Abstract
A 12 month in situ experiment-assessed colonisation behaviour of Nereis diversicolor (Nereis) using cores of defaunated sediment; either raised 5 cm above the mudflat surface (colonisation by swimming only) or flush with it (colonisation by crawling and swimming). Recruitment and dispersal occurred throughout the year but were highest in the summer. During the summer peak dispersal period the densities of worms in the raised cores were about half of those in the flush cores indicating approximately equal levels of dispersal by swimming and crawling. Colonisation of the raised cores, by swimming only, was by Nereis less than 6 cm in length. The length frequencies of worms in the flush treatments were similar to those of the natural population, including worms up to 12 cm in length indicating that larger worms disperse only by crawling. In the spring and summer the abundances of the smallest Nereis in the raised cores were between 60 and 80% of the total abundances in the flush cores, indicating that most small worms had also colonised the flush treatments by swimming. The densities of worms in the flush treatments were lower than, but not significantly different to, the natural population (with the exception of November and December). It is concluded that in most months worms undergo dispersal, mainly by swimming when small, and by crawling thereafter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The polychaete Nereis diversicolor (hereafter Nereis) is an abundant and widespread member of the infauna of temperate Atlantic intertidal sediments. It is a significant prey species of fishes and often supports internationally important populations of birds (Scaps, 2002; Rosa et al., 2008) with commercial relevance as it is also harvested for the bait industry (Fowler, 1999). It is an important ecosystem engineer (Widdows et al., 2009) as through its bioturbatory activities it will re-work and irrigate the sediment thus affecting chemical fluxes (pollutants, nutrients), affect microbial processes, and the stability of sediment surface (de Deckere et al., 2001; Kristensen, 2001; Kristensen & Mikkelsen, 2003). In the estuaries of SE England, it is often found high in the intertidal zone, at the saltmarsh–mudflat interface, where through bioturbation and herbivory it can increase sediment erosion, reduce sediment elevation, restrict the successional development of saltmarshes and increase the internal erosion of saltmarshes by facilitating creek expansion (Paramor & Hughes, 2004, 2005, 2007). Several aspects of the biology and ecology of Nereis are not fully understood including its dispersal behaviour (Abrantes et al., 1999).
Unlike some other nereids, N. diversicolor does not produce epitokous heteronereids (a specialised reproductive pelagic morph) and does not possess a planktotrophic larval stage. Instead, it has been described as having a holobenthic life cycle where the fertilised lecithotrophic eggs and developing larvae are brooded for a period of 10–14 days in the maternal burrow after which the female dies a few days later (Dales, 1950; Bartels-Hardege & Zeeck, 1990; Marty & Retiére, 1999). A holobenthic lifecycle may restrict the dispersal potential of a species compared to those with a planktonic stage that can disperse widely (Palmer et al., 1996). However, direct or brooded larval development allows a greater flexibility in the level of development of the released progeny and, the extent to which dispersal may occur. Infaunal species that are not committed to long term dispersal by having a long planktotrophic (larval) stage can develop specific dispersal behaviours. Generally they have short term dispersal, which reduces the mortality rate while out of the sediment, and increases the chance of finding a suitable habitat. For example the amphipod Corophium volutator, also common in the upper mudflats of Atlantic estuaries, has no larval stage and dispersal occurs by brief periods of swimming at the times of spring tides at night (Hughes, 1988, Drolet & Barbeau, 2009). Nereis populations may exhibit some genetic isolation concomitant with low levels of dispersal (Smith, 1958; Hateley et al., 1992, Breton et al., 2003), and in species with low dispersal, variability in reproductive mechanisms is often observed between different populations (Gudmundsson, 1985; Bolam, 2004).
Species lacking a pelagic larval dispersal stage can be rapid colonisers (Shull, 1997) and despite having an apparent low dispersal potential Nereis can rapidly recolonise defaunated sediments (Hall & Frid, 1998; Lewis et al., 2003; Bolam et al., 2004). Nereis may become established within new habitats rapidly; for example, the newly intertidal substrata within the managed realignment site at Wallasea Island, Essex, held a dense population after only a few weeks (RGH, personal observation), and it was a dominant species in the nearby Tollesbury managed realignment site after only 2 months (Garbutt et al., 2006). Studies that have concentrated on the whole assemblages generally use too large a sieve mesh size which does not retain larvae and juveniles (Santos & Simon, 1980), thus the life stages of colonists cannot be identified. The dispersal of marine polychaetes with a holobenthic lifecycle has rarely been studied in sufficient detail to ascertain the precise dispersal mechanisms (Marty & Retíere, 1999).
The aims of this study were to investigate the stage(s) involved in dispersal of Nereis (larval, juvenile and/or adult), whether dispersal occurs by swimming and/or crawling, and the season(s) that dispersal occurs, by assessing colonisation of defaunated sediment over 1 year.
Materials and methods
Study site
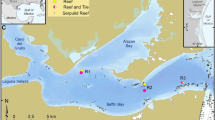
In situ experiments were conducted at Bridgemarsh Creek, on the north side of the Crouch Estuary (Essex, SE England), which is a coastal plain estuary with a spring tidal range of 4.9 m (Worley & Simpson, 1998) (Fig. 1). The site, described in detail by Bolam et al. (2004), is at the top of the mudflat west of Bridgemarsh Island in an area sheltered from strong tidal currents. The sediments were >90% silt/clay with an organic content of 1.5–1.7%.
Location of the experimental site at Bridgemarsh Creek on the Crouch estuary, SE England (after Nicholls & Trimmer, 2009)
Experimental design and sampling
The approach was to assess the colonisation of defaunated sediment by Nereis that dispersed by swimming only, and by both swimming and crawling. Six perspex cores, 20 cm in length and internal diameter 8 cm, were completely filled with sediment collected from the site and then defaunated using a repeated freeze-thawing method (Bolam et al., 2004). In addition, six cores 25 cm in length were filled with sediment to 5 cm from the top. The bottoms of all 12 cores were covered by 300 μm nylon mesh to prevent worms colonising sediment from beneath. Every 4 weeks twelve cores were inserted into holes of the same size in the sediment, with the tops of the six 20-cm cores flush with the surface sediment (Flush treatment) and the tops of the 25 cm cores 5 cm above the surface (Raised treatment). In both treatments the defaunated sediment surfaces were level with that of the surrounding sediment (Fig. 2a). To allow drainage of water trapped within the top 5 cm of the raised cores, a pair of 1 mm drainage holes were drilled on each side, just above the sediment surface and covered with 300 μm nylon mesh. The flush cores could be colonised by swimming and crawling individuals but the raised cores only by swimming individuals (Fig. 2a). The assumption was that colonisation by swimming would be the same in the two treatments and higher densities in the flush cores would be attributable to dispersal by crawling. After 4 weeks (a lunar month), the twelve cores were replaced with similar pre-prepared cores and six cores (8 cm diameter, 20 cm deep) were taken from the surrounding sediment to estimate natural abundances (Control treatment). The cores were arranged as a replicated (randomised) block design with three blocks stationed at least 10 m apart to test within and between block variability (Fig. 2b). As repeated sampling of soft-sediments unavoidably causes some localised disturbance (Skilleter, 1996), a pair of non-sampled control plots were designated within each of the three blocks and these were sampled at the end of the experiment to assess the effect of repeated sampling of the Control plots. Thirteen 4-weekly samples were collected to provide data on the seasonal variations in dispersal by Nereis.
Laboratory methods
The sediment cores were sectioned horizontally, with the top 3 cm and bottom 17 cm separated and fixed in 10% formalin solution. The top 3 cm sections were washed through 250 and 125 μm sieves, to retain larval and juvenile Nereis, and the remainder of the cores were sieved through a 500 μm sieve, to retain the larger Nereis that burrow deeper. All individuals were picked out under a binocular microscope and stored in a preservative of 70% ethanol, 10% glycerol and 20% water. As Nereis is sometimes damaged during processing it is not possible to record total lengths for all specimens. Several partial length measurements can be used instead and these include: jaw length (Chambers & Milne, 1975; Olive & Garwood, 1981; Möller, 1985; Abrantes et al., 1999), width of the 1st, 3rd or 10th chaetigerous segment (Kristensen, 1984; Davey & George, 1986; García-Arberas & Rallo, 2002); and the L3 length, which is the total length of the prostomium, peristomium and chaetiger 1 (Fidalgo e Costa, 2003; Gillet & Torresani, 2003; Durou et al., 2008). In this study, the L3 measurement (to the nearest 0.1 mm) was used. When nereid worms are fixed in formalin the muscles achieve an equal state of contraction (Kristensen, 1984) and the recorded lengths of preserved animals are more consistent than those of living worms. For each complete specimen, the total body length, the total number of segments and the L3 length were recorded and for all incomplete individuals only the L3 was recorded. Measurements were made using an eyepiece micrometer. The predicted size of damaged specimens was calculated using the relationship between L3 and total length and number of segments. The biomass of worms was estimated by selecting 98 intact fixed specimens of various sizes and recording their blotted wet weight (mg) using a Metler Toledo balance (precision = 0.0001 mg). The relationship between length L3 (mm) and blotted wet weight was calculated and used to estimate the biomass of the incomplete and remaining intact worms. These values were converted into dry weight (mg) using the wet to dry conversion factor of 0.181 specific for Nereis (Rumohr et al., 1987).
Statistical analysis
The differences in Nereis densities between treatments and time were analysed using repeated measures analysis of variance (RM-ANOVA), in which treatment and time were fixed factors and block was considered as a random factor. Prior to the analyses all density data were checked for normality using the Anderson–Darling test and homogeneity of variances using the Bartlett test. Data not satisfying the assumptions of ANOVA were square root-transformed. For some months there were low densities of Nereis and zeros in the data set. To overcome this difficulty when using RM-ANOVA, a test to determine approximate significance of results was also done by conducting a randomization (permutation) test using the R Statistical Software package v2.7.0 (after Hall & Harding, 1997). Firstly, R performs RM-ANOVA on the observed data set and stores the four resultant F values (treatment, time, block and treatment x time interaction). Secondly, the observations are then randomised to create a non-significant event, RM-ANOVA performed and the four F values stored. This randomisation was conducted 10,000 times storing all F values. The H o can be rejected if the F value for the actual data set fell in the upper 95% of the distribution of 10,000 F values from the randomisation (a situation with no effects). A post-hoc ANOVA for each sampling date was performed using the non-parametric Tukey multiple pairwise comparison test to identify significant differences (with P < 0.05) between treatments and controls. This test allows multiple testing without increasing the risk of Type 1 errors. L3 length frequency histograms were constructed and length distributions were compared using the non-parametric Kolmogorov–Smirnov (KS) test to assess the probability that two distributions are the same (Dytham, 2002). Differences in estimated mean biomass values over time were analysed using the non-parametric Mann–Whitney and Kruskall–Wallis tests.
Results
Densities of colonisers
The seasonal variations in densities of Nereis in all three treatments are shown in Fig. 3. The highest natural densities occurred from August to December and the lowest were in March and April. Colonisation of the flush and raised cores occurred throughout the year, except in February when no Nereis were found in the raised cores. The densities of individuals in both the flush and raised treatments increased during the summer, but declined in the autumn to low densities in the winter. The densities of Nereis in the flush cores were significantly lower than the natural (control) densities only in November and December. The densities of Nereis in the raised cores were significantly lower than the natural densities from September to February.
Table 1 summarises the results of the randomisation test for analysis of all three treatments (Test no. I), and of the analysis between only the flush and raised (Test no. II). In Test I, there was a significant treatment, time, block and treatment × time interaction, in Test II, there was only a significant result for treatment and time. The randomisation has given confidence in interpreting the results of the RM-ANOVA when stating if a factor has a significant effect. RM-ANOVA revealed that the mean densities were significantly different between treatments, over time, and a significant treatment × time interaction (Table 2a). This interaction term indicates that the relationship over time was not consistent between treatments. The results of the Tukey multiple comparison test (Fig. 3) indicates that this significant interaction term is because of the seasonal variations in densities of colonists due to a decline in the autumn and winter. RM-ANOVA analysis excluding the control data and only analysing the two treatment data sets flush and raised showed that there was no significant treatment × time interaction (Table 2b). This indicates that the differences in densities between the treatments (flush and raised) are consistent over time. There was a significant block effect (P < 0.05), but only when the control data were included in the analysis (Table 2a). Therefore, the block effect does not affect any differences relating to colonisation. Furthermore, there was no significant effect of repeatedly sampling the control plots over time as there were no significant differences between the mean densities in the control and non-sampled control plots, (one-way ANOVA F = 0.00, P = 1.000) sampled at the end of the experiment.
Biometric analysis
The results of the regression analyses between L3 length and total length, numbers of segments and wet weight are summarised in Table 3.
Size of colonisers
Figure 4 shows the L3 length frequency distributions for Nereis in each of the three treatments throughout the year. In the natural population the worms varied in L3 length up to 3.30 mm (body length 123 mm), but in most months the smallest size class (L3 < 0.50 mm body length < 7 mm) was the most abundant, indicating recruitment throughout the year. Consequently no distinct cohorts could be identified and traced from month-to-month. The worms found in the flush cores were of a similar size range to those in the control population, but those from the raised cores were smaller with L3 lengths no longer than 2.10 mm (body length 61 mm), indicating that larger worms do not disperse by swimming. Analysis of the L3 data for the samples of 4 August and 31 August, the dates of highest dispersal, identified a significant difference between the control and flush treatments (KS, D = 0.26, P = 0.011), because a greater proportion of the worms in the flush cores were larger than in the natural population. There were also significant differences between the raised treatment and both the control (KS, D = 0.36, P = 0.006) and flush (KS, D = 0.39, P = 0.001) treatments, in both cases because of the absence of large worms in the raised cores.
The relative abundance of the worms of different sizes in the raised cores, expressed as a % of those in the flush cores, is shown in Fig. 5, with 100% indicating no differences between them. In all seasons, there was a decrease in the colonisation of the raised cores, relative to the flush ones, with increased sizes of worms. In the summer (July and August), peak dispersal period the relative abundance of the smallest two size groups of Nereis in the raised cores was 60 and 80% of that in the flush cores, indicating that the smallest worms mostly colonised the flush cores via the water column and not by crawling across the sediment surface. Conversely, in the autumn and winter most of the small worms colonised the flush cores by crawling across the sediment.
Seasonal differences in the proportions of total numbers of Nereis recorded in the Raised cores relative to the numbers recorded for the Flush cores (as a %) for each length L3 size class. Spring is inclusive of sample months: March to June, Summer: July to August, Autumn: September to November and Winter: December to February
In some previous studies (see “Discussion” section), the sizes of the smallest worms have been expressed as the number of segments and this practise is continued here, including the numbers counted directly (72%) and those estimated from the L3 length. No individuals with fewer than 6 segments were observed during this study, and only a few individuals with 6–10 segments were found in the control and flush samples (Fig. 6). Those worms colonising from the water column (raised cores) had between 11 and 81 segments, with most having 21–40. In the control and flush treatments, worms up to 150 segments were found.
Biomass
There was no significant seasonal variation in the biomass of Nereis colonising via the water column (Kruskall–Wallis: H = 11.48, P = 0.40). The biomass of Nereis in the raised cores was always low compared to the natural population (Fig. 7) because of colonisation by small worms and at low densities. The biomass of worms in the flush cores showed a significant temporal change (Kruskall–Wallis: H = 33.19, P = 0.001), being high in the summer and low in winter. The biomass was higher in the flush cores than the natural population only in August and September, because of a higher density of large worms (Fig. 4), but lower from November to February because of lower densities and a higher proportion of small worms.
Temporal variation of the mean biomass of Nereis (histogram) with mean densities (full data shown Fig. 3) overlain for comparison
Discussion
Colonisation of hard substrata by invertebrates is largely by larvae, but soft-sediments have differing and often very independent sources of colonists (Whitlatch et al., 1998). This study has confirmed the importance of all life stages of Nereis in dispersing and colonising sediments. Dispersal by young worms throughout the year was a reflection of the continuous (but not uniform) recruitment. Females were not examined for the presence of oocytes, but the occurrence of young chaetigerous larvae throughout the year indicates extended or multiple spawning periods (Möller, 1985). Continuous or multiple spawning events have been reported for southern European populations (Abrantes et al., 1999; García-Arberas & Rallo, 2002; Fidalgo e Costa, 2003).
Hall & Frid (1998) reported Nereis to colonise defaunated sediment within several weeks, which was independent of season. In this study the colonisation of the flush cores was sustained during most of the year with densities significantly lower than controls only during November and December. Relatively high dispersal activity in the summer may partly result from high natural densities, but the decline in the numbers recovered from the flush and raised treatments in the autumn was despite continued high natural densities and indicates this change was caused by a seasonal variation in behaviour, rather than a reduction in density dependent dispersal. The decline in the colonisation of the flush cores in autumn and winter was by larger worms (Fig. 4). Larger Nereis may disperse less in the autumn and winter (Fig. 3) to remain deeper in their galleries when the risk of predation from birds is greater. Nereis are often found in greatest abundance in the upper intertidal zone, and while this reduces their exposure to predation by fish and crabs (Davey & George, 1986) they are more vulnerable to predation by wading birds. In SE England, bird predation is particularly high in the autumn and winter when densities of wading birds are high because resident birds are joined by migrants from northern nesting sites that overwinter here or are on passage to wintering grounds further south. The decline in natural mean densities from over 1500 m−2 in September to less than 400 m−2 by March (Fig. 3) indicates a winter mortality rate of Nereis of about 75%. The reduction in dispersal by larger worms in the autumn and winter (Fig. 4) may also be related to the increase in oocyte development in females, which occurs at this time of the year (Dales, 1950; Fidalgo e Costa, 2003) and when their ability to regenerate during maturation is reduced (Lawrence & Soame, 2004) By January, the densities of Nereis in the control and flush treatments were similar again because of dispersal into the latter by small worms (Fig. 4).
In summer, when dispersal activity was at its highest, the overall ratio of Nereis densities in the raised to flush treatments was ~1:2. This indicates that colonisation of the flush cores, and therefore the natural sediments, was by an equal number of swimming and crawling Nereis. Water column dispersal, into the raised cores, was only by larvae and juveniles. Some of the water column dispersal may be completely passive, by larvae and juveniles being washed into the water column by waves, but this is considered of little importance at this wave-sheltered site. The minimum lengths of mature females in the Thames estuary was 70 mm (Dales, 1950) but no worms longer than 61 mm (L3 lengths greater than 2.1 mm) colonised the raised cores. Therefore, no adults colonised the raised cores, and while worms can be stimulated to swim, Nereis are not efficient swimmers (Gray, 1939). They have been observed swimming just above the sediment surface with their body clear of the substratum but their head remaining in contact with it (Hesselberg & Vincent, 2006). No worms with fewer than 11 chaetigers were recorded in the raised cores. Nereis trochophore larvae are weak swimmers and 3-chaetiger neochaete larvae can crawl, but can also swim when they have four or five segments by folding back their chaetae and using their ciliated bands for propulsion (Dales, 1950; Smith, 1964). Dales (1950) reported that larvae became more active when seven weeks old (nine segments). Larvae with three chaetigerous segments have been observed in the upper 5 cm of the sediment but only over a short period in the spring (Möller, 1985). In this study, no larvae with fewer than six segments colonised the defaunated sediments. Here, Nereis emergence occurs at the six chaetiger stage, when they are 5- to 6-weeks-old (Dales, 1950), as larvae of this stage were recorded in the surface sediments of the flush and control treatments during the summer. These results are consistent with laboratory observations of emergence from the maternal burrow at this stage, coinciding with the fusion of the first segment to the peristomium, which forms the dorso-posterious tentacular cirri (sensory organs) (Bartels-Hardege & Zeeck, 1990; Marty & Retíere, 1999).The cores were left in situ for a period of 4 weeks at a time and during this period any newly settled larvae may have grown, because in young worms the rate of segment proliferation may be one segment per week (Dales, 1950; Durou et al., 2007). At Bridgemarsh Creek, the data indicate that the smallest chaetigerous larvae remain deep (>3 cm) within the sediment, protected in the parental gallery prior to dispersing at the six chaetiger stage, after which they construct burrows from the surface downwards (Bartels-Hardege & Zeeck, 1990; Davey, 1994).
That the densities of worms in the flush cores were generally lower than, but not significantly different to, those in the control sediments (except during November and December) indicates that in each month almost all of the population undergoes dispersal, and that individual Nereis will disperse several times in their lives. Dispersal from the maternal burrow by swimming larvae increases the risk of predation while in the plankton, but has the advantage of reducing interference and exploitation competition with siblings and the parent (Hughes, 1988) and predation by the parent. On the upper mudflats, Nereis are predominantly non-selective deposit feeders, and they feed by partially emerging from their burrows to engulf mouthfuls of surface sediment before withdrawing (Paramor & Hughes, 2004). Reise (1979) reported juveniles to colonise spaces between the feeding territories around the burrows of established worms. Larger Nereis disperse by crawling, when presumably they are not at risk of predation from conspecifics, but the advantages of doing so are not clear. Dispersal may be to find better feeding opportunities, because Nereis may deplete the food resources in the sediment around their burrow.
References
Abrantes, A., F. Pinto & M. H. Moreira, 1999. Ecology of the polychaete Nereis diversicolor in the Canal de Mira (Ria de Aveiro, Portugal): population dynamics, production and oogenic cycle. Acta Oceanologica 20: 267–283.
Bartels-Hardege, H. D. & E. Zeeck, 1990. Reproductive behaviour of Nereis diversicolor (Annelida: Polychaeta). Marine Biology 106: 409–412.
Bolam, S. G., 2004. Population structure and reproductive biology of Pygospio elegans (Polychaeta: Spionidae) on an intertidal sandflat, Firth of Forth, Scotland. Invertebrate Biology 123: 260–268.
Bolam, S. G., P. Whomersley & M. Schratzberger, 2004. Macrofaunal recolonization on intertidal mudflats: effect of sediment organic and sand content. Journal of Experimental Marine Biology & Ecology 306: 157–180.
Breton, S., F. Dufresen, G. Desrosiers & P. U. Blier, 2003. Population structure of two northern hemisphere polychaetes, Neanthes virens and Hediste diversicolor (Nereididae), with different life-history traits. Marine Biology 142: 707–715.
Chambers, M. R. & H. Milne, 1975. Life cycle and production of Nereis diversicolor O.F.Müller in the Ythan Estuary, Scotland. Estuarine and Coastal Marine Science 3: 133–144.
Dales, R. P., 1950. The reproduction and larval development of Nereis diversicolor O. F. Müller. Journal of the Marine Biological Association UK 29: 321–360.
Davey, J. T., 1994. The architecture of the burrow of Nereis diversicolor and its quantification in relation to sediment-water exchange. Journal of Experimental Marine Biology and Ecology 179: 115–129.
Davey, J. T. & C. L. George, 1986. Factors in the distribution of intertidal, estuarine polychaetes: a field experiment with Nereis (Hediste) diversicololor and Nephtys hombergi in the Tamar at Plymouth. Estuarine, Coastal & Shelf Science 22: 603–618.
de Deckere, E. M. G. T., T. J. Tolhurst & J. F. C. de Brouwer, 2001. Destabilization of cohesive intertidal sediments by infauna. Estuarine, Coastal & Science 53: 665–669.
Drolet, D. & M. A. Barbeau, 2009. Differential emigration causes aggregation of the amphipod Corophium volutator (Pallus) in tide pools on mudflats of the upper Bay of Fundy, Canada. Journal of Experimental Marine Biology & Ecology 370: 41–47.
Durou, C., C. Mouneyrac & C. Amiard-Triquet, 2008. Environmental quality assessment in estuarine ecosystems: Use of biometric measurements and fecundity of the ragworm Nereis diversicolor (Polychaeta, Nereididae). Water Research 42: 2157–2165.
Durou, C., B. D. Smith, M. Romeo, P. S. Rainbow, C. Mouneyrac, M. Mouloud, M. Gnassia-Barelli, P. Gillet, B. Deutch, & C. Amiard-Triquet, 2007. From biomarkers to population responses in Nereis diversicolor: assessment of stress in estuarine ecosystems. Ecotoxicology and Environmental Safety 66: 402–411.
Dytham, C., 2002. The tests 1: tests to look at differences, Chap 7. In: Choosing and Using Statistics. A Biologist’s Guide. Blackwell Science Ltd, Oxford: 70.
Fidalgo e Costa, P., 2003. The oogenic lifecycle of Nereis diversicolor (O. F. Müller, 1776). (Annelida: Polychaeta) in shallow water environments in southwestern Portugal. Boletin Instituto Español De Oceanografía 19: 17–29.
Fowler, S. L., 1999. Guidelines for managing the collection of bait and other shoreline animals within UK European marine sites. English Nature (UK Marine SACs Project): 132 pp.
Garbutt, R. A., C. J. Reading, M. Wolters, A. J. Gray & P. Rothery, 2006. Monitoring the development of intertidal habitats on former agricultural land after the managed realignment of coastal defences at Tollesbury, Essex, UK. Marine Pollution Bulletin 53: 155–164.
García-Arberas, L. & A. Rallo, 2002. Life cycle, demography and secondary production of the polychaete Hediste diversicolor in a non-polluted estuary in the Bay of Biscay. Marine Ecology 23: 237–251.
Gillet, P. & S. Torresani, 2003. Structure of the population, secondary production of Hediste diversicolor (O. F. Müller, 1776), (Polychaeta, Nereidae) in the Loire estuary, Atlantic Coast, France. Estuarine Coastal and Shelf Science 56: 621–628.
Gray, J., 1939. Studies in animal locomotion: VIII. The kinetics of locomotion of Nereis diversicolor. The Journal of Experimental Biology 16: 9–17.
Gudmundsson, H., 1985. Life history patterns of polychaete species of the family Spionidae. Journal of the Marine Biological Association of the United Kingdom 65: 93–111.
Hall, J. A. & C. L. J. Frid, 1998. Colonisation patterns of adult macrobenthos in a polluted North Sea estuary. Aquatic Ecology 33: 333–340.
Hall, S. J. & M. J. C. Harding, 1997. Physical disturbance and marine benthic communities: the effects of mechanical harvesting of cockles on non-target benthic fauna. Journal of Applied Ecology 34: 497–517.
Hateley, J. G., A. Grant, S. M. Taylor & N. V. Jones, 1992. Morphological and other evidence on the degree of genetic differentiation between populations of Nereis diversicolor. Journal of the Marine Biological Association, UK 72: 365–381.
Hesselberg, T. & J. F. V. Vincent, 2006. The function of parapodial setae in a nereidid polychaete moving on two different substrata. Journal of Experimental Marine Biology and Ecology 335: 235–244.
Hughes, R. G., 1988. Dispersal by benthic invertebrates—the in situ swimming behaviour of the amphipod Corophium volutator. Journal of the Marine Biological Association of the United Kingdom 68: 565–579.
Kristensen, K., 1984. Life cycle, growth and production in estuarine populations of the polychaetes Nereis virens and N diversicolor. Holarctic Ecology 7: 249–256.
Kristensen, E., 2001. Impact of polychaetes (Nereis spp and Arenicola marina) on carbon biogeochemistry in coastal marine sediments. Geochemical Transactions 2: 92–103.
Kirstensen, E. & O. L. Mikkelsen, 2003. Impact of the burrow-dwelling polychaete Nereis diversicolor on the degradation of fresh and aged macroalgal detritus in a coastal marine sediment. Marine Ecological Progress Series 265: 141–153.
Lawrence, A. J. & J. M. Soame, 2004. The effects of climatic change on the reproduction of coastal invertebrates. Ibis 146: 29–39.
Lewis, L. J., J. Davenport & T. C. Kelly, 2003. A study of the impact of a pipeline construction on estuarine benthic invertebrate communities. Part 2. Recolonization by benthic Invertebrates after 1 year and response of estuarine birds. Estuarine Coastal and Shelf Science 57: 201–208.
Marty, R. & C. Retiére, 1999. Larval-to-juvenile mobility activity of a holobenthic species. Nereis diversicolor (O. F.Müller) (Polychaeta: Nereidae)—their involvement in recruitment. Bulletin of Marine Science 65: 761–771.
Möller, P., 1985. Production and abundance of juvenile Nereis diversicolor and oogenic cycle of adults in shallow waters of western Sweden. Journal of Marine Biological Association 65: 603–616.
Nicholls, J. C. & M. Trimmer, 2009. Widespread occurrence of the anammox reaction in estuarine sediments. Aquatic Microbial Ecology 55: 105–113.
Olive, P. J. W. & P. R. Garwood, 1981. Gametogenic cycle and population structure of Nereis (Hediste) diversicolor and Nereis (Nereis) pelagica from North-East England. Journal of Marine Biological Association 61: 193–213.
Palmer, M. A., J. D. Allan & C. A. Butman, 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. TREE 11: 322–326.
Paramor, O. A. L. & R. G. Hughes, 2004. The effects of bioturbation and herbivory by the polychaete Nereis diversicolor on loss of saltmarsh in south-east England. Journal of Applied Ecology 41: 449–463.
Paramor, O. A. L. & R. G. Hughes, 2005. Effects of invertebrate infauna on early saltmarsh plant colonisation of managed realignment areas in south-east England. Marine Ecology Progress Series 303: 61–71.
Paramor, O. A. L. & R. G. Hughes, 2007. Restriction of Spartina anglica (C.E. Hubbard) marsh development by the infaunal polychaete Nereis diversicolor (O. F. Müller). Estuarine Coastal & Shelf Science 71: 202–209.
Reise, K., 1979. Spatial configurations generated by motile benthic polychatetes. Helgoland Marine Research 32: 55–72.
Rosa, S., J. P. Granadeiro, C. Vinagre, S. Franςa, H. N. Cabral & J. M. Palmeirim, 2008. Impact of predation on the polychaete Hediste diversicolor in estuarine intertidal flats. Estuarine, Coastal & Shelf Science 78: 655–664.
Rumohr, H., T. Brey & S. Anker, 1987. Compilation of biometric conversion factors for benthic invertebrates of the Baltic Sea. Baltic Marine Biologists Publication 9: 1–56.
Santos, S. L. & J. L. Simon, 1980. Marine soft-bottom community establishment following annual defaunation: larval or adult recruitment? Marine Ecology Progress Series 2: 235–241.
Scaps, P., 2002. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O. F. Müller) (Annelida: Polychaeta). Hydrobiologia 470: 203–218.
Shull, D. H., 1997. Mechanisms of infaunal polychaete dispersal and colonization in an intertidal sandflat. Journal of Marine Research 55: 153–179.
Skilleter, G. A., 1996. An experimental test of artefacts from repeated sampling in soft-sediments. Journal of Experimental Marine Biology &.Ecology 205: 137–148.
Smith, R. I., 1958. On reproductive pattern as a specific characteristic among Nereid polychaetes. Systematic Zoology 7: 60–73.
Smith, R. I., 1964. On the early development of Nereis diversicolor in difference salinities. Journal of Morphology 114: 437–464.
Whitlatch, R. B., A. M. Lohrer, S. F. Thrush, R. D. Pridmore, J. E. Hewitt, V. J. Cummings & R. N. Zajac, 1998. Scale-dependant benthic recolonization dynamics: life stage-based dispersal and demographic consequences. Hydrobiologia 375–376: 217–226.
Widdows, J., M. D. Brinsley & N. D. Pope, 2009. Effect of Nereis diversicolor density on the erodability of estuarine sediment. Marine Ecological Progress Series 378: 135–143.
Worley, A., & M. Simpson, 1998. Littoral and sublittoral biotope mapping and data capture exercise for the Essex estuaries candidate Marine Species Area of Conservation. English Natural Research Reports No 305. English Nature, UK.
Acknowledgements
This work was conducted as part of a Natural Environment Research Council funded studentship (NER/S/A/2006/14028) with additional CASE support from the Centre for the Environment, Fisheries and Aquaculture Science (CEFAS). We would like to thank P. Whomersley, M. Frost and R. Hayes for assistance in the field, and J. Barry for his invaluable statistical guidance and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: R. J. Uncles & S. B. Mitchell / The Thames Estuary and Estuaries of South East England
Rights and permissions
About this article
Cite this article
Aberson, M.J.R., Bolam, S.G. & Hughes, R.G. The dispersal and colonisation behaviour of the marine polychaete Nereis diversicolor (O. F. Müller) in south-east England. Hydrobiologia 672, 3–14 (2011). https://doi.org/10.1007/s10750-011-0752-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0752-y