Abstract
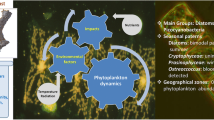
This article summarizes the outcomes of the 16th Workshop of the International Association for Phytoplankton Taxonomy and Ecology. Four major issues dealing with the impact exerted by human activities on phytoplankton were addressed in the articles of this special volume: climate change and its impacts on phytoplankton, the role of land use in shaping composition and diversity of phytoplankton, the importance of autecological studies to fully understand how phytoplankton is impacted by stressors and the role of ecological classification to evaluate community changes due to the different impacts. Case studies from different types of aquatic environments (rivers, deep and shallow lakes, reservoirs, mountain lakes, and temporary ponds) and from diverse geographical locations (not only from the Mediterranean and temperate regions, but also from subtropical and tropical ones) have shown that a complex spectrum of human impacts, not exclusively linked to eutrophication, severely conditions structure and dynamics of phytoplankton assemblage both in the short and long terms. Moreover, the trade-offs between climate change and other human-induced stresses as eutrophication, agricultural and urban land use or water overexploitation contribute to make more severe the impact exerted by humans on phytoplankton and, in turn, on the functioning of aquatic ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Though it accounts for less than 1 % of the photosynthetic biomass on Earth—representing something poorly visible and tangible compared with the terrestrial vegetation—phytoplankton fuels trophic webs and biogeochemical cycling, playing a key role in global climate regulation, C-sequestration and oxygen production (Boyce et al., 2010; McQuatters-Gollop et al., 2011; Winder & Sommer, this volume). Compared with current published estimates for land plants and for coastal vegetation, the production of phytoplankton accounts for nearly 50 % of the global net primary production (Longhurst et al., 1995; Field et al., 1998). Nevertheless, biomass development and phytoplankton composition have a huge impact on water quality, affecting not only the recreational and landscape value of water bodies, but also the availability of drinking water.
Short- and long-term environmental changes in lake ecosystems are closely paralleled by changes in the composition and biomass of phytoplankton communities. Changes most likely result from several overlapping stressors and are measured at the population and community level (at different taxonomical detail), including modifications in biodiversity patterns, phenology, dominance and frequency of algal blooms. Human alterations include the effects due to eutrophication and climate change, as well as other impacts connected with human activities and management, e.g. hydrological alterations (damming of rivers, water overexploitation), introduction of alien species, food–web manipulations, over-fishing, shoreline landscaping, aquatic plant management, dredging, introduction of pollutants among the others. Many examples about the effects of these alterations have been documented in this special issue. However, in the plethora of anthropogenically driven environmental stressors, changes connected with nutrients and climatic dynamics are considered having paramount importance.
At division or class level, a number of long-term ecological investigations and synoptic studies comparing a large number of lakes have emphasized the raising importance of cyanobacteria and, to a lesser extent, diatoms over other algal groups coupled with the increasing phosphorus concentration (Watson et al., 1997). In rivers or in reservoirs, conditions of high-nutrient concentrations associated with the stability of the water column can lead to high increase of algal biomass and huge populations of cyanobacteria (e.g. Naselli-Flores & Barone, 2003). At the other extreme of physical gradients, high flow in large rivers or in reservoirs is detrimental for the development of high-phytoplankton biomasses and cyanobacteria (Salmaso & Braioni, 2008), influencing the community structure and the presence of meroplanktic and metaphytic species (Abonyi et al., this volume).
At species level, the role of trophic state of water bodies as a selective mechanism was investigated during the 11th IAP meeting. Resuming assembly rules, Reynolds et al. (2000) highlighted that nutrient availability represented only one of the environmental dimensions defining algal niches. At the same time, they argued that the mechanisms of species selection remained controversial, expressing a positive perception about the relationship between defined species-clusters and particular physical conditions (Padisák et al., 2010). The utilization of association labels (coda) in the study of phytoplankton ecology underwent a remarkable progress in recent years (Reynolds et al., 2002; Padisák et al., 2009). Contemporarily, the definition of ecological indices based on the trophic preferences of single phytoplankton species or groups received an increasing interest, particularly in Europe, under the demands of EU Water Framework Directive (e.g. Kaiblinger et al., 2009; Ptacnik et al., 2009). Nevertheless, the inability of many indices to track closely the trophic state in different lakes reminds us the hypervolumetric nature of algal niches and the need of more reliable ecology in applied and management-oriented studies.
In the last decade, several investigations documented a significant increase of temperature in marine and inland waters (Dokulil et al., 2006). Knowledge on the effects of warming on phytoplankton is still at an early stage, but it is accelerating (Keller, 2007; Matzinger et al., 2007). Strong effects of warmer years on the development of cyanobacterial biomass have been demonstrated (Paerl & Huisman, 2009), and these results were confirmed by modelling studies, which showed a tradeoff between nutrient loading and temperature, with a dominance of cyanobacteria in water bodies characterized by comparable nutrient loadings but with higher water temperatures (Elliott et al., 2006; Jöhnk et al., 2008). In general, algae capable of buoyancy regulation are favoured by increased water stability and reduced mixing, with important effects on the structure of phytoplankton assemblages (Winder & Hunter, 2008; Tolotti et al., this volume). Further complexity is added by geographic position, lake morphometry and ecological interactions in modulating the climate effects. For example, in northern temperate lakes, an earlier timing of the spring phytoplankton growth is favoured by earlier ice melting, stratification and light availability (Weyhenmeyer et al., 2008). Conversely, it was widely demonstrated that the effects of temperature fluctuations and thermal stability in large deep lakes may involve a cascade of causal factors which include the extent of mixing, the epilimnetic nutrient replenishment and the algal growth (Goldman et al., 1989; O’Reilly et al., 2003; Verburg et al., 2003; Salmaso, 2011). The disentangling of the interactions between the North Atlantic Oscillation and trophic state over Northern and Central Europe was recently resumed by George (2010). The study of the impact of large scale climatic fluctuations in the Mediterranean area, including the southern subalpine lake district, has begun only recently (Salmaso, 2012; Salmaso & Cerasino, this volume).
Climate change
Several contributions in this special issue highlighted the importance of climate fluctuations on phytoplankton structure and development. Climate fluctuations are understood in a broad sense, from high frequency, annual/seasonal changes (Dokulil & Teubner, this volume; Salmaso & Cerasino, this volume; Morabito et al., this volume) to long-term climate trends (Tolotti et al., this volume).
Both Dokulil & Teubner (this volume) and Salmaso & Cerasino (this volume) investigated the effects of large scale atmospheric patterns on the development of phytoplankton in two deep lakes at the northern (Lake Mondsee) and southern (Lake Garda) side of the Alps, respectively. Fluctuations in phytoplankton biovolumes in Lake Mondsee were mostly due to the development of Planktothrix rubescens (de Candolle ex Gomont) Anagnostidis and Komárek. During the stratification months, this species formed deep chlorophyll maxima well below the thermocline at low temperatures and dim light. Decadal changes in the biomass of P. rubescens were controlled by phosphorus availability and eutrophication, while the interannual fluctuations over the baseline long-term trend were, in turn, influenced by the North Atlantic Oscillation, through its effects on the timing of the onset of stratification.
Similarly, in Lake Garda, the interannual fluctuations of cyanobacteria and P. rubescens showed a strong link with the extent of vertical mixing and the upward transport of phosphorus from the hypolimnion to the trophogenic layers. In turn, interannual fluctuations in P-replenishment were strictly controlled by lake and air winter temperature and, ultimately, by the winter fluctuations of large scale atmospheric modes of circulation [East Atlantic (EA) and Eastern Mediterranean Patterns (EMP)] (Salmaso & Cerasino, this volume). In this regard, the EA and EMP could become two emerging and valuable ‘climatic tools’ helpful in explaining the effects of winter climate variability on terrestrial and aquatic ecosystems over the Mediterranean area.
Indirect effects exerted by climate in Lake Maggiore were investigated by Morabito et al. (this volume). Silica, phosphorus, temperature and wind were the key explanatory variables in species selection. Specific climate-linked events driven by deep mixing and floods were shown to increase the Si:P ratio, favouring good-P/poor-Si diatom competitors. Based on these findings, these authors argued that the long-term development of key species, such as Tabellaria flocculosa (Roth) Kützing was driven by climate fluctuations and physical factors rather than nutrient availability.
Long-term effects due to changes in nitrogen and silica availability, and to rising water temperature and thermal stability were documented in Lake Piburger (Tolotti et al., this volume). The increase in nitrogen during the late 1990s was regarded as a major trigger of recent phytoplankton changes, followed by an increase of taxa such as gelatinous species and small centric diatoms able to overcome sinking losses in the more stable environments as represented by the summer epilimnion.
Winder & Sommer (this volume) reviewed the direct and indirect effects of increasing lake temperatures. Direct effects act on physiology and phenology, while indirect effects influence phytoplankton through modifications in water column stratification, availability of nutrients, light and grazing intensity. These modifications favour shifts in phytoplankton composition and structure, with straight consequences for ecosystem functioning.
Based on field and laboratory experiments with phytoplankton populations from an oligotrophic, low-altitude lake in Central Spain, Rojo et al. (this volume) analysed specific effects of climate change affecting PAR and the light spectrum. Under enhanced UVR, phytoplankton biomass was one-third lower than the biomass reached under only PAR due to a lower growth and contribution of autotrophic picoplankton to total biomass.
Implications of environmental conditions, including thermal regime, on the sedimentation and horizontal distribution of phytoplankton were analysed by Yacobi & Ostrovsky (this volume).
Modelling approach
Climate fluctuations at different temporal and spatial scales were instrumental to interpret phytoplankton changes observed in the water bodies analysed so far. A careful selection of explanatory variables and parameters was compulsory in the development of ecological models aimed at determining the factors governing the spatial distribution of P. rubescens in a southern subalpine lake (Lake Pusiano; Carraro et al., this volume). The distribution of the species in this lake was strongly influenced by lake hydrodynamics. Thus, the application of coupled physical/biological models (ELCOM/CAEDYM) showed how the characteristics of this species could be well suited to a re-oligotrophication phase occurring concurrently with the strengthening stratification of a warming climate. However, the use of these models can be affected by the lower performances for nutrient and biological variables, therefore advocating for a more comprehensive, multidisciplinary approach in the definition and development of modelling tools in phytoplankton ecology. This is considered an urgent task, because the integration of simple mechanistic models and the use of complex modelling represent promising ways to improve predictability and a tool to formulate and test hypotheses.
Land use and phytoplankton diversity
Since the development of Vollenweider’s models, it was clear that the use and management of watersheds were deeply connected to water quality. As a consequence, the importance of anthropogenic activities in determining lake and stream water chemistry was unanimously recognized, and several papers have been published since then showing the effects of land use on nutrient enrichment (e.g. Harper & Stewart, 1987). However, the recognition that biodiversity in lakes is influenced by land use in the watershed is relatively new (Hoffman & Dodson, 2005). By studying phytoplankton from an ecological point of view, i.e. as a complex system of mutually interacting populations, rather than as simple chlorophyll a concentration, the impact of land use on biodiversity clearly appears as shown by several papers included in this volume.
In particular, by analysing phytoplankton assemblages in 18 Mediterranean lakes and reservoirs, Katsiapi et al. (this volume) found that despite differences in hydrological regime and morphometric/topographic variables, land use type was strongly correlated with phytoplankton community structure. Moreover, they showed that phytoplankton biomass was significantly higher in water bodies having a watershed with agricultural and artificial land cover exceeding 30 %. This threshold was much lower than what had been set in temperate lakes (Alvarez Cobelas et al., 2005) and it is probably linked to the higher catchment area/lake area ratio experienced by Mediterranean freshwater ecosystems. In addition, the authors highlighted that in Mediterranean reservoirs the effects of land use, although masked by the operational use (e.g. Naselli-Flores, 2003; Naselli-Flores & Barone, 2005), can be even greater than that observed in natural lakes due to the higher catchment area/lake area ratios.
Analogous results were obtained by Paul et al. (this volume) who studied 11 lakes in the Rotorua region (New Zealand). In particular, these authors demonstrated that Cyanoprokaryota were negatively correlated with native forest and positively with pasture, whereas Chlorophyta were positively correlated with native forest and urban land use and negatively with pasture.
Other effects of land use on aquatic ecosystem integrity, and ultimately on phytoplankton structure, were shown by Naselli-Flores & Barone (this volume) who studied phytoplankton dynamics in Mediterranean temporary ponds. These ecosystems can be recognized as aquatic environments only during their water phase which can last from a few days to a few weeks. In the rest of the year, they appear as land depressions perfectly suitable to be filled up with garbage or to be appointed for agricultural and urban development. In addition, due to their small dimensions, they are highly subjected to pollution by fertilizers, pesticides or garbage, to water overexploitation and/or to deepening for conversion into permanent water bodies to fulfil irrigation needs.
Overexploitation of water resources and land use changes are also important in large lakes. As shown by Zohary et al. (this volume), the disappearing of the recurrent Peridinium gatunense Nygaard spring bloom in Lake Kinneret is due to a modification in the amount of water from its main inflow, the Jordan River. The seasonal patterns of phytoplankton in this lake and the very regular spring bloom of its flagship dinoflagellate are famous among phytoplankton ecologists as a paradigm of the seasonal succession of phytoplankton in lakes. A significant reduction in the amount of fresher waters from Jordan River to fulfil agricultural needs resulted in the interruption of the regular appearance of P. gatunense in Lake Kinneret from 1996 onward.
Analogously, modifications in the hydrological cycle were shown to be effective in explaining the interannual fluctuation of cyanobacterial blooms and their different distribution in side-arms of a large, dendritic reservoir by O’Farrell et al. (this volume).
Water regulation of freshwater ecosystems can deeply interfere with phytoplankton structure and dynamics as shown by Abonyi et al. (this volume). These authors analysed data collected in the River Loire. The catchment of this river covers almost 20 % of France and is the most extended among the European rivers flowing into the Atlantic Ocean. Several reservoirs interrupt the water flowing and interfere with potamoplankton dynamics by releasing lacustrine species into the river waters. In addition, urban and industrial wastewaters reaching the river contribute to further alter the composition of the phytoplankton assemblages.
Autoecology
Identifying phytoplankton is a time consuming work. Moreover, the reliability of identification (at least for those organisms which can be recognized without genetic analyses) is supported by the experience of the operator which, in turn, is based on the amount of time spent observing phytoplankton at the microscope. In addition, methodological correctness is of paramount importance since observations carried out on fixed material not always allow to correctly identify microalgae. On the other hand, live samples cannot be easily managed and maintained when routine monitoring is performed. Apart the aforementioned skills, all those dealing professionally with phytoplankton cannot avoid to have a good knowledge on autoecology of species. Achieving this kind of knowledge is a fundamental support to the correct identification of microalgae. At the same time, a clear account of the environmental conditions at the time of species collection (and even during the weeks before collection) can provide a strong help in species identification.
Knowledge on autoecology is also important when species have to be attributed to functional groups (coda). This work cannot be done a priori and requires some caution. As an example, it cannot be easy for an inexperienced observer to identify Aulacoseira or Cyclotella at species level. However, knowledge on the autoecological requirements of the different species can help in attributing the organism to the correct codon and in reducing the possibilities of species misinterpretation. Thus, if Aulacoseira is dominating in poor light conditions, it will likely belong to the granulata species rather than to the italica or to the ambigua ones. In the same way, Cyclotella comensis is unlikely to occur in eutrophic waters where most probably C. meneghiniana will be present.
Autoecology of selected phytoplankton species was investigated in six papers of this volume. Even for very well-known species, autoecological studies may provide new insights that can be helpful to better understand the dynamics and distribution of planktic algae. This was demonstrated by Zohary et al. (this volume) who found that the occurrence of P. gatunense in Lake Kinneret depends on a yet unknown microelement or/and an organic compound which reach the lake from the Hula valley during flood periods.
Dokulil & Teubner (this volume) presented an almost exhaustive account on deep living P. rubescens which confirms the preference of this organism for low temperature and dim light. Moreover, these authors argue that survival of P. rubescens during stratified period is possible because of heterotrophic subsistence and that life below the thermocline is aided by physiological acclimation of photosynthesis and buoyancy regulation. Analogous findings on metalimnetic adaptations are shown in the paper by Carraro et al. (this volume).
Adaptation to dim light was also investigated by Üveges et al. (this volume), who studied the photosynthetic characteristics and physiological plasticity of Aphanizomenon flos-aquae (L.) Ralfs blooming in winter under the ice cover of Lake Stechlin. The study underlined the importance of trade-off among different traits in Cyanobacteria. In particular, the authors showed that temperature optimum for growth depended on light intensity. At low light (7.5 μmol m−2 s−1), the temperature optimum was found at 2°C, allowing A. flos-aquae to bloom under the ice cover.
Adaptation to low temperature implies the existence of peculiar metabolic pathways and a well-defined composition of metabolites. Flaim et al. (this volume) studied the changes in galactolipid composition of the cold-adapted dinoflagellate Borghiella dodgei Moestrup, Hansen & Daugbjerg in response to temperature. Galactolipids are a class of molecules which constitute one of the structural components of the thylakoid membrane in eukaryotic chloroplasts. As shown by the authors, the relative abundance of the different galactolipids changes in response to temperature in order to ensure to optimal fluidity to thylakoid membrane and to allow the correct functioning of the photosynthetic systems. This allows Borghiella to outcompete other algae when water temperature is between 3 and 5°C.
Stoyneva et al. (this volume) investigated the causes of the phenotypic variability of Tetraëdron minimum Kützing ex Korshikov isolated from Lake Kivu. In this lake, T. minimum was characterized by an unusual ‘lemon shape’ instead of the typical polygonal one. The authors carried out a detailed investigation on the morphological and ecological features of this species and suggested that the peculiar lemon shape of T. minimum represented a defense against grazing by small zooplankton. Actually larger zooplankton disappeared from the lake because of the introduction of a planktivorous fish; this caused a disproportionate increase of small-bodied herbivores and an increase in the grazing pressure on T. minimum which stimulated the occurrence of the ‘lemon shape’.
Functional group approach
Increasing need for substitute traditional taxonomic approach to understand phytoplankton patterns has recently resulted in the emergence of three approaches of morpho-functional classifications (Reynolds et al., 2002; Salmaso & Padisák, 2007; Kruk et al., 2010). All these methods are in the focus of the papers in this volume. Here we partition the discussion on functional grouping to the following sections: notes on nomenclature, modifications in and additions to the three classifications, comparisons, use for diversity analysis of community structure and ecological status assessment.
Notes on the nomenclature
There is no written consensus on abbreviation of these functional approaches. In order to avoid the future confusion, the authors of this article advise to remain consequent to the following form:
-
functional group concept by Reynolds et al. (2002)—FG
-
morpho-functional group concept by Salmaso & Padisák (2007)—MFG and
-
the morphology-based functional group concept by Kruk et al. (2010)—MBFG
For example, the MFG abbreviation in Stanković et al. (this volume) should be read as MBFG.
Modifications in and additions to the three methods
The FG method underwent a number of modifications earlier [see summary by Padisák et al., (2009)].
Concerning the MFG, Tolotti et al. (this volume) suggested to include another category (6c-ColoPenn) into the original group 6 (large diatoms) for chain-forming pennatae species since nutrient and climate-related long-term changes in phytoplankton of the Piburger See, Austria, was possible to handle only with the separation of large Pennales into chain-forming and single-celled species. This addition seems very reasonable since shape, size and complexity (and even symmetry) highly determine competitive performance of species (Padisák et al., 2003; Naselli-Flores et al., 2007; Naselli-Flores & Barone, 2011).
With detailed statistical analyses on a database comprising data of 711 species from 925 samples taken in 211 lakes, Kruk & Segura (this volume) clarified some environmental variables as main driving forces leading to dominance of MBFGs. They found that for Group I (small organisms with high S/V), TP and TN were the most important variables. For Group II (small flagellated organisms with siliceous exoskeletal structures), no clear environmental factors could be selected because of low-explained variation. Probably the group is too complex, because, for example, the Dinobryon visualized on Fig. 1 in the paper by Kruk & Segura (this volume), though have loricae, is not a siliceous flagellate. For Group III (large filaments with aerotopes), light attenuation and TP proved to be the main drivers. According to these authors, TN and TZ (total zooplankton) were equally important for Groups IV (organisms of medium size lacking specialized traits), V (unicellular flagellates) and VI (non-flagellated organisms with siliceous exoskeletons), additionally temperature proved to be an important driver for both Groups V and VI. SRSi as a main explanatory variable for group VII (large colonies with mucilage) was supposed to be, as pointed out by the authors clearly, a proxy of catchment properties since these species do not have direct silica demand.
Comparison of approaches
So far, only few papers addressed the comparison of the above three grouping methods or at least their pair wise comparisons. Izaguirre et al. (this volume) applied all the three methods to phytoplankton of three different types of pampa lakes (clear vegetated, phytoplankton turbid and inorganically turbid). As a major result, they demonstrated that all the three classification systems separated the clear and the turbid lakes. One of the disadvantages of the MFBG approach with respect to the other two methods was its incapability of discrimination of potentially mixotrophic and non-mixotrophic flagellates having different representation in the studied pampa lakes (both in Group V). Though Kruk et al. (2011) argued that species in any particular group are basically interchangeable, this was not the case in this example and Kruk and Segura (this volume) also commented on this particular point of the MBFG grouping. The other weakness of the MBFG was its low sensitivity to light conditions (KdPAR) which (together with mixing depth) are key drivers of phytoplankton species selection in lakes (Zohary et al., 2010).
All the three grouping systems were developed for lakes, and in their original descriptions, application for river phytoplankton was either considered (FG; Reynolds et al., 2002) or at least not excluded (Salmaso & Padisák, 2007; Kruk et al., 2010). Stanković et al. (this volume) compared the FG and MFBG groupings on large lowland rivers. Since diatoms typically dominate in these ecosystems, the MFBG approach with its single group for all siliceous non-flagellated species failed to discriminate between eu- and tychoplanktonic diatoms which are crucial in evaluation of riverine phytoplankton (Borics et al., 2007) unlike the FG concept that allows clear discrimination.
Diversity approach
Phytoplankton diversity patterns along with their environmental drivers have been a recurrent research subject (Reynolds et al., 1993; Sommer et al., 1993). Recently, diversity is understood at a much more complex way than the simple compositional diversity as it was treated earlier. The morpho-functional approaches (any of the three methods) have the potential to assess functional diversity of phytoplankton as challenged by Borics et al. (this volume). The achievement of this approach has been manifold. These authors in accordance with what was found for the development of phytoplankton equilibrium patterns (Naselli-Flores et al., 2003) explored that in biomass ranges > 20 mg L−1 only some FGs (H1, SN, M, WS, J, Lo) were able to develop dominance, and if so, a sharp decline in diversity can be predicted. The unusual behaviour of bloom-forming cyanobacteria (H1, SN, M) was demonstrated by their ‘deviant’ behaviour from what can be theoretically expected: the maximum diversity occurs when relative abundance of these groups is zero. In other words, it means that even an occurrence of these species in the flora results in an immediate drop in diversity probably due to their invasive nature. They attributed this behaviour to the strong competitiveness of these species for light and to certain other features like (for WS) mixotrophy. This study partly explains why just relationships to light and potential mixotrophy were identified as weakness of the MFBG approach (Izaguirre et al., this volume; Kruk & Segura, this volume).
Ecological status assessment
Of the three morpho/functional grouping methods, the FG was developed further to be used as a metrics in the Water Framework Directive (European Parliament and Council, 2000) by developing the Q (Padisák et al., 2006) and the QR (Borics et al., 2007) indices. Performance of the QR indices was tested annually and on whole river stretch scale on River Loire (Abonyi et al., this volume). Apart that the FG classification allowed following both seasonal and spatial changes in phytoplankton assemblages of River Loire, it proved to be suitable to detect interruptions of the river continuum (Vannote et al., 1980) by reservoirs and large inflows and, moreover, alterations at lower sections by non-point nutrient loads due to agricultural land use. This analysis represents a significant addition to the River Continuum Concept.
Abonyi et al. (this volume) call the attention to some limitations of the QR index, namely that it does not discriminate between natural and human-affected benthic diatom dominance and the difficulty in sorting diatoms to B-C-D coda in monitoring practice. Implications for WFD are also mentioned in Borics et al. (this volume) emphasizing that the threshold value of 20 mg l−1 algal biomass indicated by diversity patterns corresponds to the poor ecological quality threshold for German shallow lakes (Mischke et al., 2002).
References
Abonyi, A., M. Leitão, A. M. Lançon & J. Padisák, this volume. Phytoplankton functional groups as indicators of human impacts along the River Loire (France). Hydrobiologia. doi:10.1007/s10750-012-1130-0.
Alvarez Cobelas, M., C. Rojo & D. Angeler, 2005. Mediterranean limnology: current status, gaps and the future. Journal of Limnology 64: 13–29.
Borics, D., G. Várbíró, I. Grigorszky, E. Krasznai, S. Szabó & K. T. Kiss, 2007. A new evaluation technique of potamoplankton for assessment the ecological status of rivers. Archiv für Hydrobiologie, Supplement Large Rivers 161: 465–486.
Borics, G., B. Tóthmérész, B. A. Lukács & G. Várbíró, this volume. Functional groups of phytoplankton shaping diversity of shallow lake ecosystems. Hydrobiologia. doi:10.1007/s10750-012-1129-6.
Boyce, D. G., M. R. Lewis & B. Worm, 2010. Global phytoplankton decline over past century. Nature 466: 591–596.
Carraro, E., N. Guyennon, D. Hamilton, L. Valsecchi, E. C. Manfredi, G. Viviano, F. Salerno, G. Tartari & D. Copetti, 2012. Coupling high-resolution measurements to a three-dimensional lake model to assess the spatial and temporal dynamics of the cyanobacterium Planktothrix rubescens in a medium-sized lake. Hydrobiologia. doi:10.1007/s10750-012-1096-y.
Dokulil, M. & K. Teubner, this volume. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. Hydrobiologia. doi:10.1007/s10750-012-1020-5.
Dokulil, M. T., A. Jagsch, G. D. George, O. Anneville, T. Jankowsky, B. Wahl, B. Lenhart, T. Blenckner & K. Teubner, 2006. Twenty years of spatially coherent deepwater warming in lakes across Europe related to the North Atlantic Oscillation. Limnology and Oceanography 51: 2787–2793.
Elliott, J. A., I. D. Jones & S. J. Thackeray, 2006. Testing the sensitivity of phytoplankton communities to changes in water temperature and nutrient load, in a temperate lake. Hydrobiologia 559: 401–411.
European Parliament and Council, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, PE-CONC 3639/1/00, REV 1, ENV 221, CODEC 513, Brussels: 152 pp.
Field, C. B., M. J. Behrenfeld, J. T. Randerson & P. Falkowski, 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281: 237–242.
Flaim, G., U. Obertegger & G. Guella, this volume. Changes in galactolipid composition of the cold freshwater dinoflagellate Borghiella dodgei in response to temperature. Hydrobiologia. doi:10.1007/s10750-012-1070-8.
George, G. (ed.), 2010. The Impact of Climate Change on European Lakes. Springer, Dordrecht.
Goldman, C. R., A. D. Jassby & T. Powell, 1989. Interannual fluctuations in primary production: meteorological forcing at two subalpine lakes. Limnology and Oceanography 34: 310–323.
Harper, D. M. & W. D. P. Stewart, 1987. The effects of land use upon water chemistry, particularly nutrient enrichment, in shallow lowland lakes: comparative studies of three lochs in Scotland. Hydrobiologia 148: 211–229.
Hoffman, M. D. & S. I. Dodson, 2005. Land use, primary productivity, and lake area as descriptors of zooplankton diversity. Ecology 86: 255–261.
Izaguirre, I., L. Allende, R. Escaray, J. Bustingorry, G. Pérez & G. Tell, this volume. Comparison of morpho-functional phytoplankton classifications in human-impacted shallow lakes with different stable states. Hydrobiologia. doi:10.1007/s10750-012-1069-1.
Jöhnk, K. D., J. Huisman, J. Sharples, B. Sommeijer, P. M. Visser & J. M. Stroom, 2008. Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biology 14: 495–512.
Kaiblinger, C., O. Anneville, R. Tadonleke, F. Rimet, J. C. Druart, J. Guillard & M. T. Dokulil, 2009. Central European water quality indices applied to long-term data from peri-alpine lakes: test and possible improvements. Hydrobiologia 633: 67–74.
Katsiapi, M., A. D. Mazaris, E. Charalampous & M. Moustaka-Gouni, this volume. Watershed land use types as drivers of freshwater phytoplankton structure. Hydrobiologia. doi:10.1007/s10750-012-1095-z.
Keller, W., 2007. Implications of climate warming for Boreal Shield lakes: a review and synthesis. Environmental Review 15: 99–112.
Kruk, C. & A. M. Segura, this volume. The habitat template of morphology-based functional groups. Hydrobiologia. doi:10.1007/s10750-012-1072-6.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. Lürling, C. S. Reynolds & M. Scheffler, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Kruk, C., E. T. H. M. Peeters, E. H. van Nes, V. L. M. Huszar, L. S. Costa & M. Scheffler, 2011. Phytoplankton community structure can be predicted best in terms of morphological groups. Limnology and Oceanography 56: 110–118.
Longhurst, A., S. Sathyendranath, T. Platt & C. Caverhill, 1995. An estimate of global primary production in the ocean from satellite radiometer data. Journal of Plankton Research 17: 1245–1271.
Matzinger, A., M. Schmid, E. Veljanoska-Sarafiloska, S. Patcheva, D. Guseska, B. Wagner, B. Müller, M. Sturm & A. Wüest, 2007. Eutrophication of ancient Lake Ohrid: global warming amplifies detrimental effects of increased nutrient inputs. Limnology and Oceanography 52: 338–353.
McQuatters-Gollop, A., P. C. Reid, M. Edwards, P. H. Burkill, C. Castellani, S. Batten, W. Gieskes, D. Beare, R. R. Bidigare, E. Head, R. Johnson, M. Kahru, J. A. Koslow & A. Pena, 2011. Is there a decline in marine phytoplankton? Nature 472: E6–E7.
Mischke, U., B. Nixdorf, E. Hohn & U. Riedmüller, 2002. Möglichkeiten zu Bewertung von Seen anhand des Phytoplanktons. Aktueller Stand in Deutschland. Aktuelle Reihe %/02: 25-37, BTU, Cottbus.
Morabito, G., A. Oggioni & M. Austoni, this volume. Resource ratio and human impact: how diatom assemblages in Lake Maggiore responded to oligotrophication and climatic variability. Hydrobiologia. doi:10.1007/s10750-012-1094-0.
Naselli-Flores, L., 2003. Man-made lakes in Mediterranean semi-arid climate: the strange case of Dr Deep Lake and Mr Shallow Lake. Hydrobiologia 506(509): 13–21.
Naselli-Flores, L. & R. Barone, 2003. Steady-state assemblages in a Mediterranean hypertrophic reservoir: the role of Microcystis ecomorphological variability in maintaining an apparent equilibrium. Hydrobiologia 502: 133–143.
Naselli-Flores, L. & R. Barone, 2005. Water-level fluctuations in Mediterranean reservoirs: setting a dewatering threshold as a management tool to improve water quality. Hydrobiologia 548: 85–99.
Naselli-Flores, L. & R. Barone, 2011. Fight on plankton! Or, phytoplankton shape and size as adaptive tools to get ahead in the struggle for life. Cryptogamie Algologie 32: 157–204.
Naselli-Flores, L. & R. Barone, this volume. Phytoplankton dynamics in permanent and temporary Mediterranean waters: is the game hard to play because of hydrological disturbance? Hydrobiologia. doi:10.1007/s10750-012-1059-3.
Naselli Flores, L., J. Padisák & M. Albay, 2007. Shape and size in phytoplankton ecology: do they matter? Hydrobiologia 578: 157–161.
Naselli-Flores, L., J. Padisák, M. T. Dokulil & I. Chorus, 2003. Equilibrium/steady-state concept in phytoplankton ecology. Hydrobiologia 502: 395–403.
O’Farrell, I., F. Bordet & G. Chaparro, this volume. Bloom forming cyanobacterial complexes co-occurring in a subtropical large reservoir: validation of dominant eco-strategies. Hydrobiologia. doi:10.1007/s10750-012-1102-4.
O’Reilly, C. M., S. R. Alin, P. Plisnier, A. S. Cohen & B. A. McKee, 2003. Climate change decreases aquatic eco-system productivity of Lake Tanganyika, Africa. Nature 424: 766–768.
Padisák, J., É. Soróczki-Pintér & Zs. Rezner, 2003. Sinking properties of some phytoplankton shapes and relation of form resistance to morphological diversity of plankton – an experimental study. Hydrobiologia 500: 243–257.
Padisák, J., I. Grigorszky, G. Borics & É. Soróczki-Pintér, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblage index. Hydrobiologia 553: 1–14.
Padisák, J., L. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Padisák, J., É. Hajnal, L. Naselli Flores, M. T. Dokulil, P. Nõges & T. Zohary, 2010. Convergence and divergence in organization of phytoplankton communities under various regimes of physical and biological control. Hydrobiologia 639: 205–220.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental and Microbiological Report 1: 27–37.
Paul, W. J., D. P. Hamilton, I. Ostrovsky, S. D. Miller, A. Zhang & K. Muraoka, 2012. Catchement land use and trophic state impacts on phytoplankton composition: a case study from the Rotorua lake’s district, New Zealand. Hydrobiologia. doi:10.1007/s10750-012-1147-4.
Ptacnick, R. A., A. G. Solimini & P. Brettum, 2009. Performance of a new phytoplankton composition metric along a eutrophication gradient in Nordic lakes. Hydrobiologia 633: 75–82.
Reynolds, C. S., J. Padisák & U. Sommer, 1993. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: a synthesis. Hydrobiologia 249: 183–188.
Reynolds, C., M. Dokulil & J. Padisák, 2000. Understanding the assembly of phytoplankton in relation to the trophic spectrum: where are we now? Hydrobiologia 424: 147–152.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rojo, C., G. Herrera, M. A. Rodrigo, M. J. Ortíz-Llorente & P. Carrillo, this volume. Mixotrophic phytoplankton is enhanced by UV radiation in a low altitude, P-limited Mediterranean lake. Hydrobiologia. doi:10.1007/s10750-012-1214-x.
Salmaso, N., 2011. Interactions between nutrient availability and climatic fluctuations as determinants of the long term phytoplankton community changes in Lake Garda, Northern Italy. Hydrobiologia 660: 59–68.
Salmaso, N., 2012. Influence of atmospheric modes of variability on the limnological characteristics of a deep lake south of the Alps. Climate Research 51(125–133): 2012.
Salmaso, N. & M. Braioni, 2008. Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Italy (River Adige). Aquatic Ecology 42: 533–545.
Salmaso, N. & L. Cerasino, this volume. Long-term trends and fine year-to-year tuning of phytoplankton in large lakes are ruled by eutrophication and atmospheric modes of variability. Hydrobiologia. doi:10.1007/s10750-012-1068-2.
Salmaso, N. & J. Padisák, 2007. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Sommer, U., J. Padisák, C. S. Reynolds & P. Juhász-Nagy, 1993. Hutchinson’s heritage: the diversity-disturbance relationship in phytoplankton. Hyrdobiologia 249: 1–8.
Stanković, I., T. Vlahović, M. Gligora Udivič, G. Várbíró & G. Borics, this volume. Phytoplankton functional and morpho-functional approach in large floodplain rivers. Hydrobiologia. doi:10.1007/s10750-012-1148-3.
Stoyneva, M. P., J.-P. Descy, V. Balagué, P. Compère, M. Leitao & H. Sarmento, this volume. The queer Tetraëdron minimum from Lake Kivu (Eastern Africa): is it a result of a human impact? Hydrobiologia. doi:10.1007/s10750-012-1092-2.
Tolotti, M., H. Thies, U. Nickus & R. Psenner, this volume. Temperature modulated effects of nutrients on phytoplankton changes in a mountain lake. Hydrobiologia. doi:10.1007/s10750-012-1146-5.
Üveges, V., K. Tapolczai, L. Krienitz & J. Padisák, this volume. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia. doi:10.1007/s10750-012-1103-3.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 24: 277–304.
Verburg, P., R. E. Hecky & H. Kling, 2003. Ecological consequences of a century of warming in Lake Tanganyika. Science 301: 505–507.
Watson, S. B., E. McCauley & J. A. Downing, 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnology and Oceanography 42: 487–495.
Weyhenmeyer, G. A., A.-K. Westöö & E. Willen, 2008. Increasingly ice-free winters and their effects on water quality in Sweden’s largest lakes. Hydrobiologia 599: 111–118.
Winder, M. & D. A. Hunter, 2008. Temporal organization of phytoplankton communities linked to physical forcing. Oecologia 156: 179–192.
Winder, M. & U. Sommer, this volume. Phytoplankton response to a changing climate. Hydrobiologia. doi:10.1007/s10750-012-1149-2.
Yacobi, Y. Z. & I. Ostrovsky, this volume. Sedimentation of phytoplankton: role of ambient conditions and life strategies of algae. Hydrobiologia. doi:10.1007/s10750-012-1215-9.
Zohary, T., J. Padisák & L. Naselli-Flores, 2010. Phytoplankton in the physical environment: beyond nutrients, at the end, there is some light. Hydrobiologia 639: 261–269.
Zohary, T., A. Nishri & A. Sukenik, this volume. Present-absent: a chronicle of the dinoflagellate Peridinium gatunense from Lake Kinneret. Hydrobiologia. doi:10.1007/s10750-012-1145-6.
Acknowledgments
This paper got support from the CENTRAL EUROPE Programme (European Lakes Under Environmental Stressors, 2CE243P3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: N. Salmaso, L. Naselli-Flores, L. Cerasino, G. Flaim, M. Tolotti & J. Padisák / Phytoplankton responses to human impacts at different scales: 16th workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP)
Rights and permissions
About this article
Cite this article
Salmaso, N., Naselli-Flores, L. & Padisák, J. Impairing the largest and most productive forest on our planet: how do human activities impact phytoplankton?. Hydrobiologia 698, 375–384 (2012). https://doi.org/10.1007/s10750-012-1253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1253-3