994). Average TN, TP, NH4 +-N, and SD during 1987–1988 were 3.1 mg l−1, 0.076 mg l−1, 0.61 mg l−1, and 0.50 m, respectively (Sun & Huang, 1993), and the situation continued to worsen into the early 1990s. Average TN, TP, NH4 +-N, and SD during the period 1990–1993 were 4.5 mg l−1, 0.213 mg l−1, 1.76 mg l−1, and 0.41 m, respectively (Li et al., 1994).
-
(4)
From the mid-1990s, Lake Wuli was in a hypereutrophic state. Diatoms (Cyclotella spp. and Melosira spp.) and cryptophytes (Chroomonas sp. and Cryptomonas spp.), rather than cyanobacteria, dominated the phytoplankton community. Average TN, TP, NH4 +-N, and SD during 1994–1995 were 4.9 mg l−1, 0.0825 mg l−1, 2.42 mg l−1, and 0.47 m, respectively (data from CNERN, Taihu Laboratory for Lake Ecosystem Research).
In 1999, the government of Wuxi City closed hotels, restaurants, sanatoriums, and factories along the lake. Then, from 2002 to 2005, a series of active restoration measures were implemented including sediment dredging, reduction of external nutrient loading, removal of in-lake fish ponds, and reconstruction of the bank area. The latter involved the removal of concrete banks, planting with emergent macrophytes, and the construction of a small wetland. It was estimated that sediment dredging removed 41-t TN and 90-t TP from the lake and that the reduction in external loading and the removal of in-lake fish ponds accounted for further reductions of 93.1-t TN, 8.6-t TP, and 852-t CODMn per year (Gu & Lu, 2004).
Data source and analysis
The data were obtained from CNERN, the Taihu Laboratory for Lake Ecosystem Research. Samplings were conducted at Site 1 from 1999 to 2004 and at Sites 1 and 2 from 2005 to 2010 (Fig. 1). Average depth was 2.75 m at Site 1 and 2.40 m at Site 2. Samplings took place in winter (February), spring (May), summer (August), and autumn (November) each year. The variables analyzed included temperature, SD, CODMn, TN, TP, ammonia, soluble reactive phosphorus (SRP), dissolved inorganic nitrogen (DIN = nitrite + nitrate + ammonia), total SS, chlorophyll a (Chl a), and biological community compositions.
Water temperature and SD were measured using a thermometer and a Secchi disk. TN and TP concentrations were analyzed by colorimetry after digestion (Ebina et al., 1983). Samples were filtered through pre-dried Whatman GF/C filters, dried (105°C for 4 h) and weighed to measure the concentration of SS. Chl a was extracted with 90% acetone for 24 h, and its concentration was subsequently determined by colorimetry and calculated according to the equation of Jin & Tu (1990).
For biological community analyses, microcrustaceans were collected by filtering 7.5 l of mixed water collected at three depths through a 64-μm net and preserved in 4% formaldehyde. For phytoplankton and rotifer microscopic counting, 1-l water samples mixed from the three depths were treated with 10 ml Lugol’s iodine and sedimented for 48 h. The supernatant was removed and the residue was collected. Crustacean zooplankton (cladocerans and copepods) and rotifers were counted at 40× magnification. Species identification was made according to Wang (1961), Chiang & Du (1979), and Shen & Du (1979). Zooplankton biomass (dry weight) was estimated using equations from Huang (1999). Phytoplankton biomass was estimated from algal counts using the nearest geometric volume of each taxon, assuming a mean density of phytoplankton of 1, i.e., 1-g wet weight of phytoplankton has a volume of 1 × 103 mm3.
In order to evaluate the effectiveness of restoration, the time series was divided into three periods: pre-restoration (1999–2001), during restoration (2002–2005), and post-restoration (2006–2010). Annual averages of the biotic and abiotic parameters were calculated from data on Site 1 from 1999 to 2004 and on Sites 1 and 2 from 2005 to 2010. T tests for annual data on Sites 1 and 2 showed no significant difference (P > 0.05). We therefore averaged the Sites 1 and 2 data before analyses. To evaluate the effect of zooplankton grazing on phytoplankton, the ratios of zooplankton biomass to phytoplankton biomass (ZB:PB) and of Chl a:TP concentrations were calculated. ZB:PB was calculated as zooplankton biomass/Chl a concentration/66 (Jeppesen et al., 2005a). To assess changes over time, Spearman’s correlations were conducted between the various environmental variables and years after log transformation of the data. T tests were used to compare the environmental variables and ratios between the pre- and the post-restoration period.
Results
Changes in physico-chemical variables
Average water temperatures from 1999 to 2010 were 8.1 ± 1.7, 22.2 ± 1.8, 29.6 ± 1.8, and 13.5 ± 2.4°C in February, May, August, and November, respectively. Annual water temperatures calculated using four-month averages fluctuated between 17.3 and 19.7°C, but no obvious trend was discernible during the study period.
Annual mean concentrations of TN, TP, and CODMn decreased significantly over the years (Fig. 2; Table 3). Winter TN decreased more dramatically than summer values. Summer TP tended to decrease, though not significantly. However, winter TP declined pronouncedly during the study period. CODMn changes followed the pattern of TP. There were statistically significant changes in annual average concentrations of TN, TP, and CODMn. Summer TN was significantly lower in the post-restoration period than in the pre-restoration period, whereas summer TP and CODMn showed no statistically significant changes between the two periods (Table 2). There was a dramatic drop in TN, with post-restoration concentrations being less than half of those observed prior to restoration. Since 2007, summer TN remained below 2 mg l−1, while summer and annual TP remained above 0.10 mg l−1 after restoration. CODMn decreased gradually to <5 mg l−1 after 2007 (Fig. 2).
Annual average Chl a decreased significantly after restoration. Annual SD showed no significant change, while SS increased significantly (Fig. 2; Table 3). There were no statistically significant changes in the ratios of summer SRP:TP and DIN:TN, but the Chl a:TP and Chl a:SS ratios decreased significantly (Table 2).
Changes in phytoplankton and zooplankton
Marked changes were also observed in the phytoplankton community (Fig. 3). Annual phytoplankton biomass averages decreased by 90.7%. Cryptophytes (Cryptomonas ovata, C. erosa, Chroomonas acuta) and diatoms (Cyclotella sp., Melosira granulata) generally dominated the phytoplankton community prior to restoration. Cryptophyte biomass decreased from 81.7 to 17.1% of the total biomass between 1999 and 2010, whereas the proportion of cyanobacteria of the annual total phytoplankton biomass increased from 0.3 to 29.9%. The cyanobacterial community was mainly represented by Anabaena sp. and Aphanizonmenon sp. without heterocysts.
Lake Wuli plankton dynamics from 1999 to 2010. (1) Annual average, summer (August) biomass of crustacean plankton (cladocerans + copepods), rotifers (dry weight, μg l−1), and phytoplankton (wet weight, mg l−1); (2) percentage biomass of different zooplankton and phytoplankton taxa; (3) zooplankton (Zoo):phytoplankton (Phy) biomass ratio in summer
Distinct trends were observed in the abundance of crustaceans and rotifers during the study period (Fig. 3). After 2005, cladoceran biomass increased markedly during the warm seasons, in particular, the large-sized cladoceran Daphnia spp., occurring in abundance only in spring (May) (Fig. 4a). In summer (August), the cladoceran communities became dominated by small-sized species (Bosmina longirostris, Ceriodaphnia cornuta, Moina micrura, Diaphanosoma dubium). At the same time, the predatory cladoceran Leptodora kindti appeared, with a peak density of 1.4 ind. l−1 in summer 2006 (Fig. 4b). The average body lengths of summer cladocerans and copepods varied markedly between 2004 and 2010, but with an overall tendency of showing increased length by the end of the study (Fig. 4c). The ratios of calanoid:cyclopoid and nauplii:copepod increased significantly (P = 0.040 and 0.027, respectively) from 2004 to 2010 (Fig. 4d). Prior to restoration, the rotifer community was dominated by the genera Brachionus, Polyarthra, Filinia, and Trichocerca.
Variations of zooplankton in Lake Wuli from 1999 to 2010. a density and body length (±SD) of Daphnia spp. in May (spring); b density of Leptodora kindti in August (summer); c average body length of cladocerans and copepods in summer; d density ratios of calanoids to cyclopoids and nauplii to copepods in summer
The crustacean proportion of total summer zooplankton biomass increased from 0.04 before restoration to 0.30 afterward. However, total copepod and cladoceran biomass declined dramatically after 2007; thus, total summer zooplankton biomass declined from 2342 μg l−1 before restoration to 507 μg l−1 in the post-restoration period.
Spearman’s correlation analysis indicated that the total phytoplankton biomass for a given year exhibited a significant positive correlation with CODMn (r = 0.72, P = 0.009). The ratio of cyanobacteria biomass to total phytoplankton biomass correlated negatively with TN (r = − 0.85, P < 0.001), TP (r = − 0.69, P = 0.014), TN:TP (molar ratio, r = − 0.75, P = 0.005), and CODMn (r = − 0.83, P = 0.001). Conversely, the ratio of cryptophyte biomass to total phytoplankton biomass was positively correlated with TN (r = 0.60, P = 0.04), TP (r = 0. 72, P = 0.008), and CODMn (r = 0.60, P = 0.04). The summer ratio of zooplankton to phytoplankton biomass increased significantly (r = 0.74, P = 0.009) over the years (Fig. 3).
Discussion
The physico-chemical and biological variables of Lake Wuli responded dramatically to the reduction of external nutrient loading and the removal of sediment from the lake basin. Significant winter decreases in TN and much lower summer values indicate a rapid response to external N loading, though lack of input data hinders a firm conclusion. However, a cross comparison of a number of European and North American case studies also suggests a general fast response of TN to external load reduction in mainly temperate lakes, where a new equilibrium is reached typically in less than 5 years (Jeppesen et al., 2005a, Table 4), perhaps even faster in warm regions due to higher denitrification rates (Lewis, 2000). TP decreased in winter and on an annual basis as expected from other studies (Table 4), but remained high in summer, likely reflecting a continuing high rate of P release from the sediments as seen in other studies (Köhler et al., 2005; Søndergaard et al., 2005, 2013). Contrary to most other studies of temperate lakes (Jeppesen et al., 2005b), TN:TP and DIN:SRP ratios decreased in summer, whereas summer SRP:TP exhibited no change after restoration (Table 4). These differences are attributed to the particularly strong decrease of TN and DIN summer in Lake Wuli and high level of TP and SRP summer.
SS increased despite a clear decline in summer Chl a suggesting that the turbidity is largely due to inorganic suspended matter and detritus, most likely in consequence of sediment resuspension. Lakes with a dynamic ratio (square root of lake surface area in square kilometers divided by mean depth in meters) >0.8 are prone to wave disturbance (Håkanson, 1982; Bachmann et al., 2000). The dynamic ratio of Lake Wuli, however, only decreased from 1.34 in the pre-restoration years to 1.25 after restoration due to the increase in lake area, a change that is too low to explain the increase in SS. The similar water contents observed in the surface sediment before (55.0%) and after (57.6%) restoration (Fan & Zhang, 2010) also suggest no important change in the risk of wind-induced resuspension. Moreover, the wind speed was similar in winter (3.1 m s−1) and summer (3.0 m s−1), and there were no significant changes in water levels between summer and winter (Zhu & Zhang, 2009), but still SS varied markedly, being much higher in summer. Benthivorous fish, on the other hand, can substantially enhance sediment resuspension in shallow lakes (Breukelaar et al., 1994). In Lake Wuli, prior to restoration, the annual fish yield reached 650–880 t, equivalent to 714–967 kg ha−1 (Chen, 2007). The dominance of planktivorous and benthivorous species, such as Aristichthys nobilis, Hypophthalmichthys molitrix, Cyprinus carpio, and Carassius auratus, of the fish community (Duan et al., 2009; Zhang et al., 2010) provides a likely explanation for the observed increase in SS. Fish-induced sediment resuspension may also explain the higher concentrations of SS in summer, as fish become more active in warm water (Hernandez et al., 2002). Nothing has so far been done to reduce the abundance of fish in the lake, and fish fry continues to be stocked periodically (Q. J. Luo pers. communication). Higher fish disturbance may also explain that TP in summer actually increased after restoration and that SRP:TP, contrary to observations from many other studies (Table 4), did not decline.
Major changes occurred in the zooplankton community structure, shifting from rotifer to crustacean dominance. High rotifer abundance is typical of eutrophic lakes with high levels of fish predation (Shao et al., 2001; May & O’Hare, 2005). The increased abundance of Daphnia spp., the appearance of the predatory Leptodora kindti, and the increased ratios of zooplankton:phytoplankton and calanoid:cyclopoid after the restoration process suggest a reduced fish predation pressure on large-bodied crustacean zooplankton (Adrian, 1997; Liu, 2001; Jeppesen et al., 2005b). The observed increase in SS after restoration might have contributed to the reduction in fish predation pressure as turbidity may offer refuge from visual predators (Jeppesen et al., 1999; Horppila et al., 2009; Nurminen et al., 2010). In an enclosure experiment, increasing SS reduced the predation efficiency of a visually oriented hunter, perch (Perca fluviatilis), on two large-sized cladoceran species (Nurminen et al., 2010).
Despite the elevated summer levels of TP, the phytoplankton biomass and Chl a in summer declined over time. This may in part be attributed to increased zooplankton grazing as indicated by an increase in the biomass ratio of zooplankton:phytoplankton and a reduction of summer Chl a:TP ratios, as seen in other studies of lakes in recovery (Jeppesen et al., 2005a, b, Table 4), but the higher concentration of inorganic SS may also have contributed by shading the phytoplankton (Kang et al., 2013). We also found marked changes in the phytoplankton community. Cryptophytes declined from 68% of phytoplankton biomass prior to restoration to 27% in recent years, while the proportion of cyanobacteria increased from 0.3% to 29.9% during the same period. While a decline of cryptophytes following nutrient loading reduction may be appear counterintuitive as eutrophic lakes are often considered as cyanobacteria dominated, there is substantial evidence now that hypereutrophic shallow lakes with high TN and TP, sometimes associated with high organic loading, are often dominated by green algae, cryptophytes, or euglenophytes (Leah et al., 1980; Jensen et al., 1994; Mataloni et al., 2000), and several have shown a decline in such algae groups and an increase of cyanobacteria in the early phase of recovery from loading reduction (Krienitz et al., 1996; Jeppesen et al., 2005a, b, 2007). Low TN:TP (molar ratio) (Smith, 1983) and low light levels (Scheffer et al., 1997) due to increased turbidity may further have favored cyanobacteria in Lake Wuli in recent years, although the role of the TN:TP ratio for phytoplankton dominance is ambiguous, not least for the shallow lakes (Jensen et al., 1994).
Contrary to findings from temperate Danish lakes (Jeppesen et al., 2005a, b), we did not observe an intermediate state with dominance of Microcystis (despite dominating in Lake Taihu) during recovery before the shift to dominance by Anabaena sp. and Aphanizonmenon sp. This may reflect the fast reduction in nutrients (notable TN) in Lake Wuli as a consequence of the multiple restoration measures initiated. Also, higher grazing may favor filamentous cyanobacteria over Microcystis as seen in lakes and ponds with high abundance of Daphnia magna (Fott et al., 1980; Lynch, 1980).
The present environmental state of Lake Wuli is unfavorable for the rehabilitation of a submerged macrophyte community. The high biomass of benthivorous fish is detrimental to submerged macrophytes in several ways: The macrophytes suffer directly by consumption by fish and indirectly through the effects of reduced light levels caused by sediment resuspension and the proliferation of phytoplankton in water enriched with excreted nutrients (Haas et al., 2007; Gulati et al., 2008; Bajer et al., 2009). In lakes with a mean depth <3 m, submerged macrophyte growth is adversely affected when SS exceeds 15 mg dw l−1 (Søndergaard et al., 2010). In Lake Wuli, having a mean depth of only 2.25 m, SS reached as much as 40 mg dw l−1 after restoration. Fish removal is likely to increase the light available to submerged macrophytes, and rehabilitation efforts might be further improved by a reduction of the water level. A demonstration project in a branch of Lake Wuli involving fish removal and lowering of the water level has resulted in clear water and extensive growth of macrophytes (Zhang et al., 2012).
To improve water clarity and thus enhance the rehabilitation of submerged macrophytes in Lake Wuli, removal of benthivorous fish should be considered as a restoration measure. Biomanipulation has been extensively used in the restoration of temperate waterbodies (Søndergaard et al., 2008), but its effect in subtropical and tropical lakes is less well studied (Jeppesen et al., 2007, 2012). It is to be expected that the top-down effect might be less dramatic in warm lakes due to the greater abundance of small fish (Meerhoff et al., 2007; Jeppesen et al., 2010, 2012). However, recent studies suggest that this may not be a barrier to success, at least in the short term, as removal of large benthivorous fish may reduce a resuspension-induced increase in turbidity. Beklioglu & Tan (2008) reported successful short-term restoration of a subtropical lake using biomanipulation, and successful restoration via biomanipulation was also achieved in the Huizhou West Lake, a tropical shallow eutrophic lake in south China (Chen et al., 2010; Jeppesen et al., 2012; Z. Liu et al., unpublished results). We therefore recommend removal of fish, particularly benthivores, in order to re-enforce ecological restoration and further improvement of the water quality of Lake Wuli, and we further argue that fish removal may also be a relevant restoration measure in many other Asian lakes used for fish production in either the past or the present.
References
Adrian, R., 1997. Calanoid–cyclopoid interactions: evidence from an 11-year field study in a eutrophic lake. Freshwater Biology 38: 315–325.
Attayde, J. L. & L. A. Hansson, 2001. Fish-mediated nutrient recycling and the trophic cascade in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58: 1–8.
Bachmann, R. W., M. V. Hoyer & D. E. Canfield Jr., 2000. The potential for wave disturbance in shallow Florida lakes. Lake and Reservoir Management 16: 281–291.
Bajer, P. G., G. Sullivan & P. W. Sorensen, 2009. Effects of a rapidly increasing population of common carp on vegetative cover and waterfowl in a recently restored Midwestern shallow lake. Hydrobiologia 632: 235–245.
Beklioglu, M. & C. O. Tan, 2008. Restoration of a shallow Mediterranean lake by biomanipulation complicated by drought. Fundamental Applied Limnology 171: 105–118.
Bilotta, G. S. & R. E. Brazier, 2008. Understanding the influence of suspended solids on water quality and aquatic biota. Water Research 42: 2849–2861.
Breukelaar, A. W., E. H. R. R. Lammens, I. G. P. K. Breteler & I. Tatrai, 1994. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshwater Biology 32: 113–121.
Chen, K. L., 2007. Mechanism and technology of ecological restoration in eutrophic, shallow lake: a case of Lake Wuli, a northern bay of Lake Taihu, China. Doctor Dissertation, Nanjing Institute of Geography and Limnology, CAS.
Chen, K. L., X. M. Bao, L. X. Shi, W. M. Chen, C. J. Lan, H. Xu & H. Y. Hu, 2006. Ecological restoration engineering in Lake Wuli, Lake Taihu: a large enclosure experiment. Journal of Lake Sciences 18: 139–149.
Chen, L., X. F. Zhang & Z. W. Liu, 2010. The response of a phytoplankton community to ecosystem restoration in Huizhou West Lake. Journal of Wuhan Botanical Research 28: 453–459.
Chiang, S. C. & N. S. Du, 1979. Fauna Sinica, Crustacea, Freshwater Cladocera. Science Press, Beijing.
Cooke, G. D., E. B. Welch, S. Peterson & S. A. Nichols, 2005. Restoration and Management of Lakes and Reservoirs. CRC Press, Boca Raton.
Duan, J. R., H. Y. Zhang, K. Liu, D. P. Xu, M. Y. Zhang & W. G. Shi, 2009. Community biodiversity of fishery resources in Lihu. Journal of Shanghai Ocean University 18: 243–247.
Ebina, J., T. Tsutsui & T. Shirai, 1983. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Research 17: 1721–1726.
Fan, C. X. & L. Zhang, 2010. Lake Taihu: Principles of Sediment Pollution and Remediation. Science Press, Beijing.
Fott, J., L. Pechar & M. Pražáková, 1980. Fish as a factor controlling water quality in ponds. In Barica, J. & L. R. Mur (eds), Hypertrophic Ecosystems. Developments in Hydrobiology, Vol. 2. Dr. W. Junk Publishers, The Hague: 255–261.
Gu, G. & G. F. Lu, 2004. On the integrated control of water environment of Wuli Lake, Lake Taihu. Journal of Lake Science 16: 56–60.
Gulati, R. D., P. L. M. Dionisio & E. Van Donk, 2008. Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38: 233–247.
Haas, K., U. Köhler, S. Diehl, P. Köhler, S. Dietrich, S. Holler, A. Jaensch, M. Nidermaier & J. Vilsmeier, 2007. Influence of fish on habitat choice of water birds: a whole system experiment. Ecology 88: 2915–2925.
Håkanson, L., 1982. Lake bottom dynamics and morphometry: the dynamic ratio. Water Resources Research 18: 1444–1450.
Hansson, L. A., H. Annadotter, E. Bergman, S. F. Hamrin, E. Jeppesen, T. Kairesalo, E. Luokkanen, P. A. Nilsson, M. Søndergaard & J. Strand, 1998. Biomanipulation as an application of food chain theory: constraints, synthesis and recommendations for temperate lakes. Ecosystems 1: 558–574.
Havens, K. E. & J. B. Beaver, 2011. Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia 668: 49–60.
Hernandez, C. E., P. E. Neill, J. M. Pulgar, F. P. Ojeda & F. Bozinovic, 2002. Water temperature fluctuations and territoriality in the intertidal zone: two possible explanations for the elevational distribution of body size in Graus nigra. Journal of Fish Biology 60: 472–488.
Horppila, J., P. Eloranta, A. Liljendahl-Nurminen, J. Niemistö & Z. Pekcan-Hekim, 2009. Refuge availability and sequence of predators determine the seasonal succession of crustacean zooplankton in a clay-turbid lake. Aquatic Ecology 43: 91–103.
Huang, X. F., 1999. Survey Observation and Analysis of Lake Ecology. Standards Press of China, Beijing.
Iglesias, C., N. Mazzeo, M. Meerhoff, G. Lacerot, J. Clemente, F. Scasso, C. Kruk, G. Goyenola, J. Garcia, S. L. Amsinck, J. C. Paggi, S. José de Paggi & E. Jeppesen, 2011. High predation is the key factor for dominance of small-bodied zooplankton in warm lakes – evidence from lakes, fish enclosures and surface sediment. Hydrobiologia 667: 133–147.
Investigation Team of Environmental Quality of Lake Taihu (IEQLT), 1983. A special investigation of the environmental quality of Lake Taihu. Transaction of Shanghai Normal Academy Supplement: 27–49.
Jensen, J. P., E. Jeppesen, K. Olrik & P. Kristensen, 1994. Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish lakes. Canadian Journal of Fishery and Aquatic Science 51: 1692–1699.
Jeppesen, E., J. P. Jensen, M. Søndergaard & T. Lauridsen, 1999. Trophic dynamics in turbid and clearwater lakes with special emphasis on the role of zooplankton for water clarity. Hydrobiologia 408(409): 217–231.
Jeppesen, E., J. P. Jensen, M. Søndergaard & T. Lauridsen, 2005a. Response of fish and plankton to nutrient loading reduction in 8 shallow Danish lakes with special emphasis on seasonal dynamics. Freshwater Biology 50: 1616–1627.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, K. Kangur, J. Köhler, S. Körner, E. Lammens, T. L. Lauridsen, M. Manca, R. Miracle, B. Moss, P. Nõges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005b. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Jeppesen, E., M. Søndergaard, M. Meerhoff, T. L. Lauridsen & J. P. Jensen, 2007. Shallow lake restoration by nutrient loading reduction-some recent findings and challenges ahead. Hydrobiologia 584: 239–252.
Jeppesen, E., M. Meerhoff, K. Holmgren, I. González-Bergonzoni, F. T. Mello, S. A. J. Declerck, L. De Meester, M. Søndergaard, T. L. Lauridsen, R. Bjerring, J. M. Conde-Porcuna, N. Mazzeo, C. Iglesias, M. Reizenstein, H. J. Malmquist, Z. W. Liu, D. Balayla & X. Lazzaro, 2010. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 646: 73–90.
Jeppesen, E., M. Søndergaard, T. L. Lauridsen, T. A. Davidson, Z. W. Liu, N. Mazzeo, C. Trochine, K. Özkan, H. S. Jensen, D. Trolle, F. Starling, X. Lazzaro, L. S. Johansson, R. Bjerring, L. Liboriussen, S. E. Larsen, F. Landkildehus & M. Meerhoff, 2012. Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Advances in Ecological Research 47: 411–487.
Jin, X. C. & Q. Y. Tu, 1990. The Standard Methods for Observation and Analysis in Lake Eutrophication. Chinese Environmental Science Press, Beijing: 138–272.
Kang, Y., X. Song & Z. Liu, 2013. Sediment resuspension dampens the effect of nutrient inputs on the phytoplankton community: a mesocosm experiment study. Hydrobiologia 710: 117–127.
Köhler, J., S. Hilt, R. Adrian, A. Nicklisch, H. P. Kozerski & N. Walz, 2005. Long-term response of a shallow, moderately flushed lake to reduced external phosphorus and nitrogen loading. Freshwater Biology 50: 1639–1650.
Krienitz, L., P. Kasprzak & R. Koschel, 1996. Long term study on the influence of eutrophication, restoration and biomanipulation on the structure and development of phytoplankton communities in Feldeberger Haussee (Baltic Lake District, Germany). Hydrobiologia 330: 89–110.
Lazzaro, X., 1997. Do the trophic cascade hypothesis and classical biomanipulation approaches apply to tropical lakes and reservoirs? Verhandlungen der Internationale Vereiningung für Limnologie 26: 719–730.
Leah, R. T., B. Moss & D. E. Forrest, 1980. The role of predation in causing major changes in the limnology of a hyper-eutrophic lake. Internationale Revue der Gesamten Hydrobiologie 65: 223–247.
Lewis, W. M. Jr., 2000. Basis for the protection and management of tropical lakes. Lake and Reserviors: Research and Management 5: 35–48.
Li, W. C., Q. X. Yang & W. P. Zhou, 1994. Studies on eutrophication of Wuli Lake and possible restoration strategies. Journal of Lake Sciences 6: 136–143.
Li, W. C., J. Z. Pan, K. L. Chen, Y. H. Hu & Z. W. Liu, 2005. Studies and demonstration engineering on ecological restoration technique in the littoral zone of Lake Dianchi: the target and feasibility. Journal of Lake Sciences 17: 317–321.
Liu, Z. W., 2001. Changes in abundance of the icefish Neosalanx pseudotaihuensis Zhang (Salangidae) and the impact on the zooplankton community of Xujiahe Reservoir, central China. Hydrobiologia 445: 193–198.
Lynch, M., 1980. Aphanizomenon blooms: alternate control and cultivation by Daphnia pulex. American Society of Limnology and Oceanography Special Symposium, Vol. 3. University Press of New England, Hanover, NH: 299–305.
Mataloni, G., G. Tesolín, F. Sacullo & G. Tell, 2000. Factors regulating summer phytoplankton in a highly eutrophic Antarctic lake. Hydrobiologia 432: 65–72.
May, L. & M. O’Hare, 2005. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. Hydrobiologia 546: 397–404.
Meerhoff, M., C. Iglesias, F. Teixeira de Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of contrasting climates and habitat complexity on community structure and predator avoidance behaviour of zooplankton in the shallow lake littoral. Freshwater Biology 52: 1009–1021.
Nanjing Institute of Geography and Limnology (NIGLAS), 1965. Preliminary Report of Comprehensive Investigation in Lake Taihu. Science Press, Beijing.
Nurminen, L., Z. Pekcan-Hekim, S. Repka & J. Horppila, 2010. Effect of prey type and inorganic turbidity on littoral predator–prey interactions in a shallow lake: an experimental approach. Hydrobiologia 646: 209–214.
Sas, H., 1989. Lake Restoration and Reduction of Nutrient Loading: Expectations, Experiences, and Extrapolations. Academia Verlag Richarz, St Augustin.
Scheffer, M., R. Sergio, G. Alessandra, R. M. Luuc & H. van Nes Egbert, 1997. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78: 272–282.
Shao, Z. J., P. Xie & G. Y. Zhu, 2001. Long-term changes of planktonic rotifers in a subtropical Chinese lake dominated by filter-feeding fishes. Freshwater Biology 46: 973–986.
Shen, J. R. & N. S. Du, 1979. Fauna Sinica, Crustacea, Freshwater Copepoda. Science Press, Beijing.
Smith, V. H., 1983. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221: 669–671.
Søndergaard, M., J. P. Jensen, E. Jeppesen & P. H. Møller, 2002. Seasonal dynamics in the concentrations and retention of phosphorus in shallow Danish lakes after reduced loading. Aquatic Ecosystem Health & Management 5: 19–29.
Søndergaard, M., J. P. Jensen & E. Jeppesen, 2005. Seasonal response of nutrients to reduced phosphorus loading in 12 Danish lakes. Freshwater Biology 50: 1605–1615.
Søndergaard, M., L. Liboriussen, A. R. Pedersen & E. Jeppesen, 2008. Lake restoration by fish removal: short- and long-term effects in 36 Danish lakes. Ecosystems 11: 1291–1305.
Søndergaard, M., L. S. Johansson, T. L. Lauridsen, T. B. Jørgensen, L. Liboriussen & E. Jeppesen, 2010. Submerged macrophytes as indicators of the ecological quality of lakes. Freshwater Biology 55: 893–908.
Søndergaard, M., R. Bjerring & E. Jeppesen, 2013. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 710: 95–107.
Sun, S. C. & Y. P. Huang, 1993. Lake Taihu. Ocean Press, Beijing.
Van de Bund, W. J. & E. Van Donk, 2002. Short-term and long-term effects of zooplanktivorous fish removal in a shallow lake: a synthesis of 15 years of data from Lake Zwemlust. Freshwater Biology 47: 2380–2387.
Wang, J. J., 1961. Fauna Sinica, Rotifer. Science Press, Beijing.
Wu, X. W., 1962. Limnological survey of Lake Wuli during 1951. Acta Hydrobiologica Sinica 1: 63–113.
Yang, G. S., R. H. Ma, L. Zhang, J. H. Jiang, S. C. Yao, M. Zhang & H. A. Zeng, 2010. Lake status, major problems and protection strategy in China. Journal of Lake Sciences 22: 799–810.
Zhang, X. Z., H. Y. Hu, X. D. Cao, Y. P. Shen & X. W. Bing, 2010. Spatial-temporal analysis of biodiversity and community structure in fishes in Wulihu Lake. Journal of Dalian Ocean University 25: 314–319.
Zhang, M., J. Yu, H. He, K. Li, F. Chen, B. Guan, Y. Hu, Y. Su, Y. Du & Z. Liu, 2012. Effects of ecological restoration on water quality of Wuli Bay, Lake Taihu. Ecological Science 31: 240–244.
Zhu, X. & Y. W. Zhang, 2009. Control of water pollution in Wuli Lake. Water Resources Protection 25: 86–89.
Acknowledgments
We thank CNERN TLLER for supplying data on physico-chemical parameters and phytoplankton. This study was supported by the National Natural Science Foundation of China (31170440, 41271523), the Key Project of 135 program of Nanjing Institute of Geography and Limnology, the CAS/SAFEA International Partnership Program for Creative Research Teams, the Jiangsu Province Science and Technology Support Program (BE2011820), EU-REFRESH, CLEAR (a Villum Kann Rasmussen Centre of Excellence project), CRES, CIRCE, and the Danish Council for Independent Research: Natural Sciences (272-08-0406).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karl E. Havens
Rights and permissions
About this article
Cite this article
Chen, F., Shu, T., Jeppesen, E. et al. Restoration of a subtropical eutrophic shallow lake in China: effects on nutrient concentrations and biological communities. Hydrobiologia 718, 59–71 (2013). https://doi.org/10.1007/s10750-013-1603-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1603-9