Abstract
Hydroelectric reservoirs can stratify, producing favorable conditions for mercury methylation in the hypolimnion. The methylmercury (MeHg) can be exported downstream, increasing its bioavailability below the dam. Our objective was to assess the mercury levels in plankton, suspended particulate matter (SPM) and fish collected upstream (UP) and downstream (DW) from the Reservatório de Samuel dam, an Amazonian reservoir that stratifies during half of the year. Mercury concentrations in both SPM and plankton were similar between the two sites, which could indicate there are no conditions favoring methylation at the moment of sampling (absence of stratification). Almost all mercury found in the muscle of fishes was in organic form, and differences of mercury levels between sites were dependent on the fishes trophic level. Herbivores showed similar mean organic mercury levels (UP = 117 μg g−1; DW = 120 μg g−1; n = 12), whereas omnivores (UP = 142 μg g−1; DW = 534 μg g−1; n = 27) and carnivores (UP = 545 μg g−1; DW = 1,366 μg g−1; n = 69) showed significantly higher values below the dam. The absence of a reservoir effect in herbivores is expected, since they feed on grassy vegetation, near the riverbanks, which is not much influenced by mercury in aquatic systems. On the other hand, the higher mercury levels below the dam observed for omnivores and carnivores suggest a possible influence of the reservoir since they feed on items that could be contaminated by MeHg exported from upstream. The results highlight the necessity of assessing areas downstream of reservoirs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence and behavior of mercury (Hg) in aquatic systems is of great interest and importance, since it is the only heavy metal that bioaccumulates and biomagnifies through the aquatic food web (Lindqvist et al., 1991). The behavior of Hg can change in environments influenced anthropically, usually because of input of Hg (e.g., industrial wastes and atmospheric deposition) or changes in natural conditions. Impounded reservoirs, which produce about 20% of total world electric power (REN21, 2009), currently represent an important disturbance in aquatic systems.
Many studies have observed an increase of Hg levels in the local biota after reservoir impoundment (e.g., St. Louis et al., 2004; Hylander et al., 2006; Bodaly et al., 2007). This increase is associated with the inundation area, which mobilizes Hg and organic matter from submerged vegetation and soil. The microbial decomposition of organic matter, linked to limnological characteristics such as acid pH and low oxygen levels, makes reservoirs good sites for Hg methylation (Rogers et al., 1995; Hylander et al., 2006). Methylmercury (MeHg) can be assimilated by the biota, and therefore in some reservoirs the fish have high Hg contents.
Flooding an area for reservoir filling can result in a water column that is stratified during most of the year. The stratification can cause anoxic conditions in the hypolimnion that favor Hg methylation. In reservoir hypolimnetic anoxic water, an increase of MeHg concentrations can occur, and this water, passing through the dam, increases MeHg availability downstream (Canavan et al., 2000). The MeHg transported downstream is mainly in the dissolved phase, associated with suspended particulate matter (SPM) and incorporated in the biota, mostly plankton organisms (Schetagne et al., 2000; Dominique et al., 2007). Therefore, the MeHg can be incorporated and transferred through the aquatic food web downstream.
The majority of the studies on the limnology as well as heavy-metal accumulation and management in reservoirs has been carried out in the lacustrine zone, mainly near the dam (e.g., Kamman et al., 2005; Gantzer et al., 2009), since the features of this region are usually very important for energy generation and water supply. The impact of the outflow of pollutants from reservoirs on the downstream region and especially on the biota is not well known. In Boreal reservoirs, the high Hg levels in biota downstream from dams is well discussed (e.g., Schetagne et al., 2000; Anderson, 2011), but only limited information is available on Hg loads of tropical biota found in southern hemisphere reservoirs. Notably, regarding Amazon reservoirs, there are until now, only two reservoirs studied downstream of a dam for Hg levels in fish, Tucuruí (Porvari, 1995; Malm et al., 2004; Palermo et al., 2004) and Petit-Saut (Dominique et al., 2007). In the Amazon region, where there are natural sources and likely biogeochemical processes that favor Hg methylation in the environment (Silva-Forsberg et al., 1999), damming rivers for hydroelectric reservoir construction can result in high Hg concentrations in fish. Knowing that this region has the largest volume of freshwater in the world, comprising approximately 2/3 of the total hydropower potential of Brazil (Bermann, 2002), it is important to understand the effects downstream from Amazon reservoirs.
This study took place in the Reservatório de Samuel, located in the Brazilian Amazon. This reservoir is stratified during half of the year, and previous studies have shown that its hypolimnion becomes anoxic with an acidic pH, thus creating ideal conditions for methylation. Therefore, this system can be acting as a methylation site, exporting MeHg downstream from the dam that can be taken up by biota. Hence, the objectives of this study were to assess the Hg levels: (1) in SPM and plankton, two main downstream exporters of Hg, collected upstream and downstream from the Reservatório de Samuel dam, (2) in the muscle of fish that belong to different trophic levels caught upstream and downstream from Samuel dam, and (3) in the intestines of fish from different trophic levels caught in both areas in order to understand the load of Hg taken by these organisms through food.
Materials and methods
Study area
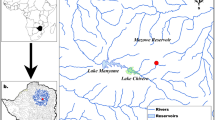
The study was conducted in Reservatório de Samuel (08°45′S, 63°26′W), a hydropower reservoir located in the state of Rondônia, Brazilian Amazon (Fig. 1). The reservoir has a surface area of 579 km2 at maximum water level (Santos, 1995). The Reservatório de Samuel was formed in 1988 by damming the Rio Jamari, an important tributary of the Rio Madeira (SEDAM, 2002). This region has a humid tropical climate with rainy (September to April) and dry (May to August) seasons. Annual precipitation ranges from 1,800 to 2,400 mm, and monthly average temperatures are between 24 and 26°C (SEDAM, 2002).
Samples were collected in March–April 2007 (at the end of the rainy season) at two sites: 1.5 km upstream from the Samuel dam, and 4 km downstream from the dam. These sites were chosen in order to compare the Hg levels above and below the dam, respecting the safe limits of the boat’s approach to the dam.
At the sample site upstream from the dam, the mean (±standard deviation) water depth is 29 ± 3 m based on 12 samplings, one per month during a year (Nascimento, 2006). This site shows thermal and chemical stratification in the dry season (Viana, 2002; Nascimento, 2006) when the difference in temperature between superficial and bottom waters can reach 6°C (Viana, 2002). During stratification, hypoxic conditions prevail at depths below 10 m, exactly the depth of the water outlet for the turbines. Consequently, the waters from the downstream region have low dissolved oxygen levels during the dry season (Viana, 2002). At the beginning of the rainy season, waters from different layers of the reservoir become mixed, and the distributions of dissolved oxygen, pH, temperature, and conductivity are relatively uniform in the water column (Viana, 2002; Nascimento, 2006). Downstream from the dam, the waters are oxygen saturated during the rainy season due to improved oxygen concentrations in reservoir bottom waters and/or due to re-oxygenation through high discharges from the spillway (Viana, 2002; Nascimento, 2006).
The reservoir is surrounded mainly by urbanized areas, farms and cattle ranches, with some remaining areas covered by tropical forest. In its basin area there are no reports of gold mining, but tin mining is an important activity. The tin ores can contain impurities such as sulfide minerals, for example. Due to the high affinity between Hg and sulfur, tin mining can be a possible source of Hg to the reservoir. This activity releases large amounts of particulate matter into the reservoir that can be rich in Hg.
Sampling and sample processing
In the upstream site, water was sampled in a vertical profile (subsurface, 5, 10, 15, 20, and 25 m) using a Van Dorn bottle. Two samples were taken at each depth and immediately filtered through a Millipore AP-40 glass fiber membrane to obtain SPM. One sample was lost during handling procedures at 10 m, so we analyzed only one membrane from this depth. Limnological variables (pH, dissolved oxygen, conductivity, and temperature) were measured at the same time and depths of water sampling. This site was 29 m total depth at the time of sampling. Exactly the same limnological and SPM sampling procedures were conducted at the subsurface waters from the downstream site, where the total depth was 4 m.
The plankton samples were taken at upstream and downstream sites by horizontal hauls at the water surface, using conical plankton nets of 20 and 68 μm mesh size. The samples collected with a net of 20 μm were considered as phytoplankton, comprising material >20 μm, and the samples collected with a net of 68 μm were considered as zooplankton, comprising material >68 μm. Since the classification was undertaken based on mesh size, some algae were probably included in the zooplankton sample, and some zooplankton were probably included in the phytoplankton sample. The filtered material obtained by hauls of each net in each site were kept together composing one sample of phytoplankton from upstream and one from downstream, and one sample of zooplankton from upstream and one from downstream. These samples were stored in polyethylene bottles pre-cleaned with acid.
Fish were collected at both sites (upstream and downstream) by means of gill-nets and hook and line. Each individual was weighed, measured (standard length), and killed by freezing immediately after collection. Their sex was determined by macroscopic examination of gonads (Vazzoler, 1996). Since fishes can modify their food habits during life stages, we selected only adult individuals, based on their standard length. Considering the relation of Hg levels and body size (Lucotte et al., 1999), we also selected individuals (for the species captured in both sites) with a maximum similarity in standard length and weight, as far as was possible, in order to obtain two similar batches from each sampling site.
We analyzed Hg levels in intestine and skinless dorsal muscle (located above the lateral line) from 108 individuals of 10 fish species: Serrasalmus rhombeus (Linnaeus, 1766); Cichla monoculus Spix & Agassiz, 1829; Rhaphiodon vulpinus Spix & Agassiz, 1829; Pinirampus pirinampu (Spix & Agassiz, 1829); Hypophthalmus marginatus Valenciennes, 1840; Hemiodus unimaculatus (Bloch, 1794); Schizodon fasciatus Spix & Agassiz, 1829; Laemolyta proxima (Garman, 1890); Leporinus friderici (Bloch, 1794); and Leporinus affinis Günther, 1864. Three fish species (R. vulpinus, L. proxima, and S. rhombeus) were caught in both sampling sites, downstream and upstream. The only R. vulpinus caught upstream had a standard length and weight within the range of this species from the downstream samples. The only L. proxima collected downstream had a standard length and weight lower than the individuals caught upstream. Specimens of S. rhombeus had the same standard length in both sampling sites (t test; t = 1.53; P = 0.15), although the mean weight of individuals from downstream was higher than from upstream (t test; t = 5.66; P < 0.001; Table 1).
The determination of the fish’s trophic level was based on specific literature. We also conducted a diet analyses on the same 108 specimens assessed for Hg concentrations. Stomach contents of fish were analyzed to identify and to estimate the relative volume of the food items (Hyslop, 1980; Branco et al., 1997).
All samples for Hg analysis (SPM membranes, phytoplankton, zooplankton, and muscle and intestine of fish) were stored at −18°C, in the laboratory they were freeze-dried and stored in hermetically sealed vessels until analytical procedures. The Hg concentrations were expressed as dry weight for SPM and plankton, and as wet weight for fish tissues. The percentage of water in the tissue (weight loss upon freeze-drying) was used for the conversion from dry to wet weight. Samples were collected, stored, and analyzed using ultra-clean techniques, including the use of polyethylene gloves, acid pre-treatment of laboratory material, pre-combusted SPM membrane at 400°C before filtration, and chemical reagents with high purity for preparing solutions (Bastos et al., 1998).
Mercury analysis
The total mercury (THg) contents were determined in all samples by hot extraction with hydrogen peroxide and acid, followed by oxidation with an aqueous solution of potassium permanganate (Bastos et al., 1998). The contents of organic mercury (OrgHg) were determined in the muscle and the intestine of fish and plankton samples by leaching with an aqueous solution of acid sodium bromide and cupric sulfate, followed by dichloromethane–hexane extraction, and hot acid-digestion (Kehrig et al., 2008). In both methods (THg and OrgHg), Hg was quantified by Cold Vapor Atomic Absorption Spectrometry with a Flow Injection Mercury System (FIMS)—FIAS 400 (Perkin Elmer), using sodium borohydride as a reducing agent. THg concentrations correspond to the sum of organic and inorganic mercury (InorgHg) concentrations. Therefore, the InorgHg concentrations were calculated by subtracting the OrgHg from the THg concentrations in each sample. The OrgHg ratio (%OrgHg) is the ratio of OrgHg in relation to THg.
The accuracy of the Hg analysis methods utilized was determined by comparison with certified reference samples (DORM-2, n = 30, and TORT-2, n = 10, both from the National Research Council of Canada). The values determined were consistently within the certified ranges, with recovery considered adequate for THg (99 ± 5% and 107 ± 2%) and OrgHg (94 ± 3% and 105 ± 5%), for DORM-2 and TORT-2, respectively. Each sample was analyzed in duplicate to assess the precision of the Hg methods utilized, and the standard errors of duplicates were <10%. The detection limits of THg and OrgHg were 0.18 and 0.14 μg l−1, respectively, corresponding to the mean of concentrations of the procedural blanks plus three times the standard deviation of the blanks (Miller & Miller, 1994).
Statistical analysis
In order to statistically compare the Hg concentrations in fish from different trophic levels and sites (upstream and downstream), we conducted a two-way ANOVA. This analysis detects the independent effect of both factors (trophic level and site) as well as their possible interaction. The ANOVA was followed by a Tukey post hoc test. To compare, within each species, THg levels between male and female we conducted a t Student test. We did not do a t test in the fish species with n < 5 per group, totalizing at least n = 10. To test for the normality and homocedasticity of data we used, respectively, a Shapiro–Wilk and a Levene’s test. When necessary, the data were log transformed. The significance level used was 0.05.
Results
Mercury levels in suspended particulate matter and plankton
The THg levels in SPM from the upstream site differed slightly with depth. These concentrations were lowest at 5 and 10 m, and uniform at other depths. THg levels of SPM downstream coincided with the concentrations in the deep water upstream (Fig. 2). The measured limnological variables confirm that the reservoir was not stratified at the time of sampling (Fig. 3). In addition, the limnological conditions were similar between the two sites.
The zooplankton from the upstream site had %OrgHg, organic and inorganic Hg levels similar to the zooplankton from downstream. The same was shown by phytoplankton, except for InorgHg levels which were higher downstream. Regardless of the sampling site, the phytoplankton showed OrgHg concentrations and %OrgHg lower than the zooplankton, while the InorgHg levels in phytoplankton were higher than those observed in zooplankton (Fig. 4).
Trophic classification of fish
According to specific literature and diet analyses, the fish species can be separated into three trophic levels: carnivores, omnivores, and herbivores (Table 1). P. pirinampu, R. vulpinus, and C. monoculus were considered carnivores, since almost all items found in their stomach were fish debris, only a small percentage (<1%) was plant debris. S. rhombeus was also considered a carnivore, with the percentage (mean ± standard deviation) of stomach items divided among fish debris (upstream: 98.0 ± 3.7%; downstream: 94.8 ± 12.1%) and plant debris (upstream: 2.0 ± 3.7%; downstream: 5.2 ± 12.1%). L. proxima, L. friderici, L. affinis, and S. fasciatus were considered herbivores, since the most important food item found in the stomach was plant debris (almost 100%). The items most frequently found in the stomach of H. marginatus were phytoplankton (50.7 ± 26.7%) and zooplankton (49.3 ± 26.7%); therefore this species was considered omnivore. H. unimaculatus was also considered omnivore, with the percentage of stomach items divided among phytoplankton (54.5 ± 26.7%), zooplankton (40.3 ± 28.1%), and filamentous algae (5.3 ± 3.6%). All the Hg analyses in the following results and discussion were based on the trophic levels, rather than on the taxonomic species. This was justified by the results of the following tests. After trophic classification of fish, an ANOVA, followed by a post hoc test (Tukey test) with THg levels in muscle from all carnivorous species, revealed two groups of species (F = 20.64, P < 0.001), those from upstream were significantly lower than those from downstream (S. rhombeus from upstream = C. monoculus from upstream < S. rhombeus from downstream = P. pirinampu from downstream = R. vulpinus from downstream). Furthermore, the only R. vulpinus caught upstream had THg levels within the range of the other carnivorous species from upstream. Therefore, we had homogeneous batches of carnivorous fish in each site.
Mercury levels in fish
The Hg concentrations in the muscle of fish increased with the trophic level at both sites (Fig. 5). THg and OrgHg levels in muscle were influenced by interaction between the two factors analyzed, trophic level and sampling site (interaction: THg: F = 9.7, P < 0.001; OrgHg: F = 10.5, P < 0.001). The differences of Hg level between sites depended on fish trophic level. The herbivorous fish showed similar muscle concentrations at the two sites. On the other hand, muscle concentrations in omnivores from downstream were, on average, 3.7 and 3.8 times higher than levels of omnivores from upstream for THg and OrgHg, respectively. The carnivores also showed higher concentrations from downstream than those from upstream (2.4 and 2.5 times, on average, for THg and OrgHg, respectively). The OrgHg concentrations in muscle were higher than those of InorgHg, independent of sampling site or trophic level, with the %OrgHg ranging from 75 to 100% (Table 2).
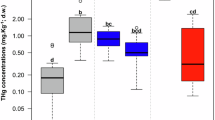
Mean mercury concentrations in tissues of fish from different trophic levels from upstream (white square with dashed line) and downstream (black square with continuous line) from the Reservatório de Samuel dam. Different letters, within each graph, indicate statistical differences. Bars indicate 95% confidence intervals. Total mercury in muscle (a) and in intestine (b), and organic mercury in muscle (c) and in intestine (d)
THg and OrgHg levels as well as %OrgHg in intestine increased according to the fish trophic level for both sampling sites (Fig. 5, Table 2). THg and HgOrg levels were not influenced by interaction between the trophic level and sampling site (interaction: THg: F = 0.2, P = 0.83; OrgHg: F = 0.2, P = 0.85). However, each factor separately was important to determine the intestine Hg levels (trophic level: THg: F = 79.4, P < 0.001; OrgHg: F = 134.9, P < 0.001 and sampling site: THg: F = 6.3, P = 0.01; OrgHg: F = 10.8, P = 0.001). The Hg concentrations in the intestines of herbivores were similar between the two sites. Different from that observed in muscle, the intestine concentrations in omnivores were similar between the two sites. The levels in intestines of carnivores from downstream were, on average, 1.5 and 1.7 times higher than those of carnivores from upstream for THg and OrgHg, respectively.
No significant differences in THg levels in muscle were observed between males and females (C. monoculus: t = 0.32, P = 0.76; P. pirinampu: t = 1.91, P = 0.08; R. vulpinus: t = 0.75, P = 0.47; S. rhombeus from upstream: t = 0.33, P = 0.75; H. unimaculatus: t = 0.02, P = 0.98).
Considering the maximum recommended limit for human consumption of fish (0.5 μg g−1 fish wet weight) established by the World Health Organization (FAO/WHO, 1991), 42 and 97% of the carnivores exceeded this limit at upstream and downstream sites, respectively. Among non-carnivorous, all analyzed specimens from upstream were safe for consumption, whereas 29% exceeded that limit at the downstream site (Fig. 6).
Total mercury (THg) concentrations in muscle of fish collected upstream (a) and downstream (b) from the Reservatório de Samuel dam. The dashed line is the maximum recommended limit established by World Health Organization (FAO/WHO, 1991) for human consumption of fish
Discussion
We hypothesized that limnological conditions in reservoir hypolimnion favor increased methylation and release of MeHg in the waters downstream from the Reservatório de Samuel dam. This MeHg can then bioaccumulate and biomagnify through the aquatic food web, resulting in high Hg levels in biota downstream. Concentrations of Hg were significantly higher below the dam in omnivorous and carnivorous fish. In contrast, Hg levels were similar between upstream and downstream sites in herbivorous fish, SPM and plankton. Studies in two other Amazon reservoirs showed the highest Hg levels in the same three compartments assessed in this study (SPM, plankton, and fish) below the dam (Malm et al., 2004; Palermo et al., 2004; Dominique et al., 2007). It is important to consider that these two reservoirs were stratified during the sampling season, and Samuel was not. The processes that favor high Hg concentrations downstream from the dam should occur mostly when the reservoir is stratified. Therefore, the Hg levels observed in this study possibly were affected by the season sampled, sampling site, compartment analyzed and trophic level of fish.
Since SPM and plankton are extremely dynamic compartments, reflecting the conditions during sampling, their similar Hg concentrations observed from both sampling sites may indicate there are no conditions favoring methylation in the absence of stratification. Studies conducted in both Elephant Butte (USA) and Petit-Saut (French Guiana) reservoirs have shown consistent changes in MeHg levels in water according to stratification–destratification dynamics (Canavan et al., 2000; Muresan et al., 2008). During stratified periods, the authors recorded much higher MeHg in the reservoir hypolimnion and, consequently, in downstream areas from the dam, compared to the surface layer of the reservoir. In the season of mixed waters, the Hg outputs decreased 25% (Muresan et al., 2008). In Tucuruí, a permanently stratified reservoir in the Amazon basin, an increase of about three times was shown in Hg levels in plankton below the dam (Malm et al., 2004).
A long-term study (between 2003 and 2005), undertaken also in Reservatório de Samuel, detected a relationship between the period of the year and THg levels in plankton from the upstream site (Nascimento, 2006; Nascimento et al., 2009). During the dry season, the authors observed low THg levels. As soon as the reservoir destratifies (early rainy season), when remobilization of hypolimnion takes place, high values were recorded; and these levels decreased until reaching low values in the dry season (Nascimento, 2006; Nascimento et al., 2009). At the downstream site, studied since the dry season in 2005, the THg levels in plankton were similar or higher than those from upstream, but without a clear relationship with season (Nascimento, 2006). Considering that this last study assessed THg levels in plankton, and MeHg can be an important species that exports Hg downstream, we suggest that further studies assess the MeHg (or OrgHg) in plankton during a seasonal cycle of Reservatório de Samuel.
Differently from SPM and plankton, fishes enable an analysis of chronic pollution of Hg (Jahanbakht et al., 2002). These organisms have high accumulation capability and low depuration rate of Hg (Wiener et al., 2002). Thus, the Hg levels in fish, particularly in muscle, are more integrative in time than the former fast-cycling compartments.
In this study, the differences found in Hg concentrations from the muscle of fish between upstream and downstream sites depend on their trophic level. The higher Hg concentrations observed in the muscle of carnivores and omnivores collected below the dam suggest that these organisms are being influenced by the reservoir. On the other hand, herbivores showed similar muscle concentrations between sites. The MeHg exported downstream is mainly associated with SPM, incorporated in the plankton, and dissolved in water (Schetagne et al., 2000; Dominique et al., 2007). The MeHg exported in the dissolved phase can be adsorbed/absorbed by plankton, adsorbed by SPM, or absorbed by fish gills (Window & Kendall, 1979; Simon & Boudou, 2001; Fishe & Hook, 2002), although this last process is considered of weak importance when compared to exposure by feeding (Boudou & Ribeyre, 1997; Wiener et al., 2002). The Hg accumulated in plankton and other suspended particles can get through the fishes food chain, transferring the Hg to higher trophic levels. Although we collected fish when the reservoir was not stratified, we suggest that the process mentioned above can occur in Samuel, resulting in the high Hg levels observed in fish from downstream. To confirm this hypothesis, Hg data from the dry season is required. It is interesting to observe that omnivores from the downstream site showed similar Hg levels in muscle to carnivores from upstream, even feeding on plankton, an item located much lower in the food chain compared to the carnivores’ prey (principally fish). The herbivorous fish, on the other hand, feed mainly on vegetation near the river banks, especially on leaves of grasses, which are not significantly influenced by Hg from aquatic systems (Stamenkovic & Gustin, 2009; Zhang et al., 2010). Studies have shown that roots act as a barrier to Hg uptake by plants, including Oryza spp. (Du et al., 2005; Sierra et al., 2009; Meng et al., 2010). These results are similar to those found in Lago Manso, another Brazilian reservoir, where fish with different carbon sources had different responses regarding the reservoir effect on Hg concentrations, with the average fish levels higher downstream except for one herbivorous fish species (Tuomola et al., 2008).
While the muscle represents an integrated sample, the intestine of fish possibly reflects the Hg levels in food consumed by the fish in the current season sampled. Since the Hg concentrations of the food of herbivores are not much influenced by the reservoir, their intestine showed similar concentrations between the two sites, as observed for the muscle. The carnivorous fishes feed mainly on fishes, which have a long Hg half-life (Wiener et al., 2002), thus both their muscle and intestine reflect integrated concentrations in time. The Hg concentrations in the intestine of omnivores were similar at both sites, following the pattern observed for plankton (their main food item), probably because this item is not influenced by the reservoir at the end of the rainy season.
Some additional factors could also contribute to explain our Hg data, and must be addressed, such as the low oxygen levels downstream during half the year. The oxygen deficiency can enhance methylation of Hg and its mobility and bioavailability (Huchabee et al., 1979). The downstream site is a lotic system, without associated large wetland areas, and the oxygen deficiency gradually improves with increasing distance from the dam (Viana, 2002). Therefore, these low oxygen levels may not have a great influence in Hg methylation downstream. However, the mobility and bioavailability of Hg can improve its bioaccumulation in the downstream area.
We selected specimens from both sampling sites in order to obtain two similar batches of fish. Even with this selection, one specimen of L. proxima from downstream was smaller than the individuals from upstream. Removing this individual from statistical analysis, the results remain the same, similar mean Hg levels between herbivores from both sites. The weight of another species, S. rhombeus, was greater downstream, even with the same length at both sites. Therefore, its different physiology could have contributed to the observed higher levels of Hg in this species downstream.
Within each trophic level, no marked differences were observed between the stomach contents of the fishes from both sites considering the categories of food items assessed. However, the food items of carnivores, mainly composed of fish debris, were not identifiable on a species specific level, biasing our interpretation. Carnivores from upstream and downstream sites could feed on different prey from different trophic levels, leading to different Hg uptake to these fish. This could also explain the higher levels in carnivores from downstream.
The diet of Amazon people is primarily composed of cassava and fish (Dorea, 2004). Along the Rio Madeira, which receives the Rio Jamari and Reservatório de Samuel waters, the consumption of fish was estimated at 250 g day−1 for adults (Bastos et al., 2006). Fish is an excellent source of good-quality protein but it can also be a source of MeHg. Considering that over 75% of Hg accumulated in freshwater fish muscle tissue is commonly MeHg (Ikingura & Akagi, 2003), the maximum tolerable weekly intake of THg is around 4.4 μg kg−1 body weight. This calculation is based on the maximum tolerable weekly intake of MeHg of 3.3 μg kg−1 body weight for adults, except women of childbearing age (FAO/WHO, 2006). With a fish consumption of 250 g day−1, a person with 60 kg should eat fish with maximum Hg levels of 0.15 μg g−1 in order to avoid exceeding the maximum tolerable intake of Hg. Even if we consider the maximum level of Hg in fish (0.5 μg g−1) as recommended by the WHO (based on approximately 400 g weekly intake of fish for a person with 60 kg of body weight), the bulk of fish in this study exceeded this limit, especially fish from downstream. These findings show that the Hg levels must be monitored in reservoir’s drainage basin, since the high Hg levels can lead to health risks, especially in areas where fish consumption is high, as in the studied region.
In this study, the %OrgHg was lowest in phytoplankton, intermediate in zooplankton, and highest in muscle of fish. This increase in ratios must be a consequence of a rise in OrgHg concentrations, mainly MeHg, through the food web. In addition, the InorgHg does not biomagnify (Kasper et al., 2009). The same tendency could also be observed for the intestines. There was a gradual increase of %OrgHg in the intestine of the fish, following an increase in the trophic level, because the intestine reflects the trophic level of food that was consumed by fish.
Conclusion
We have shown that Hg levels in omnivores and carnivorous fish are significantly higher downstream from the dam. Our hypothesis is that limnological conditions in reservoir hypolimnion favor methylation during the dry season. The MeHg produced could outflow downstream and biomagnify through the aquatic food web. Further studies are needed to confirm if this process is causing the elevated Hg levels observed. We suggest assessing Hg levels in plankton and fish intestines during the dry season at upstream and downstream sites, and in the muscle of carnivorous fish of same species at both sites (followed by a diet analyses). The results highlight the necessity of assessing areas downstream of reservoirs.
References
Anderson, M. R., 2011. Duration and extent of elevated mercury levels in downstream fish following reservoir creation. River Systems 19(3): 167–176.
Bastos, W. R., O. Malm, W. C. Pfeiffer & D. Cleary, 1998. Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon, Brazil. Ciência e Cultura 50: 255–260.
Bastos, W. R., J. P. O. Gomes, R. C. Oliveira, R. Almeida, E. L. Nascimento, J. V. E. Bernardi, L. D. Lacerda, E. G. Silveira & W. C. Pfeiffer, 2006. Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Science of the Total Environment 368: 344–351.
Bermann, C., 2002. Energia no Brasil: para quê? Para quem? Crise e alternativas para um país sustentável. Livraria da Física, São Paulo.
Bodaly, R. A. D., W. A. Jansen, A. R. Majewski, R. J. P. Fudge, N. E. Strange, A. J. Derksen & D. J. Green, 2007. Postimpoundment time course of increased mercury concentrations in fish in hydroelectric reservoirs of northern Manitoba, Canada. Archives of Environmental Contamination and Toxicology 53: 379–389.
Boudou, A. & F. Ribeyre, 1997. Mercury in the food web: accumulation and transfer mechanisms. In Sigel, A. & H. Sigel (eds), Metallons in Biological Systems—Mercury and Its Effects on Environment and Biology. Marcel Dekker, New York: 289–320.
Branco, C. W. C., T. Aguiaro, F. A. Esteves & E. P. Caramaschi, 1997. Food sources of the Teleost Eucinostomus argenteus in two coastal lagoons of Brazil. Studies on Neotropical Fauna and Environment 32: 33–40.
Canavan, C. M., C. A. Caldwell & N. S. Bloom, 2000. Discharge of methylmercury enriched hypolimnetic water from a stratified reservoir. Science of the Total Environment 260: 159–170.
Dominique, Y., R. Maury-Brachet, B. Muresan, R. Vigouroux, S. Richard, D. Cossa, A. Mariotti & A. Boudou, 2007. Biofilm and mercury availability as key factors for mercury accumulation in fish (Curimata cyprinoids) from a disturbed Amazonian freshwater system. Environmental Toxicology and Chemistry 26: 45–52.
Dorea, J. G., 2004. Cassava cyanogens and fish mercury are high but safely consumed in the diet of native Amazonians. Ecotoxicology and Environmental Safety 57: 248–256.
Du, X., Y. G. Zhu, W. J. Liu & X. S. Zhao, 2005. Uptake of Mercury (Hg) by seedlings of Rice (Oryza sativa L.) grown in solution culture and interactions with arsenate uptake. Environmental and Experimental Botany 54: 1–7.
FAO/WHO, 1991. Codex Alimentarius: Guideline Levels for Mercury in Fish (CAC/GL 7-1991). Taked by the Commission at its Nineteenth Session in Italy 1–10 July 1991.
FAO/WHO, 2006. Summary and Conclusions of the Sixty-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in Rome 20–29 June 2006.
Fishe, N. S. & S. E. Hook, 2002. Toxicology tests with aquatic animals needs to consider the trophic transfer of metals. Toxicology 181: 531–536.
Gantzer, P. A., L. D. Bryant & J. C. Little, 2009. Controlling soluble iron and manganese in a water-supply reservoir using hypolimnetic oxygenation. Water Research 43: 1285–1294.
Goulding, M., 1980. The Fishes and the Forest: Explorations in Amazonian Natural History. University of California Press, Los Angeles.
Huchabee, J. W., J. W. Elwood & S. C. Hildebrand, 1979. Accumulation of mercury in freshwater biota. In Nriagu, J. O. (ed.), The Biogeochemistry of Mercury in the Environment. Elsevier, Amsterdam: 277–302.
Hylander, L. D., J. Gröhn, M. Tropp, A. Vikström, H. Wolpher, E. C. Silva, M. Meili & L. J. Oliveira, 2006. Fish mercury increase in Lago Manso, a new hydroelectric reservoir in tropical Brazil. Journal of Environmental Management 81: 155–166.
Hyslop, E. J., 1980. Stomach content analysis—a review of methods and their application. Journal of Fish Biology 17: 411–429.
Ikingura, J. R. & H. Akagi, 2003. Total mercury and methylmercury levels in fish from hydroelectric reservoirs in Tanzania. Science of the Total Environment 304: 355–368.
Jahanbakht, S., F. Livardjani & A. Jaeger, 2002. An experimental ecotoxicological study and its application to the behavioural study of organic mercury (CH3HgCl) in the environment: influence of temperature and pH. Chemosphere 49: 1399–1405.
Kamman, N. C., N. M. Burgess, C. T. Driscoll, H. A. Simonin, W. Goodale, J. Linehan, R. Estabrook, M. Hutcheson, A. Major, A. M. Scheuhammer & D. A. Scruton, 2005. Mercury in freshwater fish of Northeast North America—a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14: 163–180.
Kasper, D., E. F. A. Palermo, A. C. M. I. Dias, G. L. Ferreira, R. P. Leitão, C. W. C. Branco & O. Malm, 2009. Mercury distribution in different tissues and trophic levels of fish from a tropical reservoir, Brazil. Neotropical Ichthyology 7: 751–758.
Kehrig, H. A., T. G. Seixas, E. F. A. Palermo, A. P. M. Di Beneditto, C. M. M. Souza & O. Malm, 2008. Different species of mercury in the livers of tropical dolphins. Analytical Letters 41: 1691–1699.
Lindqvist, O., K. Johnasson, M. Aastrup, A. Andersson, L. Bringmark, G. Hovsenius, A. Hakanson, M. Meili & B. Timm, 1991. Mercury in the Swedish environment-recent research on causes, consequences and corrective methods. Water, Air and Soil Pollution 55: 1–251.
Lucotte, M., R. Schetagne, N. Thérien, C. Langlois & A. Tremblay, 1999. Mercury in the Biogeochemical Cycle: Natural Environments and Hydroelectric Reservoirs of Northern Québec. Springer, Berlin.
Malm, O., E. F. A. Palermo, H. S. B. Santos, M. F. Rebelo, H. A. Kehrig, R. B. Oliveira, R. O. Meire, F. N. Pinto, L. P. A. Moreira, J. R. D. Guimarães, J. P. M. Torres & W. C. Pfeiffer, 2004. Transport and cycling of mercury in Tucuruí reservoir, Amazon, Brazil: 20 years after fulfillment. RMZ Materials and Geoenvironment 51: 1195–1198.
Meng, B., X. Feng, G. Qiu, Y. Cai, D. Wang, P. Li, L. Shang & J. Sommar, 2010. Distribution patterns of inorganic mercury and methylmercury in tissues of rice (Oryza sativa L.) plants and possible bioaccumulation pathways. Journal of Agricultural and Food Chemistry 58: 4951–4958.
Miller, J. C. & J. N. Miller, 1994. Statistics for Analytical Chemistry. Ellis Horwood, Great Britain.
Muresan, B., D. Cossa, S. Richard & Y. Dominique, 2008. Monomethylmercury sources in a tropical artificial reservoir. Applied Geochemistry 23: 1101–1126.
Nascimento, E. L., 2006. Concentração de mercúrio no plâncton e fatores ecológicos no Reservatório da UHE—Samuel—Amazônia ocidental (Rondônia/Brasil). Dissertation, Universidade Federal de Rondônia.
Nascimento, E. L., J. P. O. Gomes, D. P. Carvalho, R. Almeida, W. R. Bastos & K. R. Miyai, 2009. Mercúrio na comunidade planctônica do reservatório da Usina Hidrelétrica de Samuel (RO), Amazônia Ocidental. Geochimica Brasiliensis 23: 101–116.
Palermo, E. F. A., D. Kasper, T. S. Reis, S. Nogueira, C. W. C. Branco & O. Malm, 2004. Mercury level increase in fish tissues downstream the Tucuruí Reservoir, Brazil. RMZ Material and Geoenvironment 51: 1292–1294.
Porvari, P., 1995. Mercury levels of fish in Tucuruí hydroelectric reservoir and in River Mojú in Amazonia, in the state of Pará, Brazil. Science of the Total Environment 175: 109–117.
REN21, 2009. Renewables Global Status Report: Update. GTZ, Paris.
Rogers, D. W., M. Dickman & X. Han, 1995. Stories from old reservoirs: sediment Hg and Hg methylation in Ontario hydroelectric developments. Water, Air and Soil Pollution 80: 829–839.
Santos, G. M., 1995. Impactos da hidrelétrica Samuel sobre as comunidades de peixes do rio Jamari (Rondônia, Brasil). Acta Amazônica 25: 247–280.
Santos, G. M., M. Jégu & B. Merona, 1984. Catálogo de peixes comerciais do baixo Rio Tocantins. Eletronorte/INPA/CNPq, Manaus.
Santos, G. M., E. Ferreira & J. A. S. Zuanon, 2006. Peixes comerciais de Manaus. ProVárzea/IBAMA, Manaus.
Schetagne, R., J. F. Doyon & J. J. Fournier, 2000. Export of mercury downstream from reservoirs. Science of the Total Environment 260: 135–145.
SEDAM, 2002. Atlas Geoambiental de Rondônia. SEDAM, Porto Velho.
Sierra, M. J., R. Millán & E. Esteban, 2009. Mercury uptake and distribution in Lavandula stoechas plants grown in soil from Almadén mining district (Spain). Food and Chemical Toxicology 47: 2761–2767.
Silva-Forsberg, M. C., B. R. Forsberg & V. K. Zeidemann, 1999. Mercury contamination in humans linked to river chemistry in the Amazon Basin. Ambio 28: 519–521.
Simon, O. & A. Boudou, 2001. Direct and trophic contamination of the herbivorous carp Ctenopharyngodon idella by inorganic mercury and methylmercury. Ecotoxicology and Environmental Safety 50: 48–59.
St. Louis, V. L., J. W. M. Rudd, C. A. Kelly, R. A. D. Bodaly, M. J. Paterson, K. G. Beaty, R. H. Hesslein, A. Heyes & A. R. Majewski, 2004. The rise and fall of mercury methylation in an experimental reservoir. Environmental Science and Technology 38: 1348–1358.
Stamenkovic, J. & M. S. Gustin, 2009. Nonstomatal versus stomatal uptake of atmospheric mercury. Environmental Science and Technology 43: 1367–1372.
Tuomola, L., T. Niklasson, E. C. Silva & L. D. Hylander, 2008. Fish mercury development in relation to abiotic characteristics and carbon sources in a six-year old, Brazilian reservoir. Science of the Total Environment 390: 177–187.
Vazzoler, A. E. A. M., 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM/SBI, Maringá/São Paulo.
Viana, J. P., 2002. Physical and chemical post-dam alteration in the Jamari River, a hydroelectric-developed river of the Brazilian Amazon. Hydrobiologia 472: 235–247.
Wiener, J. G., D. P. Krabbenhoft, G. H. Heinz & A. M. Scheuhammer, 2002. Ecotoxicology of mercury. In Hoffman, J., B. A. Rattner, G. A. Burton & J. Cairns (eds), Handbook of Ecotoxicology. CRC, Boca Raton: 409–463.
Window, H. L. & D. R. Kendall, 1979. Accumulation and biotransformation of mercury. In Nriagu, J. O. (ed.), The Biogeochemistry of Mercury in the Environment. Elsevier/North Holland Biomedical Press, Amsterdam: 303–323.
Zhang, H., X. Feng, T. Larssen, L. Shang & P. Li, 2010. Bioaccumulation of methylmercury versus inorganic mercury in rice (Oryza sativa L.) grain. Environmental Science and Technology 44: 4499–4504.
Acknowledgments
The authors thank the financial support of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (scholarship to D Kasper), Centrais Elétricas do Norte do Brasil and Conselho Nacional de Desenvolvimento Científico e Tecnológico. We are most thankful to the staff at the laboratory Biogeoquímica Ambiental (UNIR) for their help (WR Bastos, R Almeida, JM Menezes, IBB Holanda, DP Carvalho). The authors thank CAO Ribeiro, VF Magalhães, EP Caramaschi, JRD Guimarães, JL Brito, and RP Leitão for important contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: John M. Melack
Rights and permissions
About this article
Cite this article
Kasper, D., Palermo, E.F.A., Branco, C.W.C. et al. Evidence of elevated mercury levels in carnivorous and omnivorous fishes downstream from an Amazon reservoir. Hydrobiologia 694, 87–98 (2012). https://doi.org/10.1007/s10750-012-1133-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1133-x