Abstract
The Nyirkai-Hany wetland reconstruction area in northwestern Hungary is now designated as a Ramsar and a Natura 2000 site. It was created in 2001–2002 by the Fertő-Hanság National Park Directorate to restore a part of the formerly drained large wetland called Hanság and to offer waterbirds a suitable habitat for feeding and breeding. We focused on this aim of the restoration project and studied the temporal and spatial variation in abundance of birds and their invertebrate prey in this newly created wetland. From April 2007 until May 2008, we sampled plankton, nekton and benthos of different habitats monthly and monitored waterbirds weekly on the three different areas of the Nyirkai-Hany. During our investigations, 135 invertebrate and 53 waterbird species were recorded. Benthos and macrophyte decomposition accelerating guilds were the most abundant waterbird guilds—besides the dominant grazing importer material transporter guild, represented primarily by geese—in the Nyirkai-Hany. Zooplankton assemblages primarily consisted of small species not easily used as a food by planktivorous waterbirds. The low density of zoobenthic biomass and the small extent of shallow water mudflats probably accounted for the scarcity of the bioturbing guild group of birds. Nektonic biomass varied greatly among locations having different vegetation types, was greatest in the shallow water areas dominated by Typha, Carex and Phragmites species and lowest at offshore vegetation-free sites. Chironomids, mayflies and odonates were especially abundant and their biomass significantly correlated with several waterbird species, mainly belonging to the macrophyte decomposition accelerating guild (e.g. Anas platyrynchos, Fulica atra). This guild itself, which has increased in abundance in recent years, showed an exceptionally strong correlation with odonate abundance. These results indicate the growing importance of the Nyirkai-Hany wetland area as a foraging site for waterbirds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are important contributors to biodiversity worldwide. During past centuries, their number and area have dramatically diminished on both local and global scales. Moreover, due to diversions, flow regulation and channelling, even the remaining wetlands have suffered drastic changes, and become more fragmented.
Rehabilitation of degraded areas can play a major role in biodiversity conservation and in making a link between fragmented landscapes. Although restoration has been practised in some form for a long time, restoration ecology as a field of conservation science has evolved only in recent decades (Standovár & Primack, 2001; Falk et al., 2006). But not every habitat-restoring activity is strictly restoration: creation, enhancement and reclamation can be distinguished. Restoration is literally returning a site to its natural conditions or the conditions that existed before perturbation (Middleton, 1999). Disappeared habitats cannot be re-established completely as they were, but very similar sites can be constructed with proper aims and methods and can later fulfil some of the ecological roles of former habitats (Brönmark & Hansson, 1998).
Aquatic habitats have great importance in supplying waterbirds with invertebrate food (Murkin & Kadlec, 1986; Lillie & Evrard, 1994; Cooper & Anderson, 1996; Svingen & Anderson, 1998; Arzel et al., 2009). Therefore, in the case of wetland restorations, especially those with the aim of waterbird conservation, aquatic food sources have a great influence on restoration success and monitoring of those food sources is highly desirable (Danell & Sjöberg, 1982; Lillie & Evrard, 1994; Cooper & Anderson, 1996; Sutherland et al., 2004). However, for only a few restored or newly created inland wetlands and aquatic habitats have good monitoring studies been conducted for invertebrates (e.g. Danell & Sjöberg, 1982; Andrikovics et al., 1992; Ferguson & Rakocinski, 2008) or birds (Danell & Sjöberg, 1982; Brawley et al., 1998; Moreno-Mateos et al., 2009).
Also, aquatic birds themselves can affect many characteristics of the shallow waterbodies (Gere & Andrikovics, 1994; Andrikovics et al., 1997; Boros et al., 2008), as well as their invertebrate assemblages (Hurlbert & Chang, 1983; Lopes et al., 2000; Sánchez et al., 2006). Thus, to the extent that a habitat is made more attractive to waterbirds, reductions in the standing crops of their prey or food may result.
The Nyirkai-Hany wetland reconstruction area has become an important area for migratory birds in northwestern Hungary since its creation in 2001–2002 and is now designated as a Ramsar and a Natura 2000 site. The increasing numbers of birds using this newly created wetland show that it has become an important feeding ground on their migration route (Pellinger & Takács, 2006; Ferenczi et al., 2009).
The simple objective of our study was to document the temporal and spatial variations in abundances of birds and aquatic invertebrates 6 years after the reconstruction of this wetland, and to assess any correlations between the more abundant groups of invertebrates and their waterbird consumers. This information should help inform management of the reconstruction area as well as similar projects elsewhere.
Materials and methods
Study area
The Hanság peatland formerly occupied an extensive area (55,000 ha) in northwestern Hungary. It was the eastern portion of the largest Hungarian wetland system connected to the Danube River, which included not only the Hanság but also Lake Fertő (Neusiedler See). Widely fluctuating water levels and floods stimulated the construction of drainage and channelling systems that began in the 1700s and continued until the 1960s. Increasing water withdrawals for agricultural fields and peat deposits furthered the disappearance of open waters in the Hanság.
Remains of the Hanság are now under protection belonging to the Fertő-Hanság National Park, and the Directorate has decided to restore a part of the formerly drained wetlands. The idea was to create an area that would not only increase the extent of open waters and enhance the biodiversity of the region but also offer waterbirds a suitable habitat for feeding and breeding and protect the remaining peat deposits and patches of vegetation.
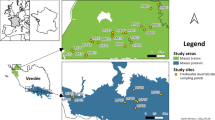
Therefore, the Nyirkai-Hany wetland reconstruction area was created in 2001–2002 (Fig. 1). It consists of three separate waterbodies covering 85 (Area No. I), 130 (Area No. II) and 215 ha (Area No. III), respectively. They were flooded by using the water of two channels and the River Rábca. The water level of each unit can be regulated independently by dams. Water depth is mainly 0–60 cm, and there are also some terrestrial and temporarily flooded parts of the area. Deep (>60 cm) waters make up only 10% of the aquatic habitat. The wetland differs from its original state primarily in receiving higher nutrient inputs and having a more stable water level (Margóczi et al., 2002; Dinka, 2006; Middleton et al., 2006). According to the chlorophyll-a content measured by Dinka (2006), the water of the Nyirkai-Hany is considered to be eu-polytrophic (Felföldy, 1974) or hypertrophic (OECD, 1982).
Open water dominates the reconstructed wetland and has increased since 2001 (Takács et al., 2007). Typha latifolia, Typha angustifolia and Phragmites australis have the highest cover. Submerged vegetation, usually represented by Ceratophyllum demersum or Myriophyllum spicatum, is scarce and can only be found in some parts of the area (Pellinger & Takács, 2006).
After flooding, some native plant (except for Area III), mollusc and fish species were introduced. These included 2,000–2,500 individuals of seven aquatic gastropods (Viviparus contectus, Bithynia tentaculata, Valvata cristata, Lymnaea stagnalis, Physa fontinalis, Planorbarius corneus, Planorbis planorbis) and three bivalve species (Anodonta anatina, A. cygnea, Unio pictorum). The fish were mostly cypriniforms, such as common rudd (Scardinius erythropthalmus), European chub (Squalius cephalus), asp (Aspius aspius), moderlieschen (Leucaspius delineatus), silver bream (Abramis bjoerkna), carp bream (Abramis brama), crucian carp (Carassius carassius), common carp (Cyprinus carpio carpio morpha hungaricus and acuminatus), European bitterling (Rhodeus amarus), common bleak (Alburnus alburnus), tench (Tinca tinca) and European weather loach (Misgurnus fossilis). Species native to the Hanság—catfish (Silurus glanis), pike (Esox lucius), perch (Perca fluviatilis), zander (Sander lucioperca) and mudminnow (Umbra krameri)—were also released. The total weight of translocated fish was 12,130 kg. A recapture survey showed that introduction was successful for all species. Some exotic fishes also eventually appeared in the Nyirkai-Hany: prussian carp (Carassius gibelio), topmouth gudgeon (Pseudorasbora parva), pumpkinseed sunfish (Lepomis gibbosus) and European eel (Anguilla anguilla). The first three of these established fast-growing populations (Kárpáti, 2003).
Waterbirds in the area were surveyed prior to our study by Pellinger (2003), Pellinger & Takács (2006) and Ferenczi et al. (2009). The total number of waterbirds, especially mallards (Anas platyrhynchos) and black-headed gulls (Larus ridibundus) has greatly increased since the creation of the area. For example, the number of mallards observed per year grew from 11,000 to 45,000 between 2002 and 2007. However, the numbers of some species, like shovelers (Anas clypeata; 536 in 2002 but only 91 in 2007) or red-crested pochards (Netta rufina) decreased over this period (Ferenczi et al., 2009).
Sampling and monitoring regimes
At roughly monthly intervals from April until October 2007 and from March until May 2008, we collected quantitative plankton, benthos and nekton samples from 10 locations representing different habitats defined on the basis of their vegetation (Carex spp.: III/1, III/2; Typha spp.: I/2, I/3; P. australis: II/1, III/3; offshore with Nymphoides peltata together with Nymphaea alba and Nuphar lutea: II/3; offshore with scarce submerged vegetation: I/1, II/2; offshore with Polygonum amphibium: III/4) on a monthly basis (Fig. 1). Takács (2003) reported that in all three areas water depth strongly correlated with dominant vegetation: 0–6 cm water depth was associated with Typha spp., 16–25 cm with Carex spp. and 33–39 cm with P. australis. This was true in our study as well, with the addition of an offshore, open water habitat averaging 80 cm deep during our samplings.
Five samples of nekton and benthos were each collected from each of the 10 sites. For benthos samples, the upper layer of sediment (10 cm) was taken out by using a plastic coring tube (diameter: 6 cm). Nekton samples were collected from the whole water column with a surface of 780 cm2. While these samples consisted mostly of nektonic invertebrates, some benthic and epiphytic animals were also caught. For zooplankton, we chose one open-water site per area (I/1, II/3, III/4), as plankton-consuming waterbirds appeared only in this habitat. Three replicate zooplankton samples were collected by filtering 30 l of water per site through a plankton net (mesh size: 60 μm). Water was collected over as wide an area as possible to compensate for the patchiness of microcrustacean assemblages. Sites were sampled in the same order each time, and collected invertebrates were stored in 70% ethanol.
Weekly waterbird monitoring was conducted separately on the three parts of the area. The numbers of individuals belonging to each species were counted on a permanent route at 11 census points around the waterbodies on the dikes. The same method was used as in Pellinger (2003), Pellinger & Takács (2006) and Ferenczi et al. (2009).
Sample analysis
Invertebrates were identified, where possible, to species level. Afterwards, in order to estimate the biomass of the aquatic macroinvertebrates, nearly all individuals were grouped by species and stages, initially dried to constant weight at 105°C, and the average dry weight of each group was then estimated (Németh, 1998). Groups represented only by a few individuals or ones too small for dry mass measurements were ignored. For calculating the dry mass of microcrustaceans, data from the literature were used for each taxon (Németh, 1998; Wolfram-Wais et al., 1999). Exuviae of chironomids were also identified but were not used for biomass calculations.
Data analysis
Biomass was calculated for each of the three areas for each sampling date, for the three major invertebrate assemblages (zooplankton, benthos, nekton) as well as for certain dominant individual taxa (Odonata, Chironomidae, Heteroptera, etc.). For zooplankton, this was done simply using the three replicate samples taken in each area. For the benthos and nekton, however, the estimates for a given area required a two-step process. First, the mean was determined separately for all those samples from a given habitat type (Phragmites, Typha, etc.) in that area. Then, a weighted average of those habitat means for the area was calculated. This used as weights the relative areal extents (G. Takács, pers. comm.) of the different habitat types within that area.
Geometric means were determined via log transformation and then back transformation. To avoid values of zero, we added the lowest possible (given our sampling and reporting protocols) non-zero value to each datum in any data set containing one or more values of zero. These constants were: 277 μg l−1 for benthic Oligochaeta and 107 μg l−1 for benthic Chironomidae. Data sets for Copepoda, Cladocera, total zooplankton, total nekton, total benthos, and nektonic Chironomidae, Odonata, Ephemeroptera and Heteroptera calculated for the three areas did not contain any zero values. In the case of the nekton and benthos data sets of the 10 sampling sites illustrated in Fig. 3, the constants of 3.88 μg l−1 for nekton and 107 μg l−1 for benthos were added prior to log transformation.
Differences among the three areas in geometric mean cladoceran and copepod biomass were assessed with separate one-way ANOVAs for each date. Differences among vegetation types in annual geometric mean nektonic biomass of different vegetation types were also tested with a one-way ANOVA.
Waterbird analyses were conducted for both individual species and for sets of species called guilds (Table 1). Oláh (2003) and Oláh et al. (2006) classified the 165 Hungarian waterbird species into three waterbird trophic guild groups comprising nine guilds defined on the basis of their metabolic and mechanical activities (Table 1). Material transporters can increase or decrease the trophic state of wetlands by transferring nutrients between terrestrial and aquatic systems thereby influencing water quality. Decomposition accelerating and bioturbing guild groups contribute to the decomposition of organic materials by digestion or mechanical activities (e.g. the prodding of waders in the mud since they consume benthic invertebrates and also accelerate aerobic inorganic decomposition at the same time). Among material transporters, grazing importers (mainly geese) and two exporter–importer guilds are established. Collecting exporter–importer birds mainly feed onshore and spend the night on the water (e.g. cranes), while nekton–consumer export–importers hunt in the water but can concentrate materials around the nest sites of their colonies (e.g. egrets or cormorants). Within decomposition accelerating guild group, guilds are distinguished by their main food types (macrophytes, nektonic, benthic or planktonic invertebrates).
For each waterbird species or guild for each area the geometric mean density in a given month was calculated using three counts, made before, during and after the week of the invertebrate samplings. This smoothed out variations due to fluctuating weather conditions. All data sets contained at least one value of zero, and so prior to log transformation the lowest possible non-zero value, 1.2 ind km−2, was added to all density values.
For the 10 most abundant invertebrate-eating waterbirds, correlation analyses were used to see if their geometric mean densities were related to the geometric mean densities any of nine different invertebrate taxa or assemblages. Each correlation analysis was based on 27 data pairs, representing the three areas each sampled nine times.
Results
Invertebrate species composition and biomass
A total of 135 invertebrate species were found in the Nyirkai-Hany wetland in this study; 98 of them are recorded here for the first time since reconstruction. No large dissimilarities were shown between the species composition of the three parts of the reconstruction area, although the number of their species was slightly different: 62 in Area I, 83 in Area II, and 95 in Area III. The species list can be found in the Online Appendix.
Zooplankton assemblages consisted mainly of Copepoda (13–96%) and Cladocera (3–86%), while Ostracoda were always negligible compared to the former two groups (having only 0–8% relative frequency in the assemblages, considering all samples). The two microcrustaceans with the highest frequencies were Acanthocyclops robustus (Copepoda) and Bosmina longirostris (Cladocera). During the study of the three areas, the latter species had the highest relative abundance in the assemblages 13 times of the total 27 samplings. Other small-sized species also reached such high relative abundance: the most frequent species were four times two Pleuroxus spp. (P. aduncus and truncatus) and once Chydorus sphaericus. In most cases, the bigger Cladocera and Copepoda species were rare, sometimes individual species could only be found as juvenile specimens. This phenomenon was the most striking in the case of Area No. I, where B. longirostris was nearly always the most abundant species (except for three samples), and its density was usually even higher than at the other two areas.
The number of dominant species was different in the three locations: 6, 8 and 11 such species were found in Areas No. I, II and III, respectively. Bosmina longirostris, Daphnia galeata and A. robustus were often dominant in all of the areas. The thermophilic Thermocyclops oithonoides also showed dominance in Areas No. II and III in summer, but in the first area, it could only reach lower relative abundance.
In addition, two adventive microcrustaceans, Pleuroxus denticulatus and Daphnia ambigua (Cladocera) were also found, although only with low abundances. The former species was found only in Area No. I in May and August while the latter was present in Areas No. II and III in May and June.
More than 90% of the total number of nektonic macroinvertebrates belonged to the five groups of Heteroptera (32.3%), Ephemeroptera (22.2%), Chironomidae (17.1%), Oligochaeta (8.4%) and Odonata (10.4%). Among all species, Caenis robusta (Ephemeroptera) had the highest abundance during the research period. It was found at all 10 sampling sites and constituted more than 15% of all nektonic macroinvertebrates. In addition, three Heteroptera species (Sigara striata, Ilyocoris cimicoides, Micronecta scholtzi), Stylaria lacustris (Oligochaeta), Ischnura elegans (Odonata), Endochironomus albipennis (Chironomidae) and another Ephemeroptera, Cloeon dipterum were also very frequent (>3% of all specimens). The latter species was common in 2007 but could not be found in the area in 2008.
Many benthic macroinvertebrates were identified only to genus or higher taxonomic level and therefore they were grouped together in the higher categories. Of these, 95% of the specimens were Chironomidae or Oligochaeta.
Both cladoceran and copepod mean biomasses differed significantly among the three areas in most occasions (Fig. 2). Both had a summer peak in 2007 and in the case of Area No. II, an even higher maximum in May 2008.
Benthic biomass was mainly composed of oligochaetes and nematodes, with some chironomids. Benthos samples were empty on many occasions, and almost all the time at the offshore sites of II/2, II/3 and III/4 (Fig. 3).
Nektonic invertebrate biomass varied greatly among locations with different types of vegetation (F 5,48 = 10.54, P < 0.001). It was most abundant in the shallow water areas dominated by Typha, Carex and Phragmites species. It was least abundant at offshore sites where submerged vegetation was scarce or dominated by P. amphibium (Fig. 4). Total nektonic biomass calculated did not differ significantly among the three areas (one-way ANOVA, P = 0.48), but tended to be lowest in Area No. I and greatest in Area No. III during the study. Biomass peaks of the dominant invertebrate groups had different timings: the maximum numbers of Chironomidae, Heteroptera, Oligochaeta and Gastropoda biomass occurred in spring, of Odonata in mid-summer, of Coleoptera in autumn, and of Ephemeroptera in early summer. Each group showed a similar temporal pattern in the three areas.
Waterbird abundances
During the study, 53 waterbird species were recorded in the wetlands (Table 1), with 37, 30 and 50 recorded for Areas No. I, II and III, respectively, the biggest area not surprisingly having the most species.
The most abundant species were geese (Anser albifrons, A. anser and A. fabalis), which made the material transporter guild group the dominant one in Nyirkai-Hany (Fig. 5). Anas species were also very frequent, of which mallard (A. platyrhynchos) was by far most abundant. Some piscivorous waterbirds also were very abundant, e.g. Egretta alba, L. ridibundus and Phalacrocorax carbo (Table 2).
Invertebrate–waterbird correlations
The biomasses of the five most frequent macroinvertebrate groups (Heteroptera, Ephemeroptera, Chironomidae, Oligochaeta and Odonata), total nekton and benthos biomass were used for correlation analysis (Table 3) with the 10 most frequent waterbird species that rely heavily on aquatic invertebrates for food. Nine positive correlations were found. Two zooplanktivorous species occurred during our investigations in the Nyirkai-Hany, shoveler (A. clypeata) and spotted redshank (Tringa erythropus), but the latter was present only once. Shovelers showed some evidence of a real correlation with Cladocera (P = 0.06) but not with Copepoda (P = 0.43), or total zooplankton biomass (P = 0.23).
In the case of the waterbird trophic guilds (excluding PDA, GIMP and CEIMT guilds), only one positive significant correlation was found with the biomass of the same macroinvertebrate groups. This was between MDA guild density and odonate biomass (P = 0.047; Fig. 6). The PDA guild, like its constituent species, showed no significant correlation with zooplankton biomass.
Discussion
The waterbird guild structure in the Nyirkai-Hany was very similar to that in Hungarian oxbows, with dominance of the material transporter birds and scarcity of the bioturbing guild group (Andrikovics et al., 2006). Four of the nine most frequent waterbird species were piscivorous (E. alba, L. ridibundus, P. carbo, Podiceps cristatus) and three were geese (Anser species) that do not actually utilize aquatic invertebrates as food. Six of our significant correlations (Table 3) may reflect to potential trophic relationships: Odonata may be successfully utilized by Fulica atra, Chironomidae by A. platyrhynchos, A. crecca and Aythya nyroca, benthic oligochaetes by A. crecca, and Ephemeroptera biomass by F. atra.
Geese, the most frequent group of waterbirds, were more abundant in Areas No. I and III, which can be a result of the larger percentage of open waters (>50%) in these two areas compared to Area No. II (<30%). The bioturbing guild group was the scarcest, which may relate to the general scarcity of benthic invertebrates and mudflats in the Nyirkai-Hany. The PDA guild was the second rarest guild during our studies. Notably, the number of shovelers has been decreasing since the creation of the area (Ferenczi et al., 2009). This may relate to increased fish predation causing a shift to dominance by zooplankters too small for zooplanktivorous waterbirds. This may explain why no significant correlation was found between microcrustaceans and this guild. A study conducted on gravel pits in northeastern France found that shovelers preferred pits where zooplankton consisted mainly of large crustaceans (Mouronval et al., 2007).
The strong correlation between the MDA guild and Odonata undoubtedly reflects a real trophic relationship. This guild—despite its name—is also a strong consumer of nekton and other macroinvertebrates living among the macrophytes (Oláh, 2003).
We cannot compare the correlations between waterbird trophic guilds and their food in the Nyirkai-Hany with other studies of reconstructed wetlands as none have employed a guild approach to analysis of relations. There are a few surveys on constructed wetlands that concentrated on particular waterbird species and their aquatic invertebrate prey, however. Danell & Sjöberg (1982) found that the number of mallards on a man-made Swedish lake declined in years when chironomid biomass was lower and that peak numbers of duck broods corresponded in time with peak Chironomidae biomass. Pöysä et al. (2000) suggested that habitat selection and distribution of mallards is highly influenced by the aquatic invertebrate food availability during the brood stage. Murkin & Kadlec (1986) also found a correlation between macroinvertebrate biomass and total number of ducks during spring in the Delta Marsh, Manitoba, Canada. Svingen & Anderson (1998) found that mallard and gadwall (Anas strepera) brood and pair use of waterbodies correlated with macroinvertebrate diversity.
The most striking result of the microcrustacean sampling was the high abundance of small cladoceran species, such as B. longirostris (most abundant crustacean in 48% of all samples), Pleuroxus spp. (15%) and C. sphaericus (4%). As large cladocerans, such as Daphnia spp. (and large predaceous copepods) are more vulnerable to predation by planktivorous fish, in the face of high fish abundance, small zooplankton species become dominant and phytoplankton increases in abundance (Hrbáček et al., 1961; Brooks & Dodson, 1965; Hurlbert et al., 1972; Dodson, 1974a,b; de Bernardi et al., 1987). Such phenomena undoubtedly occur in the Nyirkai-Hany wetlands, with the added influence of high nutrient levels in its inflow waters.
The occurrence of P. denticulatus and D. ambigua was also noteworthy. These adventive cladocerans are considered to have a North-American origin and seem to disperse in Europe (e.g. Maier, 1996; Vranovský & Terek, 1996; Hebert et al., 2003). Although they were only found in low abundance, their presence is remarkable when considering that it is a newly constructed wetland. Vranovský & Terek (1996) and Hudec & Illyová (2006) earlier noted these species to be present, often together, in Central European aquatic habitats, perhaps a result of fish stocking programs. The ecological implications of their arrival in Europe is not clear.
The predation of fish may affect not only the zooplankton, but also the macroinvertebrates. In addition, fish can have a much stronger effect on macroinvertebrate density and biomass than do waterbirds (Marklund et al., 2002). The scarcity and small size of gastropods in the Nyirkai-Hany wetlands may be a result of fish predation and potentially contributes to increased algal abundance (Brown & DeVries, 1985; Martin et al., 1992; Brönmark, 1994; Perrow et al., 2002).
The dominant waterbird trophic guild group in the Nyirkai-Hany wetland was the GIMT guild, which, together with the effects of fish, can also contribute to the high productivity of the area by the guild’s high nutrient input (Manny et al., 1994; Andrikovics et al., 1997; Mukherjee & Borad, 2001; Rönicke et al., 2008). The abundance of many piscivorous birds in conjunction with the composition of the zooplankton suggests high fish biomass in the Nyirkai-Hany. Surveys of the fish populations would be useful for better understanding of the ongoing changes in the waterbird assemblage and of management options.
Wetland restoration can play an important part in regaining the main functions or ‘ecosystem services’ of wetlands, although the structure and functioning of restored wetlands often are different from the original ones, especially in the case of projects aimed at enhancing biodiversity (Zedler, 2003). In part this can be due to governmental requirements, subjective decisions or lack of clear goals, but it is also a natural consequence of ecological succession (Zedler & Weller, 1990; Gilbert & Anderson, 1998; Grayson et al., 1999). In the early successional stages of created wetlands, there can be large fluctuations in number of species and individuals in these habitats (Zedler & Weller, 1990). Jackson et al. (1995) suggest that for achievement of high functioning of such wetlands, 10–50 years are needed. However, good results can sometimes be observed in 1–10 years, as shown by our present survey. The Nyirkai-Hany wetland already provides sufficient macroinvertebrate food supplies for several waterbird species, especially members of the MDA guild, whose numbers have increased only a few years after creation of this wetland.
References
Andrikovics, S., A. Bankovics, T. Csörgő, G. Gere, M. Sass & J. Török, 1992. Hydrozoological characters of a reconstructed wetland. Miscellanea Zoologica Hungarica 7: 65–70.
Andrikovics, S., G. Gere & E. Futó, 1997. The nutrition of Greylag Goose and its effect on the eutrophication of Kisbalaton (Hungary). In Faragó, S. & J. Kerekes (eds), Limnology and Waterfowl. Monitoring, Modelling and Management, Vol. 43. Proceedings of a Symposium on Limnology and Waterfowl held in Sopron/Sarród, Hungary, November 21–23, 1994. Wetlands International Publication: 199–210.
Andrikovics, S., L. Forró, G. Gere, Gy. Lakatos & L. Sasvári, 2006. Water bird guilds and their feeding connections in the Bodrogzug, Hungary. Hydrobiologia 367: 31–42.
Arzel, C., J. Elmberg, M. Guillemain, M. Lepley, F. Bosca, P. Legagneux & J.-B. Nogues, 2009. A flyway perspective on food resource abundance in a long-distance migrant, the Eurasian teal (Anas crecca). Journal of Ornithology 150: 61–73.
Boros, E., S. Andrikovics, B. Kiss & L. Forró, 2006. Feeding ecology of migrating waders (Charadrii) at sodic-alkaline pans in the Carpathian Basin. Bird Study 53: 86–91.
Boros, E., T. Nagy, Cs. Pigniczki, L. Kotymán, K. V. Balogh & L. Vörös, 2008. The effect of aquatic birds on the nutrient load and water quality of soda pans in Hungary. Acta Zoologica Academiae Scientiarum Hungaricae 54: 207–224.
Brawley, A. H., R. S. Warren & R. A. Askins, 1998. Bird use of restoration and reference marshes within the Barn Island Wildlife Management Area, Stonington, Connecticut, USA. Environmental Management 22: 625–633.
Brönmark, C., 1994. Effects of Tench and Perch on interactions in a freshwater, benthic food chain. Ecology 75: 1818–1828.
Brönmark, C. & L. A. Hansson, 1998. The Biology of Lakes and Ponds. Oxford University Press, Oxford.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size and composition of plankton. Science 150: 28–35.
Brown, K. M. & D. R. DeVries, 1985. Predation and the distribution and abundance of a pulmonate pond snail. Oecologia 66: 93–99.
Cooper, C. B. & S. H. Anderson, 1996. Significance of invertebrate abundance to dabbling duck brood use of created wetlands. Wetlands 16: 557–563.
Danell, K. & K. Sjöberg, 1982. Successional patterns of plants, invertebrates and ducks in a man-made lake. Journal of Applied Ecology 19: 395–409.
De Bernardi, R., G. Guissani & M. Manca, 1987. Cladocera: predators and prey. Hydrobiologia 145: 225–243.
Dinka, M., 2006. A Fertő, valamint a Nyirkai-Hany és a Keleti Mórrétek rekonstrukciós területének hidrobiológiai vizsgálata. [Hydrobiological investigation on the Fertő and the Nyirkai-Hany and Keleti Mórrétek reconstruction areas—in Hungarian.] Research Report, Institute of Ecology and Botany, Hungarian Academy of Sciences, Vácrátót: 4–40.
Dodson, S. I., 1974a. Zooplankton competition and predation: an experimental test of the size-efficiency hypothesis. Ecology 55: 613–695.
Dodson, S. I., 1974b. Adaptive change in plankton morphology in response to size-selective predation: a new hypothesis of cyclomorphosis. Limnology and Oceanography 19: 721–729.
Falk, D. A., M. A. Palmer & J. B. Zedler (eds), 2006. Foundations of Restoration Ecology. Society for Ecological Restoration International, Island Press, Washington, DC.
Felföldy, L., 1974. A biológiai vízminősítés. [Biological water qualification—in Hungarian.] Vízügyi Hidrobiológia 3. VIZDOK, Budapest.
Ferenczi, M., A. Pellinger & T. Csörgő, 2009. Vízimadár közösség monitorozása a Nyirkai-Hany élőhely-rekonstrukció területén. [Waterbird monitoring of the Nyirkai-Hany wetland reconstruction area—in Hungarian with English abstract.] Természetvédelmi Közlemények 15: 446–456.
Ferguson, H. J. & C. F. Rakocinski, 2008. Tracking marsh restoration using macrobenthic metrics: implementing a functional approach. Wetlands Ecology and Management 16: 277–289.
Gere, G. & S. Andrikovics, 1994. Feeding of ducks and their effects on water quality. Hydrobiologia 279/280: 157–161.
Gilbert, O. L. & P. Anderson, 1998. Habitat Creation and Repair. Oxford University Press, Oxford.
Gilbert, G., G. Tyler & K. W. Smith, 2003. Nestling diet and fish preference of Bitterns Botaurus stellaris in Britain. Ardea 91: 35–44.
Grayson, J. E., M. G. Chapman & A. J. Underwood, 1999. The assessment of restoration of habitat in urban wetlands. Landscape and Urban Planning 43: 227–236.
Hebert, P. D. N., J. D. S. Witt & S. J. Adamowicz, 2003. Phylogeographical patterning in Daphnia ambigua: regional divergence and intercontinental cohesion. Limnology and Oceanography 48: 261–268.
Hrbáček, J., M. Dvořakova, V. Kořínek & L. Procházkóva, 1961. Demonstration of the effect of the fish stock on the species composition of zooplankton and the intensity of metabolism of the whole plankton association. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie 14: 192–195.
Hudec, I. & M. Illyová, 2006. Pleuroxus denticulatus (Crustacea: Anomopoda: Chydoridae): a new invader in the Danube Basin. Hydrobiologia 368: 65–73.
Hurlbert, S. H. & C. C. Y. Chang, 1983. Ornitholimnology: effects of grazing by the Andean Flamingo (Phoenicoparrus andinus). Proceedings of the National Academy of Sciences of the United States of America 80: 4766–4769.
Hurlbert, S. H., J. Zedler & D. Fairbanks, 1972. Ecosystem alteration by mosquitofish (Gambusia affinis) predation. Science 175: 639–641.
Jackson, L. L., N. Lopoukhine & D. Hillyard, 1995. Ecological restoration: a definition and comments. Restoration Ecology 3: 71–75.
Kárpáti, L., 2003. Vizes élőhelyrekonstrukció a Hanságban. [Wetland reconstruction in the Hanság—in Hungarian.] Thesis, University of Debrecen.
Kovács, B., 1971. A búbosvöcsök (Podiceps cristatus L.) Hortobágyon gyűjtött gyomortartalmainak táplálékösszetétele. [Zusammensetzung der Nahrung der im Gebiete Hortobágy gesammelten Mageninhalte der Haubentaucher (Podiceps cristatus L.)—in Hungarian with German Abstract.] Debreceni Agrártudományi Főiskola Tudományos Közleményei: 112–187.
Kubetzky, U. & S. Garthe, 2003. Distribution, diet and habitat selection by four sympatrically breeding gull species in the south-eastern North Sea. Marine Biology 143: 199–207.
Lillie, R. A. & J. O. Evrard, 1994. Influence of macroinvertebrates and macrophytes on waterfowl utilization of wetlands in the Prairie Pothole Region of northwestern Wisconsin. Hydrobiologia 279/280: 235–246.
Lopes, R. J., M. A. Pardal & J. C. Marques, 2000. Impact of macroalgal blooms and wader predation on intertidal macroinvertebrates: experimental evidence from the Mondego estuary (Portugal). Journal of Experimental Marine Biology and Ecology 249: 165–179.
Maier, G., 1996. Daphnia invasion: population dynamics of Daphnia assemblages in two eutrophic lakes with particular reference to the introduced alien Daphnia ambigua. Journal of Plankton Research 18: 2001–2015.
Manny, B. A., W. C. Johnson & R. G. Wetzel, 1994. Nutrient additions by waterfowl to lakes and reservoirs: predicting their effects on productivity and water quality. Hydrobiologia 279/280: 121–132.
Margóczi, K., G. Takács, A. Pellinger & L. Kárpáti, 2002. Wetland reconstruction in Hanság area (Hungary). Restoration Newsletter 15: 14–15.
Marklund, O., H. Sandsten, L.-A. Hansson & I. Blindow, 2002. Effects of waterfowl and fish on submerged vegetation and macroinvertebrates. Freshwater Biology 47: 2049–2059.
Martin, T. H., L. B. Crowder, C. F. Dumas & J. M. Burkholder, 1992. Indirect effects of fish on macrophytes in Bays Mountain Lake: evidence for a littoral trophic cascade. Oecologia 89: 476–481.
Middleton, B., 1999. Wetland Restoration, Flood Pulsing, and Disturbance Dynamics. Wiley and Sons, New York.
Middleton, B., A. Grootjans, H. Jensen, H. Olde Venterink & K. Margóczi, 2006. Fen management and research perspectives: an overview. In Bobbink, R., B. Beltman, J. T. A. Verhoven & D. F. Whigham (eds), Wetlands: Functioning, Biodiversity Conservation and Restoration. Springer, Berlin, Heidelberg: 247–268.
Moreno-Mateos, D., C. Pedrocchi & F. A. Comín, 2009. Avian communities’ preferences in recently created agricultural wetlands in irrigated landscapes of semi-arid areas. Biodiversity Conservation 18: 811–828.
Mouronval, J. B., M. Guillemain, A. Canny & F. Poirier, 2007. Diet of non-breeding wildfowl Anatidae and Coot Fulica atra on the Perthois gravel pits, northeast France. Wildfowl 57: 68–97.
Mukherjee, A. & C. K. Borad, 2001. Effects of waterbirds on water quality. Hydrobiologia 464: 201–205.
Murkin, H. R. & J. A. Kadlec, 1986. Relationships between waterfowl and macroinvertebrate densities in a northern prairie marsh. The Journal of Wildlife Management 50: 212–217.
Németh, J., 1998. A biológiai vízminősítés módszerei. [Methods for biological qualification of surface waters guilds—in Hungarian with English Abstract.] Vízi természet- és környezetvédelem 7. Környezetgazdálkodási Intézet, Budapest.
OECD, 1982. Eutrophication of Waters. Monitoring, Assessment and Control. OECD, Paris.
Oláh, J. Jr., 2003. Vízimadár anyagforgalmi guildek. [Waterbird trophic guilds—in Hungarian with English Abstract.] Magyar Vízivad Közlemények, Nyugat-magyarországi Egyetem, Vadgazdálkodási Intézet, Magyar Vízivad Kutató Csoport: 381–423.
Oláh, J. Jr., Gy. Lakatos, B. Kovács, S. Andrikovics & J. Oláh, 2006. Waterbird guilds in Hungarian wetlands. In Hanson, A., J. Kerekes & J. Paquet (eds), Limnology and Aquatic Birds: Abstracts and Selected Papers from the Fourth Conference of the Societas Internationalis Limnologiae (SIL) Aquatic Birds Working Group. Canadian Wildlife Service Technical Report Series 474, Atlantic Region: 92–102.
Pellinger, A., 2003. Madártani monitoring. [Bird monitoring—in Hungarian.] In: Takács, G. (ed.), A dél-hansági élőhelyrekonstrukciók komplex ökológiai monitoringja. [The complex ecological monitoring of the habitat reconstruction in the South Hanság—in Hungarian.] Research Report, Fertő-Hanság National Park Directorate, Sarród: 97–129.
Pellinger, A. & G. Takács, 2006. Nyirkai-Hany vizes élőhely-rekonstrukció. [Restoration project of the wetland habitat of the Nyirkai-Hany, Fertő-Hanság National Park (North-West Hungary)—in Hungarian with English summary.] Research Report, Fertő-Hanság National Park Directorate, Sarród.
Perrow, M. R., M. L. Tomlinson & L. Zambrano, 2002. Fish. In Perrow, M. R. & A. J. Davy (eds), Handbook of Ecological Restoration: Principles of Restoration. Cambridge University Press, Cambridge: 324–354.
Pöysä, H., J. Elmberg, K. Sjöberg & P. Nummi, 2000. Nesting mallards (Anas platyrhynchos) forecast brood-stage food limitation when selecting habitat: experimental evidence. Oecologia 122: 582–586.
Rönicke, H., R. Doerffer, H. Siewers, O. Büttner, K.-E. Lindenschmidt, P. Herzsprung, M. Beyer & H. Rupp, 2008. Phosphorus input by nordic geese to the eutrophic Lake Ardensee, Germany. Fundamental and Applied Limnology, Archiv für Hydrobiologie 172: 111–119.
Sánchez, M. I., A. J. Green & R. Alejandre, 2006. Shorebird predation affects density, biomass, and size distribution of benthic chironomids in salt pans: an exclosure experiment. Journal of the North American Benthological Society 25: 9–18.
Standovár, T. & R. B. Primack, 2001. A természetvédelmi biológia alapjai. [Essentials of Conservation Biology—in Hungarian.] Nemzeti Tankönyvkiadó, Budapest: 423–437.
Sterbetz, I., 1972. Vízivad. [Waterfowl—in Hungarian.] Mezőgazdasági Kiadó, Budapest.
Sutherland, W. J., I. Newton & R. E. Green (eds), 2004. Bird Ecology and Conservation. Oxford University Press, Oxford.
Svingen, D. & S. H. Anderson, 1998. Waterfowl management on grass-sage stock ponds. Wetlands 18: 84–89.
Takács, G., 2003. Növénytani monitoring. [Botanical monitoring—in Hungarian.] In Takács, G. (ed.), A dél-hansági élőhelyrekonstrukciók komplex ökológiai monitoringja. [The complex ecological monitoring of the habitat reconstruction in the South Hanság—in Hungarian.] Research Report, Fertő-Hanság National Park Directorate, Sarród: 31–96.
Takács, G., K. Margóczi & Z. Bátori, 2007. Vegetációváltozások egy nagy kiterjedésû hansági vizes élõhely-rekonstrukción. [Vegetation changes in a large wetland reconstruction in Hanság—in Hungarian with English Abstract.] Természetvédelmi Közlemények 13: 269–279.
Vasvári, M., 1928. Adalékok a bölömbika és a pocgém táplálkozási ökológiájához. [Beiträgezur Ernährungsoekolgie von Botaurus stellaris L. und Aretta minuta L.—in Hungarian with German Abstract.] Aquila 34/35: 342–374.
Vasvári, M., 1951. A szürkegém, a nagy- és kiskócsag táplálkozási ökológiája. [Food-ecology of the Common Heron, the Great White Egret and the Little-Egret—in Hungarian with English Abstract.] Aquila 55/58: 23–38.
Vranovský, M. & J. Terek, 1996. First records of Daphnia ambigua (Crustacea, Branchiopoda) from the rivers Danube and Hron. Biologia 51: 142.
Wolfram-Wais, A., G. Wolfram, B. Auer, E. Mikschi & A. Hain, 1999. Feeding habits of two introduced fish species (Lepomis gibbosus, Pseudorasbora parva) in Neusiedler See (Austria), with special reference to chironomid larvae (Diptera: Chironomidae). Hydrobiologia 408/409: 123–129.
Zedler, J. B., 2003. Wetlands at your service: reducing impacts of agriculture at the watershed scale. Frontiers in Ecology and the Environment 1: 65–72.
Zedler, J. B. & M. W. Weller, 1990. Overview and future directions. In Kusler, J. A. & M. E. Kentula (eds), Wetland Creation and Restoration: The Status of the Science. Island Press, Washington DC: 405–413.
Acknowledgments
We would thank Klára Dózsa-Farkas, Zoltán Péter Erőss, Katalin Kovács, Tibor Kovács, Ákos Molnár, Dávid Murányi and Dávid Rédei for their help with identification, János Podani for his suggestions on statistical analysis, Gábor Takács for letting us use his unpublished data on vegetation cover, David Kosky and Gergely Horváth for the English corrections and Dávid Józsvai, Árpád Németh, Gabriella Pásti, Attila Pellinger, Attila Péntek, as well as István Horváth, Béla Horváth, Tibor Horváth and their families for their valuable help during field work. We would also like to thank the very useful and constructive comments of the anonymous reviewers and Stuart H. Hurlbert. The study was supported by the Pro Renovanda Cultura Hungariae Student Science Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Sándor Andrikovics
S. Andrikovics—deceased
Guest editors: F. A. Comín & S. H. Hurlbert / Limnology and Aquatic Birds: Monitoring, Modelling and Management
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horváth, Z., Ferenczi, M., Móra, A. et al. Invertebrate food sources for waterbirds provided by the reconstructed wetland of Nyirkai-Hany, northwestern Hungary. Hydrobiologia 697, 59–72 (2012). https://doi.org/10.1007/s10750-012-1170-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1170-5