Abstract
The metamorphosis of sea lamprey (Petromyzon marinus Linnaeus, 1758) allows young postmetamorphic individuals to migrate to the sea and start the hematophagous feeding. However, the information about this phase is very limited, especially for European populations. Herein, we provide for the first time a comprehensive study on the phenology of downstream migration, the timing and location of first feeding and the prey species in the River Ulla and its estuary (NW Spain). Results show that downstream migration occurs between October and May with a peak in March. At least for a part of the postmetamorphic lampreys this migration stops for several months when they reach the estuary, where lampreys find shelter and abundant food, before moving to coastal waters. Hematophagous feeding in the estuary allows postmetamorphics to increase their total length and weight exponentially. Our results also suggest that part of the postmetamorphics (10–30%) start the hematophagous feeding in the river, with a special affinity for anadromous species, probably because of their larger size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sea lamprey (Petromyzon marinus, Linnaeus 1758) is an anadromous species considered as ‘Vulnerable’ in Europe, listed on Annex II of the EU Habitats Directive and Annex III of the Bern Convention. It was classified as ‘Least Concern’ by IUCN in 2008 (Freyhof & Kottelat, 2008). Contrastingly, in the Great Lakes of North America a landlocked form of the sea lamprey, non-native and considered a pest, causes tremendous damage to fish stocks and the expenditure of large sums of money in their control (Berra, 2001).
Ammocoetes of this species spend from 3 to 8 years in freshwater habitats, where they are filter feeders and live burrowed in fine sediment (Beamish & Potter, 1975; Quintella et al., 2003; Taverny et al., 2005). After this period, the larvae undergo a metamorphosis that allows young postmetamorphic lampreys to migrate to the sea and start the hematophagous feeding. Metamorphosis is a highly synchronised event during which lampreys spend between 4 and 10 months without feeding (Potter, 1980; Youson, 1980). The long fasting period implies that this is a critical stage for survival (Swink, 2003), and it is very important for postmetamorphics to obtain a food source as soon as possible in order to allow the recovery of lost energy reserves. Therefore, postmetamorphics are expected to have a benefit from early feeding either by moving downstream and feeding in estuarine or coastal waters or by early feeding in river.
The most detailed studies on the postmetamorphic phase and hematophagous feeding have been made for the populations of North America (Davis, 1967; Potter & Beamish, 1977; Beamish, 1980; Farmer, 1980). In contrast, we know very little about the downstream migrations of the Atlantic sea lamprey in European rivers (Hardisty, 2006) and about the prey species during the hematophagous feeding stage (Nichols & Tscherter, 2011). Moreover, information from isolated works and comments from anglers suggests the existence of hematophagous feeding in the river for European populations (Hardisty & Potter, 1971; Bird et al., 1994; Maitland et al., 1994; Taverny & Elie, 2009). However, the occurrence of these feeding lampreys in rivers is assumed to result from attacks made on migrating teleosts in marine or estuarine waters (Bird et al., 1994). For North American populations Davis (1967) and Potter & Beamish (1977) claim that some individuals start feeding in the river before migrating downstream, especially those who migrate at the end of migration period.
The aim of this article is to contribute to the knowledge of the postmetamorphic stage of anadromous sea lamprey populations with the following objectives: corroborate the existence and start of hematophagous feeding in freshwater; identify the species that are part of the first diet of postmetamorphics and characterize the downstream migration phase, defining the period of migration and the role of the river and estuary during this phase.
Materials and methods
Study area
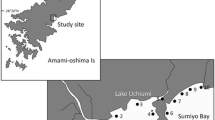
The study area was located in the River Ulla (Galicia, NW Spain) (total length of 132 km, 2,803 km2). It drains into the Atlantic Ocean through the Ría de Arousa in southwest direction. The length accessible in the main channel is 80 km, limited by the presence of a dam for hydroelectric production (UTM: 29T 561986E 4742078N), which is the first impassable barrier for anadromous species.
Data were collected in seven locations, three in the estuary-ría (E1, UTM: 29T 522247E 4723949N; E2, UTM: 29T 518953E 4716224N; E3, UTM: 29T 519039E 4715707N) and four in the river (R1, UTM: 29T 543849E 4732719N; R2, UTM: 29T 536983E 4734224N; R3, UTM: 29T 533201E 4732456N; R4, UTM: 29T 530642E 4731060N) 40, 30, 24 and 20 km away from the mouth, respectively (Fig. 1). In order to facilitate the interpretation of results, sampling locations established in the estuary and the ría are dealt together and we will refer to them as belonging to the estuary.
Map of the sampling area in River Ulla and its estuary. ( ) First insurmountable obstacle for migratory species in the main channel of the River Ulla. The area in the estuary (dashed line) was prospected for the presence of postmetamorphic individuals attached to prey. Areas (dotted line) showed high concentration of postmetamorphic individuals
) First insurmountable obstacle for migratory species in the main channel of the River Ulla. The area in the estuary (dashed line) was prospected for the presence of postmetamorphic individuals attached to prey. Areas (dotted line) showed high concentration of postmetamorphic individuals
Field work
This article is based on the use of various source of data on different life stages of P. marinus (transformers, postmetamorphic individuals in the river during their downstream migration and postmetamorphics feeding in the river and feeding in the estuary) obtained by using different methodologies. In addition, we captured and made observations on potential prey species in the river and estuary. The following section provides more detailed information about data collected, their purpose and the methodology used in each case.
River
The methodology for the capture of lampreys was different in each sampling point. R1 corresponds to the fixed trap located in the village of Ximonde, with two traps for upstream migrators (trap ‘V’ on a scale of troughs and one in a Denil-type scale with side baffles) and a smolt trap (through a set of horizontal and vertical grids that steer the fish to a channel ending in a cage trap). In the upstream traps anadromous fishes were captured during their spawning migration, so we could confirm the presence of postmetamorphics feeding on them (direct visualization of the postmetamorphic attached or identification of the relevant injury). Thus, 190 Salmo salar Linnaeus, 1758 and 69 Salmo trutta Linnaeus, 1758 (anadromous form) were examined between March and October 2011 (data provided by staff of Ximonde). In addition, the smolt trap allowed us to capture 182 lampreys during their downstream migration between April and May 2011 (PMd) (due to some technical problems, the trap was not operational during the previous months of the year). We also made use of historical data of lampreys captured during their downstream migration (PMdh), corresponding to a total of 6,839 individuals captured between 1997 and 2010 (data provided by staff of Ximonde). PMd and PMdh data were not used as a whole because PMd only correspond to the final part of the migration period. PMdh data were used to characterize the migration period and to study the variation in the biometric characteristics throughout this time. The PMdh captured in September and June were not used for statistical analysis as there were only sporadic captures. The effectiveness of the smolt trap was analysed by Caballero et al. (2006) between 1998 and 2003. Downstream catch efficiency was low but its variation was small, with an efficiency that remained between 8 and 12%. Therefore, we consider that the data from this trap are robust and reliable and provide a true picture of the pattern of migration of postmetamorphic lampreys. Both PMd and PMdh were compared with transformers and with postmetamorphics captured feeding on different prey species in the river (PMfr), in order to confirm the hypothesis of the initiation of hematophagous feeding in the river.
R2 is a spawning area of S. salar, S. trutta (resident and anadromous form) and P. marinus. In this locality, we made sporadic observations of postmetamorphics attached to some of the mentioned species between January and April 2012. The purpose of these observations was to identify other potential prey species, particularly the observation of postmetamorphic lampreys attached to resident individuals of S. trutta, which will support the hypothesis of the onset of hematophagous feeding in freshwater.
R3 and R4 correspond to deep areas we know are seating areas for adults of the species Alosa fallax (Lacépède, 1803). Thus, 89 adults of A. fallax migrating to their spawning grounds were captured in total (83 in R3 and 6 in R4) between April and June 2011 and examined to confirm the presence of postmetamorphics or corresponding wounds. We used trammel nets 40 m long, 3 m high with a mesh size of 7–10 cm. Furthermore, in R3 we captured 72 lampreys in the stage of metamorphosis, also called transformers, by electrofishing in September 2010. Transformers were identified according to criteria established by Youson & Potter (1979). They were used as control because lampreys do not feed during metamorphosis, so the larger or smaller sizes of postmetamorphics compared to the maximum and minimum values recorded for the transformers are indicative of the onset of hematophagous feeding or continuation of fasting.
Estuary
The samplings in the estuary were conducted on a weekly basis, between January and June for 4 years (2008–2011). We captured a total of 343 postmetamorphic lampreys (6 in 2008, 83 in 2009, 25 in 2010 and 229 in 2011) feeding in the estuary (PMfe) on the euryhaline species Liza aurata (Risso, 1810). This species, like other Mugilidae, perform periodic motions accompanying the tide, and approach the coast with the rise of the tide (Almeida, 1996), allowing the capture of individuals of L. aurata and postmetamorphics attached to them. This served us to corroborate that lampreys feed on this species, as well as to assess the role of this habitat during this phase by comparing the postmetamorphics caught in the same month in the estuary and river. Hand, seine and gill nets were used. For the biometric characterization of prey, we used a sample of 30 of these fish captured in 2009.
The estuary was visited on a monthly basis for the observation and identification of prey species of the sea lamprey, the identification of areas and periods of higher abundance of prey with attached lampreys, particularly L. aurata, and to find out the period in which they can be observed. This provided complementary information on the role of the estuary at this life stage of sea lamprey and further facilitated the capture of the specimens.
Data analysis
Weight (W) (±1 g) and total length (TL) for lampreys and furcal length (FL) for the rest of species (±1 mm) were recorded. The condition factor (CF) was calculated as 106 W (g)/TL3 (mm) (Henson et al., 2003) for lampreys, and as 100 W (g)/FL3 (cm) for the rest of species (White & Fletcher, 1985).
For comparison of the TL, W and CF of different groups of lampreys (transformers, PMd, PMdh, PMfr and PMfe) the nonparametric Mann–Whitney U test was used because the data did not conform to normality. Comparisons were made in total and by months. For all analyses significance tests below 0.05 indicate significant differences. Statistical analyses were performed using PASW Statistics 18.0 software.
Results
River
Migration
In the River Ulla PMdh were captured during their downstream migration between October and May, with some sporadic catches in September and June (Fig. 2). Most of the PMdh captured in R1 (81.2%) migrated between January and April, with a maximum of 33.4% in March. This peak varied depending on the year, both in the amount of PMdh and in the month in which it occurred. On average, at the end of February 50% of the population of PMdh had already migrated.
The month in which PMdh were more abundant was March 2005, with a total of 2,125 captured lampreys. In this month, lampreys were captured daily with a mean of 60 ± 12.4 ind/day (range 3–258). The maximum number of individuals captured in 1 day was 516, which were captured in January 2002.
Feeding and biometry
Sampling in the river allowed the capture of postmetamorphic lampreys feeding in the river (PMfr), particularly on S. salar, S. trutta and A. fallax (Table 1). Observed wounds, fresh and pierced, indicated the existence of feeding and not simply attachment.
The biometric parameter values corresponding to the transformers and postmetamorphic lampreys captured in the river are shown in Table 2. Significant differences were found between the PMd and transformers, with higher values for PMd in TL and W and lower in the CF (Mann–Whitney U test, P < 0.05) (Fig. 3). In fact, 42% of PMd showed a value of TL higher than the maximum recorded for transformers, 16% recorded a value of W higher and 15% exceeded the maximum values in both variables. Also, 30.2% of PMd recorded a value of CF lower than the minimum recorded for metamorphic lampreys.
PMdh showed also significant differences with transformers (Fig. 4) in the TL recorded during the months of October, April and May, in W during the months in which PMdh were captured and in CF during all months except May (Mann–Whitney U test, P < 0.05). Thus, 7.1% of all captured PMdh showed a value of TL higher than the maximum recorded for transformers and 4.8% showed a higher weight. These percentages increased progressively over the migration period, from 0% in October to more than 50% in May. Interestingly, 37.1% of PMdh showed a value of CF lower than the minimum recorded for transformers.
Box plots (maximum and minimum values, lower and upper quartiles, and median) of the total length, weight, and condition factor for transformers captured in September 2010 (grey box), postmetamorphic lampreys captured monthly during their downstream migration between 1997 and 2010 (white boxes, postmetamorphics captured in September and June not included as there were only sporadic captures) and captured feeding in the estuary (hatched boxes)
Concerning the prey species, 9.0% of captured alewives (n = 8 of 89 alewives captured) were attacked by postmetamorphic lampreys. For salmon, 4.7% of the 190 individuals captured were attacked by postmetamorphics (n = 9) and 1.6% showed healed wounds of adult lampreys (n = 3). Only one individual out of the 69 sea trouts captured at this point presented postmetamorphics feeding on it.
Estuary
Migration
PMfe were observed in the Ulla estuary at least from November to May. The distribution of PMfe throughout the estuary was not homogenous in space, as they tend to concentrate in areas safeguarded from waves such as ports, river mouths, small inlets, etc.
Postmetamorphics appear firstly on the left bank of the estuary (zone a in enlarged area of Fig. 1) and few months later, from February or March it is possible to observe them on the right bank (Fig. 1, zone b). Since April, the number of postmetamorphic lampreys observed in the estuary decreases progressively, with the latest lampreys observed in May. Due to tidal movements made by L. aurata on which they feed, lampreys move to central areas of the estuary at low tide and to the banks and even into the rivers at high tide. As a result, we could observe postmetamorphics attached to L. aurata 20 km upstream from the mouth of River Ulla (Fig. 1, zone c).
Feeding and biometry
Large amounts of postmetamorphic lampreys feeding on L. aurata were observed from November to May of the 4 years of study. The average TL of L. aurata attacked by PMfe was 337 ± 4.0 mm (range 290–380 mm). The biometric parameter values corresponding to PMfe are shown in Table 2 and Fig. 4. PMfe size increased exponentially between January and April both in terms of TL (y = 142.76e0.1632x; R 2 = 0.97) and W (y = 6.152e0.4718x; R 2 = 0.95). TL increased by 60.1% and W by 279.1%. The total length–weight relationship for PMfe adjusted very well to a potential trend (y = 2E − 06x 3.0169; R 2 = 0.93).
River–estuary differences
Unlike the estuary, where many postmetamorphics were captured feeding on their prey, in the river the capture or observation of lampreys feeding on prey was more difficult, with the exception of L. aurata shoals that go into the river from the estuary. Furthermore, the values of TL and W of PMfe and PMdh were significantly different both in total and for each month (Mann–Whitney U test, P < 0.05), with higher values for PMfe (Table 2, Fig. 4).
Discussion
River
Migration
In River Ulla downstream migration occurs between October and May, with some sporadic captures in September and June. In France, Taverny & Elie (2009) describe the downstream migration period between October and March. In Ireland, it occurs between December and June (Kelly & King, 2001) while in Siberian rivers it is restricted to the period from late May to July, reaching a peak in June (Hardisty & Potter, 1971). As a result, we suggest for European populations of sea lamprey a downstream migration period between autumn and spring, with a delay with increasing latitude. This period is also similar to that described for anadromous populations of North America (Davis, 1967; Beamish, 1980).
The downstream migration of postmetamorphics is nocturnal and is influenced by a marked increase in freshwater discharge (Potter, 1980). Davis (1967), Beamish (1980) and Kelly & King (2001) describe downstream migration with a characteristic bimodal distribution with one peak in autumn and another in spring. Contrastingly, the population of River Ulla showed a unimodal distribution with a progressive increase in the number of individuals in migration, which usually peaks in March, although there are important annual variations. The climatic conditions in North America are such as to encourage this separation of autumn and spring migrations and the same kind of seasonal pattern also occurs in Canadian populations (Hardisty, 2006). Whether they migrate in autumn or wait until the following spring appears to depend on whether the environmental stimuli, such as increased discharge, are strong enough in autumn, before the water temperature decreases causing a decrease in river discharge due to the large amount of water that remains as snow or ice (Kelly & King, 2001; Hardisty, 2006). It also depends on the completion of metamorphosis, which renders the animal susceptible to the influence of the environmental stimulus (Youson & Potter, 1979). The milder weather with higher temperatures recorded in our region in relation to the other areas of study may be those which enable a more continuous and gradual migration.
Given the moment of completion of metamorphosis, which is completed between October and November in River Ulla (unpublished data) and the monthly distribution of captured PMdh (Fig. 2), we estimate that after metamorphosis, lampreys spend an average of 3–4 months in the river before migrating into the estuary. Despite this, the migratory period is very long, and individuals can migrate at the end of the metamorphosis or 6–7 months later. In fact, the only months with no captures of postmetamorphic lampreys during their downstream migration were July and August.
Feeding and biometry
There are several references in the literature on the existence of postmetamorphics of anadromous sea lamprey populations feeding on freshwater (Hardisty & Potter, 1971; Potter & Beamish, 1977; Beamish, 1980; Bird et al., 1994; Maitland et al., 1994; Taverny & Elie, 2009). However, for European populations it had not been possible to confirm the start of this feeding in the river, which has been usually attributed to attacks in the estuary on preys that subsequently enter the river (Bird et al., 1994).
In this study collected data confirm the existence and the start of the hematophagous feeding by young lampreys in the river. The capture and observation of lampreys feeding on their prey, the significant differences found between postmetamorphics during their downstream migration (PMd and PMdh) and the transformers, as well as the finding of postmetamorphic individuals larger than the maximum registered for transformers, support the existence of hematophagous feeding in the river (Davis, 1967; Bird et al., 1994). Moreover, the distance between the river mouth and the sampling points in which the individuals were observed or captured feeding (40, 30, 24 and 20 km), the fact that PMd and PMdh were captured during their downstream migration, and the high number of animals captured, together with the observation of small postmetamorphic lampreys attached to resident S. trutta in the locality R2, corroborate that a part of the postmetamorphic population starts the hematophagous feeding in the river before migrating into the estuary, as described by Davis (1967) and Potter & Beamish (1977) for North American populations.
The non-feeding period during and after metamorphosis lasts from 4 to 10 months (Potter, 1980; Youson, 1980) and causes a decrease in the weight of the individual. Contrastingly, the hematophagous feeding allows lampreys to increase their size quickly (Beamish, 1980; Farmer, 1980). Thus, the percentages of PMd and PMdh with values of TL and W higher than the maximum value observed for transformers (5–16%) give us a rough idea of the percentage of lampreys that start the hematophagous feeding in the river. Importantly, this percentage might be underestimated because the feeding time may not have been enough to exceed the maximum value of TL or W recorded for transformers, especially in postmetamorphic lampreys which endured a more prolonged fasting. The presence of individuals that endure several months without feeding after metamorphosis was reflected in the high percentage of PMdh with values of CF less than the minimum recorded for transformers.
As a consequence, it seems reasonable to suggest that 10–30% of postmetamorphic lampreys start their hematophagous feeding in the river before downstream migration and 70–90% start it in the estuary. For A. fallax, S. trutta and S. salar captured in the river with attached lampreys we cannot exclude that these individuals have been attacked by lampreys in the estuary, as these prey species can migrate several kilometres a day (Lucas & Baras, 2001).
The analysis of wounds in attacked fishes indicated the existence of feeding and not a simple adhesion. Simple adhesion could be explained if lamprey benefited from migrating with minimum energy expenditure (Farmer, 1980; Nichols & Hamilton, 2004). However, in our case this assumption is meaningless because the migration direction of preys (upstream) is opposite to the migration direction of lampreys (downstream). As far as we know, the observations described here provide the first evidence that, for European sea lamprey populations, part of the individuals start the hematophagous feeding in the river before migrating to the estuary and to the sea.
Estuary
Migration
Concerning PMfe, the progressive increase in the range of TL, W and CF from January to March (Fig. 4) indicates the coexistence of small individuals newly arrived from the river and individuals that have already spent a certain time feeding in the estuary. The fact that lampreys arrive earlier and in higher numbers to the left bank of the estuary could be due to the influence of water currents in this sector, as the output current from the river to the sea is concentrated mainly on this side (Grajal-Blanco, 1980).
Feeding and biometry
In the estuary, lampreys find shelter and a large source of food that is exploited during several months (at least from November to May). A particularly important prey species is L. aurata, which is very abundant in this area. Mugilidae is a cosmopolitan family which is typical of coastal areas, with species all over the world (Berra, 2001), and they might be a key element in the diet of most populations of sea lamprey. As far as we know, the observations described here provide the first evidence that P. marinus actively feed on this family and in particular on L. aurata.
River–estuary differences
Thus, although part of the population start feeding in the river, the differences between PMdh and PMfe indicate that feeding of young lampreys focuses mainly on the estuary given the abundance of suitable prey, particularly L. aurata. The onset of hematophagous feeding in the river is expected to allow a recovery of lost energy reserves and facilitate the subsequent downstream migration.
References
Almeida, P. R., 1996. Estuarine movement patterns of adult thin-lipped grey mullet, Liza ramada (Risso) (Pisces, Mugilidae), observed by ultrasonic tracking. Journal of Experimental Marine Biology and Ecology 202: 137–150.
Beamish, F. W. H., 1980. Biology of the North American anadromous sea lamprey, Petromyzon marinus. Canadian Journal of Fisheries and Aquatic Sciences 37: 1924–1943.
Beamish, F. W. H. & I. C. Potter, 1975. The biology of the anadromous sea lamprey (Petromyzon marinus) in New Brunswick. Journal of Zoology 177: 57–72.
Berra, T. M., 2001. Freshwater Fish Distribution. Academic Press, London.
Bird, D. J., I. C. Potter, M. W. Hardisty & B. I. Baker, 1994. Morphology, body size and behaviour of recently-metamorphosed sea lampreys, Petromyzon marinus, from the lower River Severn, and their relevance to the onset of parasitic feeding. Journal of Fish Biology 44: 67–74.
Caballero, P., F. Cobo & M. A. González, 2006. Life history of a sea trout (Salmo trutta L.) population from the north-west Iberian Peninsula (River Ulla, Galicia, Spain). In Harris, G. & N. Milner (eds), Sea Trout: Biology, Conservation & Management. Blackwell, Oxford: 234–247.
Davis, R. M., 1967. Parasitism by newly transformed anadromous sea lampreys on landlocked salmon and other fishes in a coastal Maine lake. Transactions of the American Fisheries Society 96: 11–16.
Farmer, G. J., 1980. Biology and physiology of feeding in adult lampreys. Canadian Journal of Fisheries and Aquatic Sciences 37: 1751–1761.
Freyhof, J. & M. Kottelat, 2008. Petromyzon marinus. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. www.iucnredlist.org. Downloaded on 18 June 2012.
Grajal-Blanco, M., 1980. Las corrientes marinas y su influencia en la zona del Vado en la Isla de Arosa. Cuadernos do Laboratorio Xeolóxico de Laxe 1: 249–278.
Hardisty, M. W., 2006. Lampreys: Life Without Jaws. Forrest Text, Ceredigion.
Hardisty, M. W. & I. C. Potter, 1971. The general biology of adult lampreys. In Hardisty, M. W. & I. C. Potter (eds), The Biology of Lampreys, Vol. 1. Academic Press, London: 127–206.
Henson, M. P., R. A. Bergstedt & J. V. Adams, 2003. Comparison of spring measures of length, weight, and condition factor for predicting metamorphosis in two populations of sea lamprey (Petromyzon marinus) larvae. Journal of Great Lakes Research 29: 204–213.
Kelly, F. L. & J. J. King, 2001. A review of the ecology and distribution of three lamprey species, Lampetra fluviatilis (L.), Lampetra planeri (Bloch) and Petromyzon marinus (L.): a context for conservation and biodiversity considerations in Ireland. Biology and Environment: Proceedings of the Royal Irish Academy 101B: 165–185.
Lucas, M. C. & E. Baras, 2001. Migration of Freshwater Fishes. Blackwell Science, Oxford.
Maitland, P. S., H. Morris & K. East, 1994. The ecology of lampreys (Petromyzonidae) in the Loch Lomond area. Hydrobiologia 290: 105–120.
Nichols, O. C. & P. K. Hamilton, 2004. Occurrence of the parasitic sea lamprey, Petromyzon marinus, on western North Atlantic right whales, Eubalaena glacialis. Environmental Biology of Fishes 71: 413–417.
Nichols, O. C. & U. T. Tscherter, 2011. Feeding of sea lampreys Petromyzon marinus on minke whales Balaenoptera acutorostrata in the St Lawrence Estuary. Journal of Fish Biology 78: 338–343.
Potter, I. C., 1980. Ecology of larval and metamorphosing lampreys. Canadian Journal of Fisheries and Aquatic Sciences 37: 1641–1657.
Potter, C. & F. W. H. Beamish, 1977. The freshwater biology of adult anadromous sea lampreys Petromyzon marinus. Journal of Zoology 181: 113–130.
Quintella, B. R., N. O. Andrade & P. R. Almeida, 2003. Distribution, larval stage duration and growth of the sea lamprey ammocoetes, Petromyzon marinus L., in a highly modified river basin. Ecology of Freshwater Fish 12: 286–293.
Swink, W. D., 2003. Host selection and lethality of attacks by sea lampreys (Petromyzon marinus) in laboratory studies. Journal of Great Lakes Research 29: 307–319.
Taverny, C. & P. Elie, 2009. Bilan des connaissances biologiques et de l’état des habitats des lamproies migratrices dans le bassin de la Gironde—Propositions d’actions prioritaires. Rapport Final. Etude Cemagref, Groupement de Bordeaux.
Taverny, C., M. Urdaci, A. M. Elie, L. Beaulaton, I. Ortusi, F. Daverat & P. Elie, 2005. Biologie, écologie et pêche des lamproies migratrices (Agnathes amphihalins)—Troisième tranche fonctionnelle. Rapport Final. Etude Cemagref, Groupement de Bordeaux.
White, A. & T. C. Fletcher, 1985. Seasonal changes in serum glucose and condition of the plaice, Pleuronectes platessa L. Journal of Fish Biology 26: 755–764.
Youson, J. H., 1980. Morphology and physiology of lamprey metamorphosis. Canadian Journal of Fisheries and Aquatic Sciences 37: 1687–1710.
Youson, J. H. & I. C. Potter, 1979. A description of the stages of metamorphosis in the anadromous sea lamprey, Petromyzon marinus L. Canadian Journal of Zoology 57: 1808–1817.
Acknowledgments
Part of this study has been carried out in the laboratories of the Station of Hydrobiology of the USC ‘Encoro do Con’ in Vilagarcía de Arousa. The authors thank the staff of this station due their participation in the surveys, especially D. J. Nachón. We also thank the staff of Ximonde permanent trap for their collaboration in this study. This study has been partially supported by the project 10PXIB2111059PR of Xunta de Galicia and the project MIGRANET of the Interreg IV B SUDOE (South-West Europe) Territorial Cooperation Programme (SOE2/P2/E288).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Rights and permissions
About this article
Cite this article
Silva, S., Servia, M.J., Vieira-Lanero, R. et al. Downstream migration and hematophagous feeding of newly metamorphosed sea lampreys (Petromyzon marinus Linnaeus, 1758). Hydrobiologia 700, 277–286 (2013). https://doi.org/10.1007/s10750-012-1237-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1237-3