Abstract
Sponges are an important component of the benthic community, especially on coral reefs, but demographic data such as growth, recruitment or mortality are notably limited. This study examined the growth of the elephant ear sponge Ianthella basta, the largest and in some areas one of the dominating sponge species on Guam and other pacific reefs. We measured growth rates of the natural population on Guam over the course of one year and identified intra-individual growth patterns. Initial sponge sizes ranged from 200 to 35,000 cm2. Specific growth rates ranged from 0.08 to 6.08 with a mean specific growth rate of 1.43 ± 1.29 (SD) year−1. Furthermore, specific growth decreased with sponge size. The age estimate for the largest sponge (1.7 m height × 9.5 m circumference) was ~8 years. Intra-individual growth was mostly apical. This study demonstrated high growth rates, which has notable implications for environmental assessments, management and potential biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sponges are an important component of benthic coral reef communities (Diaz & Rützler, 2001). On Caribbean reefs, their importance has begun to be recognized, where sponge assemblages reach similar diversities and abundances as scleractinian corals (Targett & Schmahl, 1984; Suchanek et al., 1985). However, on Pacific reefs, sponges have received much less attention despite their various ecological roles. Sponges are benthic filter feeders (Reiswig, 1971; Hadas et al., 2009; Riisgard & Larsen, 2010), serve as habitat for organisms (Duffy, 1992; Henkel & Pawlik, 2005; Hultgren & Duffy, 2010) and affect the benthic community composition by competitive interactions (Suchanek et al., 1985; Engel & Pawlik, 2000).
Despite their high diversity and abundance, research just started to investigate life history traits like growth, life span, or reproduction in more detail (e.g. Turon et al., 1998; De Caralt et al., 2008; Koopmans & Wijffels, 2008; McMurray et al., 2010). While there is no doubt that growth, size, or life span affect and explain many ecological interactions and functions (Peters, 1983), more research needs to be done to reveal sponge life histories (McMurray et al., 2008).
There are many factors that may have contributed to this lack of studies. Sponges are often very slow growing organisms, which require long-time studies to estimate growth rates (Reiswig, 1973; Duckworth & Battershill, 2001). Growth can vary significantly among seasons, populations, or sites (Garrabou & Zabala, 2001; De Caralt et al., 2008), and many sponge species have a highly variable morphology, which complicates accurate size estimates (but see Koopmans & Wijffels, 2008). Sponges also lack distinct morphological structures that can be used as age indicators like otoliths in fishes or growth rings in trees.
Growth rates have been determined for a number of sponge species, including encrusting, rope-like, tubular, and massive growth forms (e.g. Turon et al., 1998; Garrabou & Zabala, 2001; Tanaka, 2002; De Caralt et al., 2008; Koopmans & Wijffels, 2008; McMurray et al., 2008). Hoppe (1988) investigated growth of the flabellate sponge Agelas clathrodes, but to our knowledge, no data are known from fan-like growing sponges.
When measuring growth in sponges it should be recognized that sponges often heal wounds much faster compared to their normal rate of growth (Ayling, 1983; Smith & Hildemann, 1986; Hoppe, 1988), and wound healing/tissue regeneration is therefore not a good predictor of normal growth rates.
Estimation of the age structure of benthic communities is essential for applied ecological assessments, which require knowledge of the age and growth of the community to assess recovery rates after habitat destruction or mitigation projects.
Ianthella basta (Pallas) is a conspicuous, fan- or funnel-like shaped sponge reaching heights of up to 2 m (Bergquist & Kelly-Borges, 1995, personal observation). I. basta is widely distributed in the Indo-Pacific (Bergquist & Kelly-Borges, 1995). Its distribution ranges from the Mascarene Islands to Vanuatu, the Philippines and Guam. However, I. basta is absent from all intervening Micronesian Islands including the Federated States of Micronesia and Palau (Kelly et al., 2003). Therefore, it has been speculated whether it colonized Guam by jump dispersal or became introduced through anthropogenic transport (Kelly et al., 2003). The fact that on Guam I. basta only occurs in Apra Harbor, where the port is situated, may support the latter. The restriction to Apra Harbor as documented by Paulay et al. (2002) has significant consequences for the population of I. basta on Guam. For construction of a new aircraft carrier wharf, large reef areas in Apra Harbor are planned to be dredged (Navy, 2010). This would reduce or potentially eliminate much of the actual habitat of I. basta on Guam. While I. basta might be an introduced species, it lacks invasiveness in that it does not seem to compete with other sessile invertebrates for limited space. It is mainly found along the edge of coral slopes where the hard substrate changes to soft bottom sediments with occasional rocky outcrops to which it is attached. In this habitat, I. basta adds to the rugosity and provides shelter for other invertebrates and fishes (Rohde and Schupp, personal observation). As one of the largest and most conspicuous sponges inside Apra Harbor, I. basta has also become an attraction for the local dive operators. Therefore, I. basta has become a biological and economical important species. Thus for both, assessments of ecological damage and potential mitigation demands, an assessment of the population characteristics (age, growth) of I. basta is essential and encouraged by local resource agencies (D. Burdick, personal communication).
Another reason to investigate the natural growth rate of I. basta is recent studies describing its unusual chitin skeleton and the potential of such skeletons in biomedical applications (Brunner et al., 2009; Ehrlich et al., 2010a, b). However, the use of marine natural products in general and of the identified chitin scaffold in particular is restricted by supply limitations (e.g. there is no synthesis available for chitin scaffolds). Consequently, a detailed knowledge of growth rates and growth patterns is necessary to evaluate if aquaculture could be viable to produce enough material for biomedical applications.
The aim of this study was to determine the growth and consequently the age of the natural population of I. basta. Beside the general lack of knowledge on sponge growth rates, the results could highlight the consequences of habitat destruction and explore whether I. basta constitutes a sustainable source for tissue harvest for extraction of chitin scaffolds.
Materials and methods
Growth of the natural Ianthella basta population
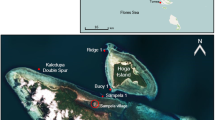
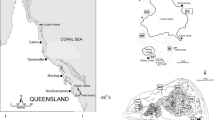
The study site was at Western Shoals, Apra Harbor, on the west coast of Guam (13°27.3′N, 144°39.2′E). This is a very sheltered location with a high density of I. basta. At depths between 8 and 11 m, we tagged 40 specimen of I. basta using numbered aluminum washers that were nailed to the reef next to the sponges. We chose specimen over the entire size range of the present population. In June 2009 and June 2010, circumference and slant height were measured by SCUBA using measuring tapes. The shape of a cone was used as a model to calculate the area of the sponge tissue, and growth was calculated as:
where G is the specific growth rate (year−1), A 1 is the initial area (cm2), A 2 is the final area (cm2), and t is the time (years).
To assess whether size affects the growth rates of the sponges, individuals were grouped into 4 size classes: 1 (from 195 to 1,000 cm2, n = 6), 2 (from 1,001 to 3,000 cm2, n = 18), 3 (from 3,001 to 10,000 cm2, n = 9) and 4 (>10,000 cm2, n = 8). Specific growth data were log10-transformed to obtain homogeneity of variances (Levene’s test). Growth differences among size-class were analyzed by a one-way ANOVA and Tukey’s posthoc test (SPSS 17).
Five commonly used growth models were fit to size-increment data to determine the best model describing growth of I. basta (McMurray et al., 2008): the general von Bertalanffy growth formula (gVBGF) (von Bertalanffy, 1938; Beverton & Holt, 1957; Pauly, 1981), specialized von Bertalanffy growth formula (sVBGF) (Richards, 1959; Pauly, 1981), Gompertz (Gompertz, 1825; Winsor, 1932), Richards (Richards, 1959; Ebert, 1980), and Tanaka (1982) growth functions. The square root of area estimates was used as an average linear size to model growth of I. basta. The difference equations of the models were fitted to final and initial linear sizes on a Walford plot by nonlinear regression (SOLVER, MS Excel 2007). To produce size-at-age curves, we used parameter estimates using the integrated forms of the growth functions, which were subsequently squared to obtain area-at-age plots. The sum of squared error (SSE), coefficient of determination and Akaike information criterion (AIC) (Akaike, 1973) were used to evaluate model fit. Because the AIC evaluates the trade-off between model biases that results from too few parameters versus variance that results from too many, it can be used to evaluate any difference between the 2- and 3-parameter models that may otherwise be neglected through use of the SSE as the sole fitting criteria (Burnham & Anderson, 2002). The model with the lowest SEE and AIC is sought to select the best fitting model. Because sample size (n) was small with respect to the number of model parameters, K, (n/K < 40), the second-order correction (AICc) was used (Burnham & Anderson, 2002). As a measure of each model relative to the best model, the model selection criteria AIC difference, ∆i, was calculated.
Regeneration and intra-individual growth patterns
To observe the intra-individual growth and regeneration patterns, we tagged ten additional specimen of I. basta with slant heights of 60–90 cm at the same site at 12 m depth in May 2010. Of these sponges, all the tissue above 50 cm slant height was cut off. At every 10 cm height, cable ties were pierced through the sponge tissue to determine whether growth occurred throughout the entire sponge body, or whether apical regeneration of tissue dominated. Additionally, two round holes (3 cm diameter) were cut at 10 and 30 cm height to identify and measure regeneration in response to injuries.
Sponge growth was remeasured two and five months later by measuring the distances between the cable ties and the diameter of the holes to estimate growth at the different portions of the thalli.
Results
Initial size estimates of the sponges ranged from 200 to 35,000 cm2 with a mean value of 5,702 cm2. Specific growth rates ranged from 0.08 to 6.08 with a mean specific growth rate of 1.43 ± 1.29 (SD) year−1. Comparisons among size-classes revealed that smaller sponges had higher growth rates (Fig. 1). Sponges in size-class one showed specific growth rates of 3.21 ± 2.16 year−1 (mean ± SD), sponges in size-class two 1.45 ± 0.88 year−1 (mean ± SD), sponges in size-class three 0.86 ± 0.47 year−1 (mean ± SD) and sponges in size-class 4 grew 0.70 ± 0.51 year−1 (mean ± SD). Differences among size-classes were significant (P < 0.001, ANOVA). Figure 1 depicts the significant differences from the posthoc comparisons.
The parameter estimates of the growth models are presented in Table 1. All models showed a high model fit. The models in order of best fit are: Tanaka > Gompertz > Richards > gVBGF > sVBGF. The SSE values were in agreement with the AICc and revealed a similar fit of Tanaka, Gompertz, Richards and gVBGF with a slightly lower fit of the sVBGF model (Table. 2). According to the models, the largest measured sponges in this study were ~8 years old (Fig. 2).
Size at age curves from parameter estimates in Table 1. Line at 82,000 cm2 refers to the largest measured sponge in this study
Specimen of I. basta that were cut off at 50 cm height regenerated apical tissue at rates of 1.03 cm/month (Fig. 3). The basal and middle parts of the sponge body did either not grow at all, or only slightly (0.25 cm/month). The holes cut in the center of the sponge (3 cm diameter) healed within 8 weeks.
Discussion
Ianthella basta is a common member on reefs in Apra Harbor, Guam, and reefs in the tropical Western Pacific. While there has been renewed interest in this species for biomedical applications (Bergquist & Kelly-Borges, 1995; Brunner et al., 2009), little is known about its ecology and demography. Because the US NAVY has large scale dredging plans for Apra Harbor, more detailed knowledge of I. basta ecology and demography is needed to adequately assess mediation for destroyed coral reef areas.
This study demonstrated that I. basta is a remarkably fast growing sponge species, which reaches sizes of up to 2 m height in less than 10 years. To our knowledge, there is only one other study, which estimated growth and age of a sponge species that reaches similar sizes. The Caribbean sponge Xestospongia muta also grows to heights of 170 cm, but growth measurements indicated that specimens of this size are around 250 years old (McMurray et al., 2008). Even though X. muta is a massive species that needs to build up much more biomass to reach these large dimensions, it is an interesting fact that X. muta needs around 25 times longer to reach similar dimensions as I. basta.
The specific growth rate of I. basta was with 1.43 year−1 around threefold higher than that of X. muta, but similar to growth of four Mediterranean sponge species with mean rates from 1.08 to 2.4 year−1 (Garrabou & Zabala, 2001). However, individuals of size-class 1 grew as much as 3.21 year−1 leading to a relative fast transition in higher size-classes. Specific growth rates are a relative measure; therefore, the absolute biomass production is higher in large sponges compared to small sponges with similar specific growth rates. Consequently, growth rates of 0.7–1.45 in size classes 2 to 4 are comparable to Mediterranean sponge species (Garrabou & Zabala, 2001), but due to the large size of I. basta specimen, the biomass production is much higher. This could be one reason supporting the establishment of many large sponge individuals.
Specific growth rates decreased with increasing size. This pattern has also been found for many sponge species (e.g. Reiswig, 1973; Garrabou & Zabala, 2001; De Caralt et al., 2008; McMurray et al., 2008; but see Duckworth & Battershill, 2001). The negative correlation of size and specific growth of I. basta is especially not surprising, since absolute growth was similar among all sizes. Growth was apical with 1 (±0.69 SD) cm month−1 along the upper edge, resulting in lower specific growth with increasing size. All measured specimen showed positive growth, but the intraspecific variation was very high and this seems to be characteristic for many sponge species (Reiswig, 1973; Dayton et al., 1974; Wulff, 1985; Duckworth & Battershill, 2001; Garrabou & Zabala, 2001; McMurray et al., 2008).
Holes that were cut in the center of the sponge healed within 2 months. This rate is very similar to the apical regeneration rates and much higher than the growth measured at this part of uninjured sponge specimens. This further demonstrates that growth is almost entirely restricted to edges of the sponge, whether they are the apical edge, edges of holes from predation or, in this case artificial injury. Some sponge species showed wound healing processes that generated tissue much faster than their normal growth (Ayling, 1983; Smith & Hildemann, 1986; Hoppe, 1988). Assuming that wound healing exerts the highest physiologically possible growth rate and the fact that natural apical growth in I. basta showed similar rates, we suggest that the rate of 1 cm month−1 is the upper limit of growth under the conditions of this study.
The question arises, what limits the size and the age of I. basta? The largest specimens of the population were estimated to be around 8 years old, but still growing with average rates of 1 cm month−1, indicating infinite growth for this species. Since no larger and therefore older specimen could be found on Guam, other biotic or abiotic factors must restrict the sponge to a maximal size and age.
Predation can significantly affect a sponge community (e.g. Wulff, 1997; Pawlik, 1998) and consequently also affect growth measurements by consuming sponge tissue. During our study, we observed no evidence of predation, such as bite marks or removal of significant amounts of biomass. The crude extract of I. basta deterred feeding by various predators (Becerro et al., 2003). Therefore, the observed growth seems not or only minimally restricted by predation.
The effect of abiotic factors on the growth and mortality of I. basta has not been studied. Apra Harbor is a geographically protected bay with low current and wave dynamics. These conditions have been described as similar to other locations where I. basta occurs (Bergquist & Kelly-Borges, 1995; Kelly et al., 2003). It therefore seems likely that high water movement restricts the distribution of I. basta and may also restrict its size. Large sponges with an area of over 80,000 cm2 offer a large resistance to waves or currents. One possibility is that this size represents a threshold where water movements rip off the sponges and in this way limit their size and age distribution. Extreme wave action, as it occurs during typhoons, could topple and kill the sponges (when they subsequently decompose in the fine sediment). With the last typhoon occurring on Guam on December 8, 2002 it seems unlikely that typhoons are the sole event restricting the maximum size, as medium size sponges would have had over 8 years to grow and therefore should have had reached a total age of 10 to 15 years, with corresponding sizes (100,000 cm2 to 500,000 cm2, depending on the model). However, the maximum size we observed was 82,000 cm2, making such a scenario unlikely. Another factor restricting the size could be the fiber dominated skeleton (chitin and spongin), which might be too flexible to support a larger (>82,000 cm2) fan-shaped skeleton against currents and wave action.
Other sponge species have shown to regrow at their bases after detachment by storms or anthropogenic effects (Schmahl, 1999; McMurray et al., 2010). This effect has not been shown for I. basta, but could contribute to the preservation of the abundance of I. basta.
The population of I. basta in Apra Harbor is very isolated. The sponge does not occur on other reefs around Guam and can also not be found on the surrounding Micronesian Islands (Kelly et al., 2003). Consequently, the loss of the population in Apra Harbor would extinct this species from the entire region. The planned dredging of large reef areas in Apra Harbor to build an aircraft carrier berthing will reduce the habitat of I. basta to a great extent. Many surveys have been done to assess the marine community, to evaluate environmental consequences and to determine the appropriate quantity of the compensatory mitigation measures that will be recommended for the project (Navy, 2010). However, to evaluate how the loss of significant parts of the I. basta population could be compensated, one requires demographic data such as growth, recruitment and mortality. None of these data have been available so far. The results of this study allow the assessment of the population structure, i.e. age-distribution curves, or size-at-age analyses, which describe the present population. In order to estimate population recovery rates after disturbances, data on recruitment and mortality are essential but non-existent so far. However, the fact that I. basta reaches high abundances despite its relative ephemerality indicates that recruitment could be high and regular. Since I. basta tissue is relatively tough and does not tear or fragment easily, asexual reproduction by fragmentation seems less likely. If the recruitment is accomplished through self-seeding by the current population, large scale dredging could diminish the larval producing population to the point that recruitment is disrupted and the population would eventually die off.
Within the last years, several studies investigated I. basta with regard to its chitin-based skeleton (Ehrlich et al., 2007a, b, 2010a, b; Brunner et al., 2009). These chitin-based scaffolds are of high interest for many biomedical applications like tissue engineering and biomedicine (e.g., Maeda et al., 2008; Jayakumar et al., 2010). However, one major obstacle that also natural product chemists face is the supply problem (Faulkner et al., 2000). The source organisms of bioactive compounds often need to be collected in large quantities to supply the necessary amount for the industry. This can often not be justified ecologically (Munro et al., 1994). The farming of marine organisms is sometimes an alternative to collecting specimens from the wild. But this can only be an economically relevant alternative if the organisms lend themselves to a cost-effective cultivation (Schupp et al., 2009). Using I. basta as source for chitinous scaffolds requires the harvest of large amounts of sponge tissue. Both, ecologically sustainable wild harvest and farming therefore rely on high growth rates that provide a sufficient supply. The growth rates of I. basta are high compared to other sponge species and preliminary experiments revealed that cultivation of I. basta can be suitable (Rohde and Schupp, in preparation). However, this would only be suitable if a healthy natural population is present to support any aquaculture settings.
Further studies on the demography of I. basta (e.g. reproduction and mortality) together with this study could provide the necessary framework to enable effective management and potential mitigation.
References
Akaike, H., 1973. Information theory and an extension of the maximum likelihood principle. In Petrov, B. N. & F. Csaki (ed.), Proceedings of the 2nd International Symposium on Information Theory. Akademiai Kiado, Budapest: 267–281.
Ayling, A. L., 1983. Growth and regeneration rates in thinly encrusting Demospongiae from temperate waters. Biological Bulletin, Marine Biological Laboratory, Woods Hole 165: 343–352.
Becerro, M. A., R. W. Thacker, X. Turon, M. J. Uriz & V. J. Paul, 2003. Biogeography of sponge chemical ecology: comparisons of tropical and temperate defenses. Oecologia 135: 91–101.
Bergquist, P. R. & M. Kelly-Borges, 1995. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellidae) in the South-West Pacific. The Beagle, Records of the Museums and Art Galleries of the Northern Territory 12: 151–176.
Beverton, R. J. H. & S. J. Holt, 1957. On the Dynamics of Exploited Fish Populations. Fisheries Investigations of the Ministry of Agriculture and Fisheries, Food in Great Britain, Series 2, Sea Fish, Vol 19. Facsimile reprint 1993. Chapman & Hall, London.
Brunner, E., H. Ehrlich, P. Schupp, R. Hedrich, S. Hunoldt, M. Kammer, S. Machill, S. Paasch, V. V. Bazhenov, D. V. Kurek, T. Arnold, S. Brockmann, M. Ruhnow & R. Born, 2009. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. Journal of Structural Biology 168: 539–547.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretical Approach. Springer, New York.
Dayton, P. K., G. A. Robilliard, R. T. Paine & L. B. Dayton, 1974. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecological Monographs 44: 105–128.
De Caralt, S., M. J. Uriz & R. H. Wifffels, 2008. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Marine Ecology Progress Series 360: 97–106.
Diaz, M. C. & K. Rützler, 2001. Sponges: an essential component of Caribbean coral reefs. Bulletin of Marine Science 69: 535–546.
Duckworth, A. R. & C. N. Battershill, 2001. Population dynamics and chemical ecology of New Zealand demospongiae Latrunculia sp nov and Polymastia croceus (Poecilosclerida : Latrunculiidae : Polymastiidae). New Zealand Journal of Marine and Freshwater Research 35: 935–949.
Duffy, J. E., 1992. Host use patterns and demography in a guild of tropical sponge-dwelling shrimps. Marine Ecology Progress Series 90: 127–138.
Ebert, T. A., 1980. Estimating parameters in a flexible growth equation, the Richards function. Canadian Journal of Fisheries and Aquatic Sciences 37: 687–692.
Ehrlich, H., M. Krautter, T. Hanke, P. Simon, C. Knieb, S. Heinemann & H. Worch, 2007a. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). Journal of Experimental Zoology Part B-Molecular and Developmental Evolution 308B: 473–483.
Ehrlich, H., M. Maldonado, K. D. Spindler, C. Eckert, T. Hanke, R. Born, C. Goebel, P. Simon, S. Heinemann & H. Worch, 2007b. First evidence of chitin as a component of the skeletal fibers of marine sponges Part I. Verongidae (Demospongia: Porifera). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 308B: 347–356.
Ehrlich, H., M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. V. Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V. Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, E. Steck, W. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke & E. Brunner, 2010a. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. International Journal of Biological Macromolecules 47: 132–140.
Ehrlich, H., E. Steck, M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. V. Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V. Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke, E. Brunner & W. Richter, 2010b. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. International Journal of Biological Macromolecules 47: 141–145.
Engel, S. & J. R. Pawlik, 2000. Allelopathic activities of sponge extracts. Marine Ecology-Progress Series 207: 273–281.
Faulkner, D. J., M. K. Harper, M. G. Haygood, C. E. Salomon & E. W. Schmidt, 2000. Symbiotic bacteria in sponges: sources of bioactive substances. In Fusetani, N. (ed.), Drugs from the Sea. Karger, Basel: 107–119.
Garrabou, J. & M. Zabala, 2001. Growth dynamics in four mediterranean demosponges. Estuarine, Coastal and Shelf Science 52: 293–303.
Gompertz, B., 1825. On the nature of the function expressive of human mortality, and on a new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society of London, Series B 115: 513–585.
Hadas, E., M. Shpigel & M. Ilan, 2009. Particulate organic matter as a food source for a coral reef sponge. Journal of Experimental Biology 212: 3643–3650.
Henkel, T. P. & J. R. Pawlik, 2005. Habitat use by sponge-dwelling brittlestars. Marine Biology 146: 301–313.
Hoppe, W. F., 1988. Growth, regeneration and predation in 3 species of large coral reef sponges. Marine Ecology Progress Series 50: 117–125.
Hultgren, K. M. & J. E. Duffy, 2010. Sponge host characteristics shape the community structure of their shrimp associates. Marine Ecology Progress Series 407: 1–12.
Jayakumar, R., D. Menon, K. Manzoor, S. V. Nair & H. Tamura, 2010. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydrate Polymers 82: 227–232.
Kelly, M., J. N. A. Hooper, V. Paul, G. Paulay, R. W. M. Van Soest & W. de Weerdt, 2003. Taxonomic inventory of the sponges (Porifera) of the Mariana Islands. Micronesica 35–36: 100–120.
Koopmans, M. & R. H. Wijffels, 2008. Seasonal growth rate of the sponge Haliclona oculata (Demospongiae: Haplosclerida). Marine Biotechnology 10: 502–510.
Maeda, Y., R. Jayakumar, H. Nagahama, T. Furuike & H. Tamura, 2008. Synthesis, characterization and bioactivity studies of novel beta-chitin scaffolds for tissue-engineering applications. International Journal of Biological Macromolecules 42: 463–467.
McMurray, S. E., J. E. Blum & J. R. Pawlik, 2008. Redwood of the reef: growth and age of the giant barrel sponge Xestospongia muta in the Florida Keys. Marine Biology 155: 159–171.
McMurray, S. E., T. P. Henkel & J. R. Pawlik, 2010. Demographics of increasing populations of the giant barrel sponge Xestospongia muta in the Florida Keys. Ecology 91: 560–570.
Munro, M. H. G., J. W. Blunt, R. J. Lake, M. Litaudon, C. N. Battershill & M. J. Page, 1994. From seabed to sickbed: what are the prospects? In Van Soest, R. W. M., T. Van Kempen & J. Braekman (eds), Sponges in Space and Time. AA Balkema, Rotterdam: 395–400.
Navy, U.S.D.o.t., 2010. Guam and CNMI Military Relocation: EIS, Vol. 4: Aircraft Carrier Berthing.
Paulay, G., L. Kirkendale, G. Lambert & C. Meyer, 2002. Anthropogenic biotic interchange in a coral reef ecosystem: a case study from Guam. Pacific Science 56: 403–422.
Pauly, D., 1981. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy’s theory of growth. Meeresforschung 28: 251–282.
Pawlik, J. R., 1998. Coral reef sponges: do predatory fishes affect their distribution? Limnology and Oceanography 43: 1396–1399.
Peters, R. H., 1983. The Ecological Implications of Body Size. Cambridge University Press, Cambridge.
Reiswig, H. M., 1971. In situ pumping activities of tropical Demospongiae. Marine Biology 9: 38–50.
Reiswig, H. M., 1973. Population dynamics of three Jamaican Demospongiae. Bulletin of Marine Science 23: 191–226.
Richards, F. J., 1959. A flexible growth function for empirical use. Journal of Experimental Botany 10: 290–300.
Riisgard, H. U. & P. S. Larsen, 2010. Particle capture mechanisms in suspension-feeding invertebrates. Marine Ecology Progress Series 418: 255–293.
Schmahl, G. P., 1999. Recovery and growth of the giant barrel sponge (Xestospongia muta) following physical injury from a vessel grounding in the Florida Keys. Memoirs of the Queensland Museum 44: 532.
Schupp, P. J., C. Kohlert-Schupp, S. Whitefield, A. Engemann, S. Rohde, T. Hemscheidt, J. M. Pezzuto, T. P. Kondratyuk, E. J. Park, L. Marler, B. Rostama & A. D. Wright, 2009. Cancer chemopreventive and anticancer evaluation of extracts and fractions from marine macro- and micro-organisms collected from twilight zone waters around Guam. Natural Product Communications 4: 1717–1728.
Smith, L. C. & W. H. Hildemann, 1986. Allograft-rejection, autograft fusion and inflammatory responses to injury in Callyspongia diffusa (Porifera, Demospongia). Proceedings of the Royal Society of London, Series B: Biological Sciences 226: 445–464.
Suchanek, T. H., R. C. Carpenter, J. D. Witman & C. D. Harvell, 1985. Sponges as important space competitors in deep Caribbean coral reef communities. In Reaka, M. L. (ed.), The Ecology of Deep and Shallow Coral Reefs. Symposia Series for Undersea Research. NOAA, Rockville: 55–59.
Tanaka, K., 1982. A new growth curve which expresses infinitive increase. Publications of the Amakusa Marine Biology Laboratory Kyushu University 6: 167–177.
Tanaka, K., 2002. Growth dynamics and mortality of the intertidal encrusting sponge Halichondria okadai (Demospongiae, Halichondrida). Marine Biology 140: 383–389.
Targett, N. M. & G. P. Schmahl, 1984. Chemical Ecology and Ddistribution of Sponges in the Salt River Canyon, St. Croix, U.S.V.I. NOAA Technical Memorandum OAR NURP-1.
Turon, X., I. Tarjuelo & M. J. Uriz, 1998. Growth dynamics and mortality of the encrusting sponge Crambe crambe (Poecilosclerida) in contrasting habitats: correlation with population structure and investment in defence. Functional Ecology 12: 631–639.
von Bertalanffy, L., 1938. A quantitative theory of organic growth (inquires on growth laws II). Human Biology 10: 181–213.
Winsor, C., 1932. The Gompertz curve as a new growth curve. Proceedings of the National Academy of Science, USA 18: 1–8.
Wulff, J. L., 1985. Patterns and processes of size change in Caribbean demosponges of branching morphology. In Rutzler, K. (ed.), New Perspectives in Sponge Biology. Smithsonian Institution Press, Washington: 425–435.
Wulff, J. L., 1997. Parrotfish predation on cryptic sponges of Caribbean coral reefs. Marine Biology 129: 41–52.
Acknowledgments
We like to thank Gitta Rohde, Ciemon F. V. Caballes and the UOG Marine Lab Techs for assistance in the field. This research was in part supported by NIH MBRS SCORE grant S06-GM-44796 to PJS. Comments of two anonymous reviewers improved the manuscript. SR was supported by a fellowship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Maldonado, X. Turon, M. A. Becerro & M. J. Uriz / Ancient animals, new challenges: developments in sponge research
Rights and permissions
About this article
Cite this article
Rohde, S., Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 687, 219–226 (2012). https://doi.org/10.1007/s10750-011-0774-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0774-5