Abstract
The ecology of cubozoans is poorly understood and there are few quantitative studies on their distribution patterns. Sampling was designed to test first for variation in abundance with distance across the continental shelf in waters of the Great Barrier Reef, Australia. Second, we tested for the possible influence of islands versus submerged reefs on the abundances of cubozoan jellyfishes. Jellyfishes were collected after attraction to tethered night lights. Additional sampling focused on turbid near-shore waters. Carybdeid jellyfishes were found at mainland, inner, and mid-shelf reefs during summers between 2007 and 2010. No cubozoan medusae were found at outer reef sites. Copula sivickisi and Carukia barnesi were more abundant near reefs with islands than at fully submerged reefs. There was no evidence of lunar periodicity in abundance for these cubozoan taxa. Chironex fleckeri medusae were only found close to shore near the mainland, but they were rarely observed when riverine runoff was high. All taxa were characterized by high spatial and temporal variation and there was some evidence for small populations at spatial scales of less than one kilometer. “Blooms” and related intensity of predation and risk to humans are most likely at small spatial scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jellyfishes of the Class Cubozoa (box jellyfish) are of great biological interest (Bentlage et al., 2010) and are a great risk to users of tropical waters (Barnes, 1966; Gershwin et al., 2010). Despite their low species diversity (40–50 species, Bentlage et al., 2010), they are morphologically diverse and have fast growth rates (Gordon et al., 2004), interesting life histories (Hartwick, 1991a; Straehler-Pohl & Jarms, 2005), strong swimming abilities (Gordon & Seymour, 2009), complex eyes that are used to hunt (Coates & Theobald, 2003; Nilsson et al., 2005), and powerful venom (e.g., Kintner et al., 2005). The nematocysts of “Stingers” (Chironex fleckeri Southcott) cause life threatening stings and have been responsible for many deaths in Australia alone (Gershwin et al., 2010). Other taxa are also a threat. For example, “Irukandji Syndrome” is an envenoming reaction in humans that results from stings of several species of box jellyfish (Little et al., 2006), which on rare occasions results in death (Pereira et al., 2010). Cubozoans that pose threats to humans occur in tropical waters of many parts of the world (Fenner & Lippmann, 2009). The threat of cubozoans has given great focus to the nature of venoms (Nagai et al., 2000; Underwood & Seymour, 2007), geographic variation in venoms (Winter et al., 2009), affects on patients (Winter et al., 2008; Tiong, 2009), and the development of antivenoms. Although there is a diversity of dangerous cubozoan medusae in tropical waters, knowledge of their ecology is poor.
Scyphozoan and cubozoan jellyfishes are notoriously patchy in distribution at spatial scales ranging from meters to tens of kilometers (Pitt & Kingsford, 2000; Gordon et al., 2004). Other species are simply very difficult to find, which has been a major problem for cubozoan research. For example, Hartwick (1991b) completed 47 cruises across the continental shelf on the Great Barrier Reef (GBR) near Townsville. On each cruise, multiple Tucker Trawls and neuston tows were done, but only eight C. fleckeri Southcott and about 82 other cubozoans were caught in total. Similarly, in 700 tows he collected only 100 C. fleckeri, with maximum densities <3 medusae per 100 m3 within four estuaries. Other approaches have included casual observations (Matsumoto, 1995) and the sampling of beach wrack for jellyfishes, which have yielded few medusae (Yamada et al., 2010). There are few data on temporal variation in abundance, but new cohorts of one species, Chiropsella bronzie, appear after rain events (Gordon et al., 2004). Furthermore, in Hawaii regular occurrences of Alatina moseri Mayer appear 9–13 days after the full moon and this is thought to relate to spawning (Thomas et al., 2001).
Physical forcing often has a significant role in the population dynamics of jellyfishes. Variation in factors such as salinity, temperature, and abundance of food can directly affect abundance of jellyfishes. These factors often correlate with variation in nutrient levels, riverine runoff, and upwelling, which may affect the release of medusae from polyps and the survival of medusae (Kingsford et al., 2000). For example, medusae of the semaestostome Phyllorhiza punctata von Lendenfeld die if the salinity drops below 12 (Rippingale & Kelly, 1995). Greater knowledge of the environmental conditions required by jellyfishes is especially important because there is growing speculation about the affects of climate change on populations of jellyfish (e.g., Lynam et al., 2005, 2010)—specifically are blooms more likely?
Many cubozoans are photopositive and anecdotal accounts suggested that they could be attracted to lights, potentially allowing quantitative measures as for pre-settlement reef fishes collected with light traps (Doherty, 1987). Our objective was to focus on shallow waters near the reefs at different distances from the coast across the GBR. Shallow waters are important biologically and also are the areas of highest risk for swimmers.
The specific aims of this project were as follows:
-
1.
To use a mensurative experimental design to test the null hypotheses that abundances of cubozoan medusae do not vary with distance across the GBR and that patterns would be consistent in multiple cross-shelf transects;
-
2.
To test that broad-scale patterns of abundance of cubozoan medusae do not vary with lunar phase;
-
3.
To use mensurative experimental designs to test the null hypothesis that cubozoan medusa abundances are not different between islands and submerged reefs;
-
4.
To use opportunistic sampling and data from Surf Life Saving Australia (SLSA) to obtain data on rarer species;
-
5.
Test if patterns of abundance of C. fleckeri and riverine runoff are correlated.
Mensurative experimental designs are used to test hypotheses about patterns, where the sites are not selected by random (Hurlbert, 1984).
Methods
Abundances cross-shelf
The hypothesis that abundances of cubozoans do not vary cross-shelf was tested within the framework of a mensurative multi-factorial experimental design. Three cross-shelf cruises (transects) were completed annually during the summers of: (1) 2007–2008, (2) 2008–2009, and (3) 2009–2010 between December and February (see Fig. 1). Transects were Lizard Island, Cairns, and the Palm Island Group, and extended from 14°35′ E to 18°33′ E, about 450 km North–South (Table 1). Three categories were defined according to distance strata across the shelf (Distance strata: inner, mid, and outer). For each transect at the three distances, sampling was completed at two sites separated by 0.7–3 km. Cubozoan medusae were sampled by light attraction (1× 1,000 W bulbs positioned within the top 1 m of the water column). At each site, two replicate 1-h samples were taken for abundance data; replicates were taken from two anchored vessels that were separated by 50–200 m so that pools of light did not overlap. Additional sampling time was to collect more jellyfishes for size frequency determinations. The physical characteristics of the water column were also measured at most sites using a CTD (Seabird, SBE 19 Plus).
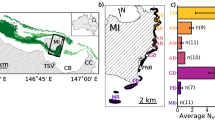
Map of areas in northeastern Australia for study of distribution patterns of cubozoan medusae. Circles indicate sites of night lighting in the cross-shelf-sampling design; squares indicate sites in the island verses reef design; the diamond is an additional site used in the temporal study. Lizard Transect (A), Cairns Transect (B), and Palms Transect (C)
Temporal variation in medusa abundances was determined from Mermaid Bay (over four summers) and Double Island (near Palm Cove, 16°43′32 S; 145°41′00 E; Fig. 1). Sampling was completed on multiple nights within each season.
The influence of geology on abundances of cubozoan medusae
Patterns found in 2007–2008 suggested that carybdeid jellyfishes would be most abundant around islands. In two summers we chose distances from shore where carybdeids were found in year one. We sampled two reefs mid-shelf on the Lizard Island transect (Lizard Island, a granite island and, the Rocky Islets) and two sites per reef. Rocky Islets are a group of reefs at a similar distance from the mainland as is Lizard Island, but the reefs rarely emerge at any state of tide and they are made up of a coral matrix. Similarly, on the Cairns transect, Green Island (a coral cay) was sampled mid-shelf to compare with Michaelmas, a largely submerged reef. In the summer of 2009–2010, a comparison was also made between Pandora Reef (Coral cay and mid to low tide) and the granitic islands of Pelorus and Orpheus (Table 1).
Sampling at mainland beaches and estuaries
Sampling by night light was not done near the mainland because the waters were very turbid. Trawling, visual counts, and opportunistic sampling were used in these waters at all cross-shelf transects. The beam trawl (1.5 m × 0.5 m mouth, 8-mm mesh) was towed in very shallow water (<2 m deep) and deeper waters (3–5 m deep) adjacent to the mainland, at the entrance of rivers, and 1 km from the rivers (n = 2 five-minute tows at each depth and location). The two depths of sampling allowed for variation in movement of jellyfishes with the tide. We measured the distance of the trawl with a General Oceanics 2030 flowmeter (200–250 m3 filtered per tow). Visual estimates and samples are also taken during the trawls (3-m-wide visual swath × distance of the trawl). Sampling was completed between December and February in the summers of: (1) 2007–2008, (2) 2008–2009, and (3) 2009–2010. The Cairns transect was not sampled near shore in 2007–2008 due to high freshwater flows and an abundance of floating logs.
Data on temporal variation in medusa abundance were collected using trawls and visual sampling from Townsville in 2008–2009 and 2009–2010 at three sites separated by 1–2 km at Rowes Bay (19°14.170 S, 146°47.379 E). An additional site was added in 2009–2010, on the Strand (19°14.762 S, 146°48.802 E); all trawl and visual count procedures were the same as above. Opportunistic sampling was done on each occasion that we boated along the edge of beaches and around the marina and harbor at the entrance to Ross Creek.
Additional sampling was undertaken at Port Douglas, Double Island near Palm Cove, Mission Beach, Balgal Beach, Townsville beaches, and Magnetic Island; some of these samples were provided by SLSA, which samples popular beaches daily between 16°28.394 S, 145°27.468 E and 19°15.638 S, 146°50.934 E. Samples were provided from Keeper Reef by the MV Kalinda. Verbal records of C. fleckeri were obtained from Palm Cove in Cairns, Mission Beach to Cardwell, the Strand in Townsville, and Magnetic Island. Data were categorized as: early (October–December), mid (January–February), and late (March–May) in the summer period.
Taxonomy
Identification of cubozoans has been problematic and uncertainty about taxonomic names still exists (Bentlage et al., 2010). We based identifications on Gershwin, (2005a, b) and Gershwin & Kingsford (2008). The majority of specimens were the carybdeids C. barnesi Southcott and Copula sivickisi Stiasny (recently changed from Carybdea sivickisi Stiasny; Bentlage et al., 2010). Some Carukia spp. were identified where we were not certain they were C. barnesi; it is possible they are undescribed taxa. Carybdea xaymacana Conant was identified according to Gershwin (2005b), but this identification has been questioned as being based on coincidental evidence (Bentlage et al., 2010); therefore, in this paper it should be considered a type. The size of cubozoans was measured as inter-pedalial distances (IPD).
Rainfall
The relationship between the abundances of jellyfishes collected near shore and riverine runoff was tested with a Pearson’s correlation. Data on freshwater outflow was obtained for major watersheds that drain onto the shelf adjacent to the areas where the abundance of C. fleckeri medusae was measured. River gauging station data were obtained from the Barron River (Cairns area) and the Tully River (between Mission Beach and the Herbert River). Data were expressed in megalitres (Ml); web source from Department of Environment and Resources Management (www.derm.qld.gov.au/watershed).
Treatment of the data
Data were analysed using a mixed model ANOVA; Distance (treatments: inner, mid, and outer shelf) was a fixed factor, and latitude (treatments: Lizard, Cairns, and Palms) and sites (nested in distance) were random factors. Data were sometimes ln(x + 1) transformed, but if they were still heterogeneous, according to Cochran’s tests, we proceeded with analyses on ln(x + 1) data as ANOVA is robust to heterogeneity (Underwood, 1997). Variance components were calculated only for raw data and with fully-nested designs and random factors (Kingsford, 1998). Because there are accounts of lunar periodicity in the occurrence of A. moseri Mayer medusae in Hawaii, we used a pattern-seeking approach with all cross-shelf data by plotting the abundance of Carukia spp. by phase of the lunar month (i.e., days 1–30; full moon on day 15). Catches of jellyfish from Double Island (part of the temporal study) were also compared with lunar phase.
Results
Abundance cross-shelf
A total of 208 cubozoans were collected during the first hour of sampling at sites and cross-shelf sampling programs over the three summer seasons December 2007 to February 2010; an additional 55 specimens were collected within the second hour (Table 2). The species breakdown was as follows (first hour sampling only): C. barnesi (76), Carukia other (6), and C. sivickisi (125); also see Table 2.
Catches of Carukia spp. were low in 2008–2009 and 2009–2010 compared to 2007–2008. In 2007–2008 we collected 69 C. barnesi (three cruises combined) in 1 h counts and an additional 43 specimens were collected. In 2008–2009, we collected four C. barnesi (three cruises combined) in 1 h counts and no additional specimens. Similarly in 2009–2010 we collected two C. barnesi (all transects combined); an additional four specimens were obtained that were not collected in 1 h counts (Fig. 2) and six C. barnesi were all caught at additional sites in the islands versus reefs design.
Although no irukandji jellyfishes were found offshore at any latitude or in any summer, cross-shelf patterns varied by latitude. When C. barnesi medusae were abundant in 2007–2008, variation across the shelf resulted in a significant latitude × distance interaction (Table 3). This was largely because no jellyfish were found in the Palm Island transect and high abundance only occurred mid-shelf on the Lizard Island transect.
Great differences were found among sites within distance strata (Fig. 3). For example, at Lizard Island (mid-shelf), means of 14.5 C. barnesi occurred at one site and 3.5 at the other. Forty-one percent of variation in abundance was explained by variation at the level of site. This suggested that C. barnesi populations may be very localised at small spatial scales such as within bays; the greatest variation was found among replicates (51%).
Mean abundance of Carukia spp. medusae (most were C. barnesi) collected in the Lizard Island cross-shelf transect at inner, mid, and outer shelf locations in northeastern Australia in 2007, All counts were done with night lighting. Variance components from nested ANOVA: distance 7.8%, df = 2, MS = 341.58; site (distance) 41.1%, df = 3, MS = 252.41; residual 51.1, df = 6, MS = 96.75. df degrees of freedom, MS mean square
Carukia spp. collected in the cross-shelf study ranged from 3 to 18 mm in IPDs (mean 8.5 mm). The majority of these jellyfish were collected at Lizard Island (95.3%) and the great variation in size suggested that the medusae had been released from polyps over many nights, rather than in a distinct pulse.
Other cubozoans were collected in the three summers and all were collected from the mainland to mid-shelf reefs (Table 2). Irukandji jellyfishes other than C. barnesi were as follows: Two C. xaymacana were caught in trawls on beaches near Cooktown. Two Alatina sp. were collected from a charter boat at a mid-shelf reef (Keeper) near the Palms transect, November 2009.
Copula sivickisi is a carybdeid cubozoan that has a mild sting that does not result in “Irukandji syndrome”. A total of 125 C. sivickisi were collected, 34 in 2007–2008 and 90 in 2008–2009, and 1 in 2009–2010. All were collected at mid-shelf reefs, Lizard Island, and Green Island. Casual counts with lights at Magnetic Island (Inner) also detected C. sivickisi in shallow water (Table 3). Although no C. fleckeri were collected around lights, many were found near the mainland in shallow water during the 3-year study.
There was no evidence for lunar periodicity in the abundances of Carukia spp. and C. sivickisi (Fig. 4). Relatively high abundances of Carukia spp. and C. sivickisi were found at more than one phase of the lunar cycle.
Where physical data were available and Carukia spp. were collected, they were found in waters with salinity ranging from 31.6 to 35.1 and temperatures of 28.1–30.0°C. With the exception of Fitzroy Island in 2009, where the water column was relatively fresh at the surface, the water columns were generally well mixed between top and bottom.
Does the presence of islands influence the abundance of cubozoans?
All carybdeid jellyfishes were collected near islands (Low Wooded Isle—Inner 1, Three Islands—Inner 2, Fitzroy Island—Inner, Lizard Island-mid) in the cross-shelf transects during the study, with the greatest numbers collected near granite islands (e.g., Lizard Island). Few C. barnesi were collected in 2008–2009 (one at Lizard Island) and 2009–2010 (one at Lizard Island, four at Pandora Reef (Inner, Palm Island Group). Pandora is not a granite-based reef, but it emerges at low tide and probably should be considered to be geologically similar to Three Islands (Inner, Lizard Transect), where we also collected carybdeids.
Multiple C. sivickisi medusae were collected for the paired comparisons. The relationship with the presence of islands was a clear; 90 were collected at islands and only one at reefs without islands (at Rocky Islets B; Fig. 5). There was great variation between replicates.
Temporal variation of C. barnesi
There was great temporal variation in abundance of Carukia spp. caught at Mermaid Bay, Lizard Island over four seasons; however, there was always a high probability of collecting Carukia spp. there. Even with this variation, differences among years, as described earlier (i.e., highest abundance in (2006–2008)), were robust (Table 4). Temporal variation in abundance also was great at Double Island (<1 km from the mainland); nevertheless, nine sampling days showed that the probability of detecting Carukia spp. was high regardless of year. We compared catches at Double Island (by day, n = 19) by lunar day (i.e., 1–30) and found no patterns, which concurred with the broad-scale study.
Sampling near the mainland
Few cubozoans were found in trawls and transects near the mainland. We collected only two C. xaymacana near Cooktown (Lizard Island Transect) and one C. fleckeri in transects over three summers; however, 255 C. fleckeri were observed or collected near the mainland (Table 2). Pooled data from near-shore surveys, our casual observations, some trained observers, and information from SLSA showed that the most C. fleckeri were collected between October and December in the summers of 2007–2008 and 2008–2009. Between 10 and just over 100 individuals were found in locations including Trinity Beach, Cairns, Hinchinbrook Channel near Cardwell, Townsville, and Magnetic Island then.
Chironex fleckeri was rare in January to February in the first two summers and absent from March to May. In contrast, C. fleckeri were sparse from October 2009 to May 2010, but a few medusae were seen early, mid, and late in the season. In all years, medusae were found in shallow water, usually 0.5–5 m deep and within 100 m of shore. An exception to this was near Townsville, C. fleckeri were found near the mainland in shallow waters, but they were also found at Magnetic Island (about 10 km from the mainland) where they appear to have colonized near-shore waters (19°06.921, 146°51.698). However, waters separating the island from the mainland are less than 5 m deep. Jellyfish were found in estuaries and marinas (e.g., Port Douglas, Hinchinbrook Island, and Townsville) and on beaches that were exposed to the sea (e.g., Townsville). At all locations temporal persistence of C. fleckeri was low.
The influence of riverine flow on C. fleckeri abundance
Chironex fleckeri was not observed when freshwater outflows were high in mid- to late-summer, in years one and two (Fig. 6). River flow varied greatly among rivers and years. Flow was low in all years near the Cairns transect (Tully River) and was lowest early in the season in all rivers. The correlation between riverine runoff and abundance of C. fleckeri was not significant (Fig. 6), probably because jellyfish mostly disappeared after the first period of sustained heavy rain. Year three had the lowest flows in all rivers and some C. fleckeri medusae were found in early-, mid-, and late-summer.
Abundance of C. fleckeri medusae in northeastern Australia during three summers (open circles 2007–2008; solid squares 2008–2009; diamonds 2009–2010). Data were pooled by area for early, mid, and last within summers. Riverine flow in ML is provided for the rivers adjacent to areas were jellyfish were observed (locations and latitudinal range for the area affected by each river); Barron River (Cairns Regions; 16°13′522 E, 145°28.309 S to 16°57.664 E, 145°50.544 S); Tully River (Mission Beach and Hinchinbrook channel; 17°51.133 S, 146°08.071 E to 18°17.041 E, 146°03.051 S); Bole and Haughton rivers (Balgal Beach to Townsville/Magnetic Island; 19°01.354 S, 146°24.938 E to 19°15.638 S, 146°50.934 E)
Discussion
There was great variation in abundance patterns of cubozoan medusae cross-shelf. C. fleckeri medusae were restricted to near-shore waters, estuarine areas, and mainland beaches. All other cubozoans, the irukandji species (C. barnesi, C. xaymacana, Alatina sp.) and the relatively innocuous C. sivickisi were found near the mainland and/or at inner and mid-shelf reefs. Variation in cross-shelf patterns have been found for some scyphomedusan jellyfish species (Lynam et al., 2005), but there were no previous data for cubozoans.
It was possible that we failed to detect some jellyfish because of the sampling design. The abundance of A. moseri medusae are most abundant on beaches of Hawaii 9–13 days after the full moon (Thomas et al., 2001). Alatina sp. medusae were found during the study, but none were collected in lights at outer reefs despite multiple samples being collected after the full moon. There are anecdotal accounts of lunar pulses of Alatina near reefs of the Coral Sea. However, it is likely that their spatial distribution is very patchy, even given possible lunar periodicity. We found no evidence of lunar periodicity in C. barnesi or C. sivickisi.
The greatest numbers of C. barnesi were found near granite islands. Although orthogonal comparisons near and away from granite islands were inconclusive, the probability that Carukia spp. would be collected was much greater at granite islands. We also received photographs, each with as many as eight carybdeids, from a site (by Macona Inlet, 20°148.21 S and 148°55.47 E) near the granitic Hayman Island, the Whitsundays (Inner); 2 Feb 2010. There was also strong evidence that C. sivickisi were most abundant around islands. Possible explanations for an island effect include: (1) there is more suitable habitat for polyps, (2) oceanographic and wind effects around islands facilitate retention (Wolanski et al., 1984), and (3) for C. sivickisi, Sargassum spp., which is the preferred substratum for the jellyfish polyps (Hartwick, 1991a) is abundant. Even near islands, aggregations of Carukia spp. were rare. The highest concentrations, and a broad size range of individuals, were found only at a few sites, such as Mermaid Bay. This suggested that populations are highly localized due to local retention and supply of medusae. High variance between sites was common and great differences were found among replicates, which is typical for jellyfishes (Pitt & Kingsford, 2000).
There was strong evidence that freshwater flow influenced the abundance of C. fleckeri medusae, primarily during the wet season on north Queensland (December–April). In the first two summers, most C. fleckeri were collected early (November–December) and few were found as the runoff of freshwater increased from January to April. Relatively high abundance in January at the Barron River was found just before the heavy rain fall starting about 10 January 2009. Although river runoff and time within a season are confounded, more C. fleckeri were found mid- and late-season when the lowest flows occurred in the final season (2009–2010). To clarify this issue, experiments are required to test the affects of salinity on different life history stages.
It well known that changes in salinity can trigger the production of jellyfish from polyps in and influence the survival of scyphozoan medusa (reviews: Kingsford et al., 2000; Purcell et al., 2007). Although the paradigm for the cubozoan C. fleckeri is that the release of medusae is triggered by an input of freshwater (Hartwick, 1991b), and it has been assumed that the source of medusae is in estuaries, our data also suggest that there is a lower limit for salinity. This concurs with occasional observations of dead C. fleckeri on beaches after periods of strong river runoff. We suggest, therefore, that seasons of high rainfall may be a high risk to C. fleckeri populations. Due to global climate change, the frequency and intensity of cyclones is predicted to increase in north Queensland (Lough, 2008). Although experimental testing of critical salinities is required, we suggest that increased rainfall may have a negative affect on C. fleckeri populations. It is also likely that the affects of climate change will vary by species and region; both positive and negative effects on population sizes probably will be found (Lynam et al., 2005).
In conclusion, our data on cubozoan distributions and abundances tested hypotheses about the possible effects of distance from shore and the influence of islands. There were clear cross-shelf patterns in the abundance of cubozoans. The risk of envenomation to humans was greatest from the mainland to mid-shelf reefs, and especially around granite islands. There was no evidence for lunar-related variation in abundance, but physical forcing by freshwater input apparently had a strong influence on the abundance of C. fleckeri. This, combined with its near-shore distribution, suggests strong possibilities for biophysical modeling. The greatest challenge for reliable long-term data on cubozoan medusae is the extreme variation in their spatial and temporal distributions.
References
Barnes, J. H., 1966. Studies on three venomous Cubomedusae. In Rees, W. J. (ed.), The Cnidaria and Their Evolution. Academic Press, London: 307–332.
Bentlage, B., P. Cartwright, A. A. Yanagihara, C. Lewis, G. S. Richards & A. G. Collins, 2010. Evolution of box jellyfish (Cnidaria: Cubozoa), a group of highly toxic invertebrates. Proceedings of the Royal Society B-Biological Sciences 277: 493–501.
Coates, M. C. & J. C. Theobald, 2003. Optimal visual parameters for a cubozoan jellyfish in the mangrove environment. Integrative and Comparative Biology 43: 1016.
Doherty, P. J., 1987. Light-traps: selective but useful devices for quantifying the distributions and abundance of larval fishes. Bulletin of Marine Science 41: 423–431.
Fenner, P. J. & J. Lippmann, 2009. Severe Irukandji-like jellyfish stings in Thai waters. Diving and Hyperbaric Medicine 39: 175–177.
Gershwin, L. A., 2005a. Taxonomy and phylogeny of Australian Cubozoa. PhD Thesis, James Cook University, Townsville.
Gershwin, L. A., 2005b. Two new species of jellyfishes (Cnidaria: Cubozoa: Carybdeida) from tropical Western Australia, presumed to cause Irukandji syndrome. Zootaxa 1084: 1–30.
Gershwin, L. A. & M. J. Kingsford, 2008. Pelagic Cnidaria and Ctenophora. In Hutchings, P., M. Kingsford & O. Hoegh-Guldberg (eds), The Great Barrier Reef: Biology, Environment and Management. CSIRO Publishing, Collingwood: 188–198.
Gershwin, L. A., M. De Nardi, K. D. Winkel & P. J. Fenner, 2010. Marine stingers: review of an under-recognized global coastal management issue. Coastal Management 38: 22–41.
Gordon, M. R., C. Hatcher & J. E. Seymour, 2004. Growth and age determination of the tropical Australian cubozoan Chiropsalmus sp. Hydrobiologia 530: 339–345.
Gordon, M. R. & J. E. Seymour, 2009. Quantifying movement of the tropical Australian cubozoan Chironex fleckeri using acoustic telemetry. Hydrobiologia 616: 87–97.
Hartwick, R. F., 1991a. Observations on the anatomy, behavior, reproduction and life-cycle of the cubozoan Carybdea sivickisi. Hydrobiologia 216: 171–179.
Hartwick, R. F., 1991b. Distributional ecology and behavior of the early life stages of the box-jellyfish Chironex fleckeri. Hydrobiologia 216: 181–188.
Hurlbert, S. H., 1984. Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54: 187–211.
Kingsford, M. J., 1998. Analytical aspects of sampling design. In Kingsford, M. J. & C. N. Battershill (eds), Studying Temperate Marine Environments: A Handbook for Ecologists. University of Canterbury Press, Christchurch: 49–83.
Kingsford, M. J., K. A. Pitt & B. M. Gillanders, 2000. Management of jellyfish fisheries, with special reference to the order Rhizostomeae. Oceanography and Marine Biology: An Annual Review 38: 85–156.
Kintner, A., S. Edwards & J. E. Seymour, 2005. Variation in lethality and effects of two Australian chirodropid jellyfish venoms, Chironex fleckeri and Chiropsalmus sp., in fish. Toxicon 46: 699–708.
Little, M., P. Pereira, T. Carrette & J. Seymour, 2006. Jellyfish responsible for Irukandji syndrome. Quarterly Journal of Medicine 99: 425–427.
Lough, J. M., 2008. A changing climate for coral reefs. Journal of Environmental Monitoring 10: 21–29.
Lynam, C. P., S. J. Hay & A. S. Brierley, 2005. Jellyfish abundance and climatic variation: contrasting responses in oceanographically distinct regions of the North Sea, and possible implications for fisheries. Journal of the Marine Biological Association of the United Kingdom 85: 435–450.
Lynam, C. P., M. J. Attrill & M. D. Skogen, 2010. Climatic and oceanic influences on the abundance of gelatinous zooplankton in the North Sea. Journal of the Marine Biological Association of the United Kingdom 90(special issue): 1153–1159.
Matsumoto, G. I., 1995. Observations on the anatomy and behaviour of the cubozoan Carybdea rastonii Haacke. Marine and Freshwater Behaviour and Physiology 26: 139–148.
Nagai, H., K. Takuwa, M. Nakao, B. Sakamoto, G. L. Crow & T. Nakajima, 2000. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (sea wasp) Carybdea alata. Biochemical and Biophysical Research Communications 275: 589–594.
Nilsson, D. E., L. Gislen, M. M. Coates, C. Skogh & A. Garm, 2005. Advanced optics in a jellyfish eye. Nature 435: 201–205.
Pereira, P., J. Barry, M. Corkeron, P. Keir, M. Little & J. E. Seymour, 2010. Intracerebral hemorrhage and death after envenoming by the jellyfish Carukia barnesi death due to Irukandji syndrome. Clinical Toxicology 48: 390–392.
Pitt, K. A. & M. J. Kingsford, 2000. Geographic separation of stocks of the edible jellyfish, Catostylus mosaicus (Rhizostomeae) in New South Wales, Australia. Marine Ecology Progress Series 196: 143–155.
Purcell, J. E., S. Uye & W.-T. Lo, 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series 350: 153–174.
Rippingale, R. J. & S. J. Kelly, 1995. Reproduction and survival of Phyllorhiza punctata (Cnidaria: Rhizotomeae) in a seasonally fluctuating salinity regime in Western Australia. Marine & Freshwater Research 46: 1145–1151.
Straehler-Pohl, I. & G. Jarms, 2005. Life cycle of Carybdea marsupialis Linnaeus, 1758 (Cubozoa, Carybdeidae) reveals metamorphosis to be a modified strobilation. Marine Biology 147: 1271–1277.
Thomas, C. S., S. A. Scott, D. J. Galanis & R. S. Goto, 2001. Box jellyfish (Carybdea alata) in Waikiki: their influx cycle plus the analgesic effect of hot and cold packs on their stings to swimmers at the beach: a randomized, placebo-controlled, clinical trial. Hawaii Medical Journal 60: 100–107.
Tiong, K., 2009. Irukandji syndrome, catecholamines, and mid-ventricular stress cardiomyopathy. European Journal of Echocardiography 10: 334–336.
Underwood, A. J., 1997. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. Cambridge University Press, Cambridge: 504 pp.
Underwood, A. H. & J. E. Seymour, 2007. Venom ontogeny, diet and morphology in Carukia barnesi, a species of Australian box jellyfish that causes Irukandji syndrome. Toxicon 49: 1073–1082.
Winter, K. L., G. K. Isbister, J. J. Schneider, N. Konstantakopoulos, J. E. Seymour & W. C. Hodgson, 2008. An examination of the cardiovascular effects of an ‘Irukandji’ jellyfish, Alatina nr mordens. Toxicology Letters 179: 118–123.
Winter, K. L., G. K. Isbister, S. McGowan, J. J. Schneider, N. Konstantakopoulos, J. E. Seymour & W. C. Hodgson, 2009. A pharmacological and biochemical examination of the geographical variation of Chironex fleckeri venom. Toxicology Letters 192: 419–424.
Wolanski, E., J. Imberger & M. L. Heron, 1984. Island wakes in shallow coastal waters. Journal of Geophysical Research 89: 553–569.
Yamada, T., T. Takeda & S. Kubota, 2010. Temporal patterns of jellyfish species occurrence at the Suma Coast, Kobe City, Hyogo Prefecture (Years 2003–2009). Kuroshio Biosphere 6: 27–30.
Acknowledgments
Counting cubozoans under lights is often arduous and we thank our many volunteers for assisting, especially Shelley Templeman and Christopher Mooney. The crews of MV Piscean, MV Kalinda, and MV Phoenix provided critical support on the cross-shelf cruises. For the Double Island samples we thank Teresa Carrette, Avril Underwood, Glenda Seymour, and Richard Fitzpatrick for their assistance. We also thank SLSA for providing data and specimens of cubozoans collected on beaches in North Queensland. We also appreciate photographs of cubozoans provided by John Sinclair. Funding was provided by a Marine Science Tropical Science Research Facility (MTSRF) and a LIONS Foundation grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: J. E. Purcell, H. Mianzan & J. R. Frost / Jellyfish Blooms: Interactions with Humans and Fisheries
Rights and permissions
About this article
Cite this article
Kingsford, M.J., Seymour, J.E. & O’Callaghan, M.D. Abundance patterns of cubozoans on and near the Great Barrier Reef. Hydrobiologia 690, 257–268 (2012). https://doi.org/10.1007/s10750-012-1041-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1041-0