Abstract
A survey was conducted for the presence of cyanobacteria toxins in Lake Kotokel due to a few cases of Haff disease registered in 2008–2009 caused by consumption of fish from Lake Kotokel, and wildlife mortality including large fish kill. The aims of this study were to determine what cyanotoxins (if any) were present in the lake, to describe phytoplankton composition including morphology, density, and species diversity of cyanobacteria, as well as to evaluate the trophic state of the lake. Samples were collected from both nearshore and central sites in August of 2009. Aphanocapsa holsatica dominated the phytoplankton. The presence of toxigenic genotypes of Microcystis spp. and Anabaena lemmermannii was detected by sequencing of PCR-amplified aminotransferase domain of microcystin synthetase gene. LR, RR, and YR microcystin (MC) variants were detected with liquid chromatography-UV mass spectrometry. The data do not shed light on the etiology of Haff disease in Lake Kotokel region, nevertheless taking into account the recreational importance of the lake and its direct connection to Lake Baikal, a necessity to monitor cyanobacteria in these water bodies is evident. This is the first report on simultaneous detection of MC-producing genotypes and MCs in the Lake Baikal region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria produce a range of secondary bioactive metabolites, including toxins. One of the best studied cyanotoxins in freshwater ecosystems is the group of microcystins (MCs). MCs are cyclic heptapeptides produced nonribosomally by peptide synthetases, polyketide synthases, and tailoring enzymes. Over 90 variants of MCs are known (Welker & Von Döhren, 2006), the most common of them differ in l-amino acids and in modification by methyl groups. MCs specifically inhibit the eukaryotic serine/threonine phosphatases 1 and 2A rendering the hyperphosphorylation of proteins in the hepatocyte cytoskeleton, which leads to massive hepatocyte necrosis and pooling of blood into the liver (MacKintosh et al., 1990). Chronic exposure to sublethal doses of MCs resulted in endemic cancer of the liver in one of the provinces of China (Chorus & Bartram, 1999).
Predominant MC-producing cyanobacteria belong to the Anabaena, Microcystis, Planktothrix, Anabaenopsis, Hapalosiphon, and Nostoc genera (Chorus & Bartram, 1999). Planktonic cyanobacteria, including unicellular colony forming Microcystis and the filamentous, heterocystous Anabaena, commonly form toxic blooms in highly productive freshwater ecosystems worldwide (Codd et al., 2005). Therefore, many countries monitor the presence and safe concentrations of highly toxic MC-LR, which contains leucine and arginine as variable l-amino acids. The LD50 (lethal dose, 50%) of MC-LR (given intraperitoneally to mice) is 50 μg kg−1, MC-RR- (arginine–arginine) and MC-YR- (tyrosine–arginine) variants are less toxic. The World Health Organization (1998) has set an advisory level for MCs in drinking water of 1.0 μg l−1 MC-LR.
Several detection methods for MCs are commonly used, including high performance liquid chromatography (HPLC), immunoassays, and protein phosphatase inhibition assays (reviewed in Carmichael & An, 1999). However, liquid chromatography–mass spectrometry (LC/MS) provides a greater specificity and sensitivity (Chorus & Bartram, 1999).
Identification of a gene locus of microcystin synthetase provided a powerful tool to detect potential toxigenic species (Nishizawa et al., 1999; Rouhiainen et al., 2004). MCs producing multienzyme complex is encoded by a 55 kb-long gene cluster containing 10 genes designated mcyA–mcyJ (Rouhiainen et al., 2004). One of the enzymes, the aminotransferase (AMT) plays a crucial role in cyanotoxin producing by transferring amino groups to the side chain of the Adda moiety (Moffitt & Neilan, 2004). The gene for AMT domain locating between microcystin synthetase gene E and nodularin synthetase gene F has been found suitable for the detection of MC or nodularin producing Microcystis, Planktothrix, Anabaena, Nostoc and Nodularia strains (Jungblut & Neilan, 2006). In phylogenetic analysis, mcyE sequences from different toxin producer genera form their own groups and are not affected by lateral gene transfer (Rantala et al., 2004). Thus, the primers for AMT region are suitable for reliable detection and identification of main cyanotoxin producers in environmental samples.
Lake Kotokel is situated close to the eastern coast of Lake Baikal (Eastern Siberia, Fig. 1). In 2008, mass mortality of fish, birds, and domestic animals was recorded in Lake Kotokel and its neighborhoods. Sixteen persons were hospitalized with symptoms of Haff disease: severe muscular pains, rhabdomyolysis (rapid breakdown of skeletal muscle), and myoglobinuria. The etiology is not yet known, but there is little doubt that the disease is due to an unknown, natural toxin. In June of 2009, the Ministry of Health and Social Development of Russia forbade eating fish caught in the lake, as well as bathing in and drinking the water. Analysis of phytoplankton samples obtained in Lake Kotokel in August of 2008 revealed the presence of potentially toxic Microcystis bearing microcystin synthetase gene E, however, the analysis for the appearance of MCs in the water samples was not performed (Belykh et al., 2010). Therefore, Lake Kotokel became a subject of the interdisciplinary investigation.
Lake Kotokel was thoroughly studied in the 1950–1980s due to its recreational and mainly commercial value for the region—the annual fish catch exceeded 1,200 tons (Kuzmich, 1988). It was a eutrophic water body, cyanobacteria were the most abundant phytoplankton group, and Gloeotrichia ehinulata was the dominant species (Korde, 1968; Kuzmich, 1988). Microcystis aeruginosa, M. pulverea, and Anabaena lemmermannii proliferated less intensively. In summer, the water transparency dropped to 0.6–0.8 m and the water temperature sometimes reached 25.8°C. Mass development of phytoplankton in July–August resulted in a decrease of phosphate and mineral nitrogen concentration; in other seasons, the content of these nutrients was higher (Kuzmich, 1988).
In Siberia, the first report on finding of microcystin synthetase genes for potentially toxic M. aeruginosa and Anabaena sp. was concerned to Ust-Ilim and Bratsk reservoirs fall within of Lake Baikal watershed (Tikhonova et al., 2006, 2007). However, mcy genes have not been detected in any samples either from the pelagic zone or the shallow areas of Lake Baikal.
The aim of this work was to comprehensively investigate the phytoplankton of Lake Kotokel including morphology, density, and species diversity of cyanobacteria, to evaluate the trophic state of the lake, to measure the concentration of different variants of the MCs (if they were), as well to find potential toxin producers by the PCR-amplified mcyE genes.
Materials and methods
Site description
Lake Kotokel is located in the south-central region of Siberia (Russia), 2 km off the eastern coast of Lake Baikal (Fig. 1). It is separated from Baikal by a low mountain chain. The elevation of Lake Kotokel is only 5 m higher than that of Lake Baikal. The Istok River flows from Lake Kotokel and joins the Turka River, which flows into Lake Baikal. The area of Lake Kotokel is 68.9 km2 and the catchment area is 150 km2. The water balance of the lake provides continuous discharge into Lake Baikal at a rate of 1.3 m3 s−1, and the time of conventional water retention is close to 7 years. The lake is 15 km long and 6 km wide. It has an average depth 3.5 m, and its maximum depth is 14 m (Kuzmich, 1988).
The lake has low-lying and swampy shores often covered with an aquatic plant community consisting of Carex, Equisetum, and Typha. Aquatic macrophytes Phragmithes, Potamogeton, Polygonum, and species Elodea canadensis grow up to 1.5 m, and Ceratophyllum occurs up to a depth of 3 m (Korde, 1968; Kuzmich, 1988).
Sample collection and processing
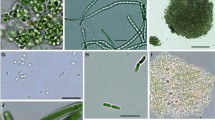
In August of 2009, plankton samples were collected with a Ruttner water sampler and Apstein net (mesh size 16 μm) at a pelagic site in the central part (52°49′543″, 108°07′357″, maximal depth 14 m) and at a littoral site near village Cheremushki (52°45′533″, 108°07′557″, depth 1 m) in the southern part of Lake Kotokel (Fig. 1). Sampling depths were 0, 6.5, and 13 m. Water temperature and Secchi depth were also measured. Bottle phytoplankton samples (0.5 l) were fixed with Lugol’s solution and concentrated by sedimentation to a volume 0.1 l. Net phytoplankton samples were fixed with 2% formalin for microscopic observations and with 80% ethanol for molecular analysis. A 0.1-ml aliquot of the settled sample was pipetted in a Nageotte chamber, and cyanobacteria were counted in triplicate, under a light microscope at a magnification of 400× and 1000× (Axiovert 200, Zeiss). The biovolume of cyanobacteria cells was calculated from microphotographs obtained using a Penguin 600CL camera (Pixera Corp., USA) and the VideoTest-Razmer 5.0 software package (www.videotest.ru).
Nutrients were measured by colorimetric methods: phosphates with phosphorous-molybdate, ammonium with Nessler’s reagent, and nitrite with the Griess reagent (Semenov, 1977). Nitrate was analyzed by HPLC. The total phosphorus was measured in water samples after oxidation with persulphate. The total phosphate was then detected by the methods described above for inorganic phosphate. Determination of permanganate and dichromate oxidizability was carried out by titration in an acid medium.
The concentration of chlorophyll a was measured by spectrophotometry. Samples (0.5 l) were filtered onto 0.45 μm Millipore polycarbonate filters, extracted using hot methanol, and then analysed with a spectrophotometer (Bio-Rad SmartSpec Plus, USA).
Microcystin analyses
Net samples of phytoplankton were frozen and thawed (5 times) and then dried (60°C) prior to toxin extraction. The dried phytoplankton biomass (178.2 mg) was extracted twice (10 ml each time) with methanol by sonication in an ultrasonic bath (1 h). The combined raw extracts were centrifuged (at 5,263 rpm for 15 min), and the supernatant was dried in a rotary vacuum evaporator IKA RV 05-ST (Werke GmbH & Co. KG, Germany). The extract obtained was redissolved in 0.5 ml of methanol prior to Liquid chromatography–mass spectrometry analysis. LC/MS analyses were performed on an Agilent HP1200 chromatographic system that was coupled to an Agilent time-of-flight mass spectrometer (ESI-TOF) equipped with an electrospray ionization source operating in the positive ionization mode. Chromatographic separation of MCs was carried out using a Zorbax 300 SB C18 column (2.1 × 150 mm, 5 μm) maintained at 35°C, at a flow rate of 0.3 ml min−1,with a gradient of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The solvent program was as follows: 10% B at time zero, 10-80% B for 30 min, 100% B for 5 min, and 100% B for 40 min. The injected sample volume was 10 μl, and the detection was performed with a diode array UV detector at 214, 222, 238, and 330 nm. Quantification was achieved by measuring the chromatographic signals of each MC against calibration curve produced from “Microcystins-LR standard” (Biosense Laboratories, Norway), as they have the same extinction coefficients as MC-LR.
Genetic analyses
Amplification, cloning, and sequencing
DNA from the phytoplankton samples was extracted using “DNA-sorb” extraction kit (InterLabService, Russia). Mcy gene fragments were PCR amplified with the HepF and HepR gene specific primers (Jungblut & Neilan, 2006) in the Peltier Thermal Cycler (MJ Research, USA). PCR was performed in 50 μl reaction mixtures containing 0.1–0.4 μM of each primer, 0.25 mM of dNTPs, 2.5 mM of MgCl2, buffer (20 mM TrisCl, pH 8.4, KCl 40 mM), Taq DNA polymerase (1.2 units) (Fermentas, MD, USA), and 10–15 ng of DNA template. Cycling conditions were as follows: initial denaturation at 95°C for 5 min, then 35 cycles of 95°C for 0.5 min, 55°C for 0.5 min, and 72°C for 1 min followed by 10 min at 72°C to complete the extension. PCR products of expected size (~470 bp) were gel-purified using the “PCR Clean-Up Gel Extraction NucleoSpin Extract II kit” (Macherey-Nagel, Germany) and subcloned into E. coli XL1BL cells using “InsTAclone” (Fermentas, MD, USA). 100 cloned amplicons were processed for sequencing. Plasmid inserts were sequenced bidirectionally by using vector-specific primers on the CEQ 8800 Genetic Analysis System and using DTSC Quick Start Kit (Beckman Coulter, USA). All unique sequences from this study have been deposited in GenBank under accession numbers GU186843–GU186846.
Phylogenetic analyses
Newly decoded mcyE gene sequences were used in a tBLASTn search against the NCBI “nr” database (http://www.ncbi.nlm.nih.gov/BLAST/) to find close relatives. The most similar reference sequences, preferably of cultivated strains, were downloaded and aligned with Kotokel isolate sequences using the Clustal W program (MEGA software; Tamura et al., 2007). The data set was analyzed by neighbor-joining (NJ), maximum likelihood (ML), and Bayesian (BI) methods to infer trees and estimate branch support. Evolutionary distances for the NJ tree (MEGA software; Tamura et al., 2007) were calculated by the Kimura-2 parameter model; the tree topology was evaluated by bootstrap support in 1,000 replicons. ML calculations were performed using PhyML software (Guindon & Gascuel, 2003), and the most appropriate evolutionary model (GTR + G) for the nucleotide sequences was set. The model was generated by the Model Generator program (http://distributed.cs.nuim.ie/multiphyl.php). BI tree topology was calculated using a Bayesian Markov chain Monte Carlo method implemented by the program Mr. Bayes v3.1.2. (Ronquist & Huelsenbeck, 2003). One million generations were run, and the likelihood function stabilized after 2,000 generations; these trees were discarded, and every hundredth of the remaining trees was used to drive the final consensus tree according to the 50% majority rule.
Results
Environmental parameters
Temperature, Secchi disk transparency, concentrations of total phosphorus, nutrients, permanganate and dichromate oxidation, and chlorophyll a in the Lake Kotokel pelagic area are presented in Table 1. Based on these data, the trophic state of Lake Kotokel was assigned as eutrophic according to the Vollenweider & Kerekes classification (1982).
Intracellular microcystin concentration
The extracts from phytoplankton samples analyzed by HPLC exhibited three major peaks with the following retention times (T R): 14.50, 16.55, and 16.86 min (Fig. 2); the UV spectra were typical of MCs. LC/MS analysis revealed the presence of three MCs with characteristic m/z values of MC-RR (m/z = 519.8089 [M + 2H]2+), MC-LR (m/z = 995.5874 [M + H]+), and MC-YR (m/z = 1045.5661 [M + H]+) (Fig. 2). The total content of MC was 53 μg g−1 of dry weight and the ratio of MCs was the following: MC-RR—49%; MC-LR—42.5%; and MC-YR—8.5%.
HPLC/MS determination of microcystins extracted from the Lake Kotokel phytoplankton (for experimental details see text). A HPLC/UV chromatogram of raw extract of microcystins: 1 microcystin-RR (T R = 14.50 min), 2 microcystin-YR (T R = 16.55 min), 3 microcystin-LR (T R = 16.86 min). B–D ESI-TOF mass spectra for the peaks 1 (B), 2 (C), 3 (D)
Species composition and abundance
Twenty-five species of planktonic cyanobacteria were identified in the pelagic samples taken in August, 2009. Half of them were considered dominant species (the number of each exceeded 50 × 103 cell l−1), namely: Aphanocapsa holsatica, Aph. delicatissima, Aph. conferta, Aph. incerta, Microcystis aeruginosa, M. viridis, Chroococcus limneticus, Coelosphaerium kuetzingianum, Snowella rosea, Romeria sp., Pseudanabaena voronichinii, and Planktolyngbya contorta. Microcystis wesenbergii, A. lemmermannii, A. scheremetievi, A. circinalis, and A. spiroides only occurred in the net samples. Diatom Aulacoseira granulata and green algae Coelastrum cambricum, Scenedesmus communis, Dimorphococcus lunatus, and Pediastrum spp. dominated in the eukaryotic phytoplankton.
The mean abundance and biomass of cyanobacteria in the 0–13 m layer were 7.13 × 109 cell l−1 and 3.87 × 103 mg m−3 that consisted of 99.97 and 83.07% of total phytoplankton values, accordingly. Aph. holsatica had the highest cell density (7.11 × 109 cell l−1) and the maximum biomass (3.77 × 103 mg m−3); its contribution to the total cyanobacterial biomass reached 97.28%. The quantitative indices of Microcystis species were low and their contribution to cyanobacteria total biomass did not exceed 0.55%. The concentration of Microcystis viridis, which was the most abundant among Microcystis spp., was 2.14 × 105 cell l−1 (biomass 14.03 mg m−3), whereas that of M. aeruginosa was 1.12 × 105 cell l−1 (biomass 7.15 mg m−3). The cell number and biomass of Anabaena spp. were low, 3.30 × 104 cell l−1, 2 mg m−3, respectively.
Genetic analysis of mcyE
Decoded sequences of one hundred of the cloned amplicons can be divided into four groups, designated as K1-09, K6-09, K7-09, and K8-09 (index “09” underlines clones obtained in the study), and each of them contained 12, 30, 30, and 28 identical sequences, respectively.
A sequence of each type served as a query in a tBLASTn search against the NCBI “nr” database. The search revealed that close homologes of K6-09, K7-09, and K8-09 sequences belong to Microcystis spp., whereas Anabaena spp. are the nearest relatives of the K1-09 sequence. Pair-wise comparisons of mcyE gene fragments of cyanobacteria isolated from geographically distinct freshwater bodies, including Lake Kotokel, show that Microcystis spp. differ by no more than 4% of nucleotide changes, whereas the dissimilarities in sequences of Anabaena and Microcystis genera reach up to 21% of the substitutions. K6-09 and K7-09 mcyE gene sequences share up to 98–99% identity with the strains of M. viridis NIES-102, M. aeruginosa NIES-843, M. aeruginosa K-139, and M. wesenbergii NIES-107 isolated during toxic bloom from Lakes Kasumigaura and Kawaguchi in Japan. K8-09 fragment shows 99% similarity with sequences of some strains of M. aeruginosa (PCC 7806, PCC 7005, UTEX B 2667) isolated from different locations in Europe and North America. K1-09 shows 100% homology with many of the sequences of Anabaena-species found in 9 Scandinavian lakes, for example Anabaena sp. SYKE 971/6 (Lake Kotojärvi, Finland) or A. lemmermannii NIVA-CYA 270/1 (Arefjordsvatnet, Norway).
As all tree-constructing methods resulted in similar topologies of the mcyE gene phylogeny, the unrooted neighbor-joining tree only is shown in Fig. 3. The branching of mcyE genes corresponds to highly supported diversification according to Microcystis, Anabaena, Nodularia, Planktothrix, and Phormidium genera. Three new mcyE sequences from Lake Kotokel (K6-09, K7-09, and K8-09 sequences) are reliable examples of the Microcystis genus. The internal relationships of the Microcystis clade are uncertain and represented by threefold polytomies. K6-09 and two more sequences from Lake Kotokel (K1-08 and K2-08), isolated in 2008 (Belykh et al., 2010), form a monophyletic group. The monophyly of K8-09 and two more Lake Kotokel sister sequences K3-08 and K5-08, as well as the relatedness of K8-09 to sequences from geographically distant water bodies has weak support (37%). K7-09 is the closest relative to the gene fragments of cyanobacteria from mesotrophic lakes of Japan. Finally, the K1-09 sequence belongs to the Anabaena genus clade and is identical to some of A. lemmermannii strains sequences from Scandinavian lakes. In general, among potentially MC-producing genotypes identified in Lake Kotokel in 2009, the single K1-09 is attributed to A. lemmermannii, whereas K6-09, K7-09, and K8-09 sequences belong to different species within the Microcystis genus.
Phylogenetic analysis of 470 bp mcyE and ndaF gene sequences. The neighbor-joining phylogenetic tree is shown. Neighbor-joining, maximum likelihood bootstrap values (BP > 50%), and Bayesian posterior probabilities (PP > 0.5) are given at common branch points (NJ/ML/BI). Sequences of the mcyE gene obtained by authors are underlined. The strain Nostoc sp. IO-102-I has been chosen as an out-group
Discussion
Lake Kotokel is a eutrophic water body, as stated earlier (Kuzmich, 1988), and confirmed by our data on the concentration of total phosphorus, content of chlorophyll a and water transparency. In the summer of 2009, the concentrations of phosphate and mineral nitrogen were lower 1.7 and 3.6 times, accordingly, in comparison with the 1990s (N. M. Pronin, personal communication). It was probably caused by some reduced anthropogenic impact on the lake due to the depletion of agricultural activity in this area. Moreover, in the summer of 2009, the low concentrations of nutrients in Lake Kotokel might be attributed not only to the phytoplankton bloom but also to the high development of aquatic plants, which are diverse in the lake.
An invasive macrophyte Elodea canadensis, unlike in most water bodies of Siberia, proliferates in Lake Kotokel, especially in the coastal zone near camp sites and residential buildings. This species facilitates the utilization of the nutrients in the lake, preventing the transition of the lake to the hypereutrophic state (Kuzmich, 1988). It has been shown that there are competitive relationships between cyanobacteria and aquatic macrophytes—the major primary producers in small eutrophic lakes (Li et al., 2009). Aquatic plants such as E. canadensis, Ceratophyllum, and Phragmithes spp. overgrown the lake are known to accumulate and transform MCs (Pflugmacher et al., 1999, 2001). Considering that the littoral zone covered by aquatic plants extends to a depth of 2 m and occupies 23% of the Lake Kotokel surface (Kuzmich, 1988), it is possible that macrophytes can decrease the content of toxins in the water.
Aphanocapsa holsatica (previously known as Microcystisholsatica) was a dominant species of planktonic cyanobacteria in the lake in August of 2009 (97.28% of total abundance), as well as in the summer of the previous year (53% of total abundance, Belykh et al., 2010). This species is nontoxic (Yasuno et al., 1998) and differs in 16S rDNA sequence from the Microcystis genus, despite minor morphometric differences (Neilan et al., 1997). Previously, the morphological similarity of Aph. holsatica and Microcystis spp. probably often led to their misidentification in Lake Kotokel samples. In August of 2008, besides Aph. holsatica, the potentially toxic M. viridis also dominated in the phytoplankton of Lake Kotokel (36% of total abundance). Other cyanobacteria species, including M. aeruginosa, made up less than 5% of the total number (Belykh et al., 2010). As there were no data on water chemistry in August of 2008, we can speculate only about the decreasing of the development of Microcystis compared to August of 2009. Heavy organic input from nearby camp sites and residential buildings might result in the dominance of M. viridis as a more resistant beta-mesosaprob to pollution (Barinova et al., 2006). Its abundance reduced in 2009 possibly due to the ban of the recreational use of the lake.
In 2009, the samples were collected in August, after the bloom. The development of cyanobacteria was low (3.87 g m−3), and the biomass was 4 times lower when compared to the maximal biomass recorded in July 1986 by other authors (Kuzmich, 1988). The chlorophyll a concentration was high due to abundant development of cyanobacteria (~80% of total biomass) and also to the impact of green and diatom algae. Previous results (Korde, 1968; Kuzmich, 1988) and our survey show that a complex of species typical of shallow well-warmed productive water bodies has been developed in Lake Kotokel over the last 50 years. It is characterized by a high abundance of diatoms, green algae and cyanobacteria.
The plankton cyanobacteria of Lake Kotokel like Microcystis aeruginosa, M. viridis, M. wesenbergii, A. lemmermannii, A. flos-aquae, and A. circinalis are known as potential MC producers. We amplified 10 diverse cyanobacterial sequences of microcystin synthetase genes from Lake Kotokel samples collected during 2 years of our observations (Belykh et al., 2010): nine of them belonged to Microcystis genus, the tenth was identical to A. lemmermannii. The total number of clones containing the Microcystis gene insert was 9 times higher than those with the Anabaena gene. Similarly, in lakes of Finland where Anabaena and Microcystis often form hepatotoxic mass occurrences, Microcystis mcyE gene copy numbers were more abundant than those of Anabaena; both toxic and nontoxic strains of Anabaena existed, whereas all strains of Microcystis spp. were toxic (Vaitomaa et al., 2003). The data correlate with records that Microcystis-species are the most common and significant MC producers in freshwater environments. Eighty to ninety percent of the samples from the water bodies of Denmark, Germany, Czech Republic, and Korea, where Microcystis dominated, contained MCs (Chorus & Bartram, 1999). Coexistence of toxigenic Microcystis and Anabaena species, like in Lake Kotokel, was detected in 15% of mesotrophic and 25% of eutrophic lakes in Finland (Rantala et al., 2006). In four other examined Siberian water bodies, the genera occurred as well (Tikhonova et al., 2006, 2007). In oligotrophic Lake Baikal and Irkutsk reservoir, cyanobacteria were nontoxic. In a mesotrophic Bratsk reservoir, the mcyA genes were found in Anabaena species. In Ust-Ilim reservoir, which is a mesotrophic also, both mcyA and mcyE genes of Microcystis genus were detected (Tikhonova et al., 2006, 2007). Thus, simultaneous development of toxic cyanobacteria of the both genera was observed only in the eutrophic Lake Kotokel.
The total value of potentially toxic Microcystis and Anabaena-species in Lake Kotokel in August 2009 was low (3.59 × 105 cell l−1 and 23.2 mg m−3) and similar to that detected in the oligotrophic Lake Baikal during the warmest season (Belykh et al., 2007). Nevertheless, the total abundance of cyanobacteria in Lake Kotokel was higher than that recommended by WHO guidelines for recreational water reservoirs averaging 7.13 × 109 cell l−1. The recreational use of water is not recommended in many countries when the concentrations of cyanobacteria exceed 20 × 106 cell l−1 (Chorus & Bartram, 1999).
However, the intra-cellular concentrations of MCs (MC-RR, MC-LR, and MC-YR) were not high in the Lake Kotokel phytoplankton (53 μg g−1 DW). For comparison, the highest total concentration of MCs detected by HPLC in phytoplankton of Kenya water bodies was 19,822 μg g−1 DW, which is similar to that found in the cyanobacterial bloom samples from Germany that contained 14,700 μg g−1 DW (Jungmann et al., 1996; Ballot et al., 2003). In general, the average MC concentration in water during the Microcystis bloom was seven times higher than that for mass development of Anabaena (Fastner et al., 1999).
Microcystis and Anabaena strains commonly produce two or more MC variants simultaneously, which may be the same for these genera (Sivonen et al., 1992; Sivonen & Jones, 1999). In the cyanobacterial blooms caused by more than one toxin-producing species, several MC types can be detected, but the maximum contribution to the total is provided by a few main types of MCs. Of the three MC variants recorded in Lake Kotokel, the most toxic MC-LR concentration was slightly lower than that of the least toxic MC-RR. MC-YR, being eight times more toxic than MC-RR, was in the minor portion of the microcystin pool.
The composition and ratio of MCs in Lake Kotokel were similar to those in field samples and Microcystis strains from Japan where variants of MC-LR, -RR, and -YR mainly dominated; however, the total concentration of MCs during cyanobacterial bloom reached 2,100 μg g−1 DW (Yasuno et al., 1998; Sivonen & Jones, 1999). MC-RR was also more abundant in the majority of Microcystis strains from Japanese lakes (Yasuno et al., 1998). The same, three MC types were also detected in Lake Wannsee, which was dominated by Microcystis (Kurmayer et al., 2002). In general, MC-LR was proposed to be the major toxin in bloom samples and strains from temperate waters of Europe and Canada (Chorus & Bartram, 1999). Based on this assumption, and on the data on blooming of Indian ponds and lakes, it was suggested that the production of MC-RR was more common under tropical conditions (Ghosh et al., 2008). The prevalence of MC-RR in temperate Lake Kotokel conflicts with this conclusion. MC-YR is generally a minor component of the MC pool, for example, in the Great Lakes its contribution ranged between 9–23% of total MCs (Hotto et al., 2007; Dyble et al., 2008; Allender et al., 2009). The low content of the dominating, the least toxic MC-RR in Lake Kotokel was not likely to lead to animal or human disease, nevertheless deaths were reported in 2008–2009. Apparently, our finding of MCs does not shed light on the etiology of Haff disease because the clinical symptoms associated with the hepatotoxic MCs and those typical for Haff disease are different (Buchholz et al., 2000). However, although the etiology of Haff disease is not clear, outbreaks of the disease usually correlate to cyanobacterial blooms (Smith, 2000).
It should be noted that Lake Kotokel is similar to small Japanese lakes in the composition of MCs, ratios of MC variants, and genetic affinity of toxigenic Microcystis strains. The Japanese strains M. wesenbergii NIES-107 and M. viridis NIES-102 contained the same three MC variants and were grouped into the same clade as the K7-09 sequence from Lake Kotokel. MC-RR dominated in these two strains, and MC-YR was a minor component (Yasuno et al., 1998).
The K1-09 sequence from Lake Kotokel is identical to A. lemmermannii strains from Finnish lakes, where MC-LR, MC-RR, and their demethylated forms are produced as dominant MCs (Sivonen et al., 1992).
Toxic cyanobacterial blooms have been well documented from over sixty-five countries, including the European part of Russia (Codd et al., 2005). However, the data from Asian regions of Russia were absent. This work and previous studies (Tikhonova et al., 2006, 2007; Belykh et al., 2010) demonstrate that toxigenic cyanobacterial mcyA and mcyE genes present in two water bodies of East Siberia, and both mcyE gene and toxins have been found in Lake Kotokel.
One of the factors facilitating the introduction of toxic cyanobacteria is the spread of MC-producing genotypes into the nearby shallow lakes and ponds due to storm-induced water currents or by external carriers like fishing boats and animals (Hotto et al., 2007). The direct water connection between Lake Kotokel, in which toxic cyanobacteria were found, and Lake Baikal may be a threat to the latter lake, which holds ~20% of the world’s fresh water reserve. The water temperature at the coastal zone of Lake Baikal rises to 20°C in the summer, i.e., there are conditions favorable for dense growth of cyanobacteria. The species composition of cyanobacteria in Lake Kotokel is similar to that described for bays and sors that are located on the eastern shore of Lake Baikal (Korde, 1968; Belykh et al., 2007). The abundance of cyanobacterial plankton is high in these areas of Lake Baikal. Therefore, the emergence of toxic genotypes in Lake Baikal is not improbable.
Conclusions
Light microscopy analysis showed the mass development of Aphanocapsa holsatica in Lake Kotokel. Potentially toxic Microcystis and Anabaena species (M. aeruginosa, M. viridis, M. wesenbergii, A. lemmermannii, A. circinalis, and A. spiroides) were recorded in small quantities (less than 24 mg m−3). Toxigenic cyanobacteria of Microcystis and Anabaena containing the mcyE gene were also present in the lake. The ratio of MC-RR:-LR:-YR found in Lake Kotokel was 49:42.5:8.5. Intra-cellular MC concentration in the phytoplankton was 53 μg g−1 DW. The presence of toxic cyanobacteria in the lake can pose a serious threat to the humans living in the Lake Baikal region. Taking into account the recreational importance of Lake Kotokel and its direct connection with Lake Baikal, it is necessary to perform regular assessment of the toxic cyanobacteria in this lake to prevent cases of mass poisoning of people and animals in this area.
References
Allender, C. J., G. R. LeCleir, J. M. Rinta-Kanto, R. L. Small, M. F. Satchwell, G. L. Boyer & S. W. Wilhelm, 2009. Identifying the source of unknown microcystin genes and predicting microcystin variants by comparing genes within uncultured cyanobacterial cells. Applied and Environmental Microbiology 75: 3598–3604.
Ballot, A., S. Pflugmacher, C. Wiegand, K. Kotut & L. Krienitz, 2003. Cyanobacterial toxins in Lake Baringo, Kenya. Limnologica 33: 2–9.
Barinova, S. S., L. A. Medvedeva & O. V. Anisimova, 2006. Diversity of algal indicators in the environmental assessment. Pilies Studio, Tel Aviv: 498 pp (in Russian).
Belykh, O. I., G. V. Pomazkina, I. V. Tikhonova & I. V. Tomberg, 2007. Characteristics of Lake Baikal summer phytoplankton and autotrophic picoplankton. International Journal on Algae 9: 247–263.
Belykh, O. I., I. V. Tikhonova, A. S. Gladkikh, E. G. Sorokovikova & Ok. V Kaluzhnaya, 2010. Detection of toxic Microcystis in Lake Kotokelskoe (Buryatia). Vestnic Tomsk State University 330: 172–175. (in Russian with English abstract).
Buchholz, U., E. Mouzin, R. Dickey, R. Moolenaar, N. Sass & L. Mascola, 2000. Haff Disease: from the Baltic Sea to the U.S. Shore. Emerging Infectious Diseases 6: 192–195.
Carmichael, W. W. & J. An, 1999. Using an enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of microcystins and nodularins. Natural Toxins 7: 377–385.
Chorus, I. & J. Bartram (eds), 1999. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. E & FN Spon, London.
Codd, G. A., S. M. F. O. Azevedo, S. N. Bagchi, M. D. Burch, W. W. Carmichael, W. R. Harding, K. Kaya & H. C. Utkilen, 2005. CyanoNet: A Global Network for Cyanobacterial Bloom and Toxin Risk Management. International Hydrologolical Programme. Initial situation assessment and recommendations. UNESCO, Paris: 138.
Dyble, J., G. L. Fahnenstiel, R. W. Litaker, D. F. Millie & P. A. Tester, 2008. Microcystin concentrations and genetic diversity of Microcystis in the Lower Great Lakes. Environmental Toxicology 23: 507–516.
Fastner, J., U. Neumann, B. Wirsing, J. Weckesser, C. Wiedner, B. Nixdorf & I. Chorus, 1999. Microcystins (hepatotoxic heptapeptides) in German fresh water bodies. Environmental Toxicology 14: 13–22.
Ghosh, S. K., P. K. Das & S. N. Bagchi, 2008. PCR-based detection of microcystin-producing cyanobacterial blooms from Central India. Indian Journal of Experimental Biology 46: 66–70.
Guindon, S. & O. Gascuel, 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704.
Hotto, A. M., M. F. Satchwell & G. L. Boyer, 2007. Molecular characterization of potential microcystin-producing cyanobacteria in Lake Ontario embayments and nearshore waters. Applied and Environmental Microbiology 73: 4570–4578.
Jungblut, A.-D. & A. B. Neilan, 2006. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Archives of Microbiology 185: 107–114.
Jungmann, D., K. U. Ludwichowski, V. Faltin & J. Benndorf, 1996. Field study to investigate environmental factors that could effect microcystin synthesis of a Microcystis population in the Bautzen reservoir. Internationale Revue der Gesamten Hydrobiologie 81: 493–501.
Korde, N. V., 1968. Bottom-Sediments Biostratigraphy of Lake Kotokel. In Galazii G. I., G. A. Dmitriev & A. P. Zhuze et al. (eds), Mesozoic and Cenozoic Lakes of Siberia. Nauka, Moscow: 150–170 (in Russian).
Kurmayer, R., E. Dittmann, J. Fastner & I. Chorus, 2002. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Berlin, Germany). Microbial Ecology 43: 107–118.
Kuzmich, V. N. (ed.), 1988. Bioproductivity of Eutrophic Lakes Irkana and Kotokel in Basin of Lake Baikal. Sbornik nauch. trudov GosNIORH 279. Promrybvod, Leningrad. (in Russian).
Li, D., G. Li, W. Chen & Y. Liu, 2009. Interactions between a cyanobacterial bloom (Microcystis) and the submerged aquatic plant Ceratophyllum oryzetorum Kom. Chinese Journal of Oceanology and Limnology 27: 38–42.
MacKintosh, C., K. A. Beattie, S. Klumpp, P. Cohen & G. A. Codd, 1990. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Letters 264: 187–192.
Moffitt, M. C. & B. A. Neilan, 2004. Characterization of the nodularin synthetase gene cluster and proposed evolution of cyanobacterial hepatotoxins. Applied and Environmental Microbiology 70: 6353–6362.
Neilan, B. A., D. Jacobs, T. Del Dot, L. L. Blackall, P. R. Hawkins, P. T. Cox & A. E. Goodman, 1997. rRNA sequences and evolutionary relationship among toxic and nontoxic cyanobacteria of the genus Microcystis. International Journal of Systematic Bacteriology 47: 693–697.
Nishizawa, T., M. Asayama, K. Fujii, K. Harada & M. Shirai, 1999. Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. Journal of Biochemistry 126: 520–529.
Pflugmacher, S., G. A. Codd & C. E. W. Steinberg, 1999. Effects of the cyanobacterial toxin microcystin-LR on detoxication enzymes in aquatic plants. Environmental Toxicology 14: 111–115.
Pflugmacher, S., C. Wiegand, K. A. Beattie, E. Krause & C. E. W. Steinberg, 2001. Uptake, effects and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (Cav.) Trin ex. Steud. Environmental Toxicology Chemistry 20: 846–852.
Rantala, A., D. P. Fewer, M. Hisbergues, L. Rouhiainen, J. Vaitomaa, T. Börner & K. Sivonen, 2004. Phylogenetic evidence for the early evolution of microcystin synthesis. Proceedings of the National Academy of Science of the USA 101: 568–573.
Rantala, A., P. Rajaniemi-Wacklin, C. Lyra, L. Lepistö, J. Rintala, J. Mankiewicz-Boczek & K. Sivonen, 2006. Detection of microcystin-producing cyanobacteria in Finnish Lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. Applied and Environmental Microbiology 72: 6101–6110.
Ronquist, F. & J. P. Huelsenbeck, 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574.
Rouhiainen, L., T. Vakkilainen, B. L. Siemer, W. Buikema, R. Haselkorn & K. Sivonen, 2004. Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Applied and Environmental Microbiology 70: 686–692.
Semenov, A. D. (ed.), 1977. Manual for Chemical Analysis of Land Surface Waters. Gidrometeoizdat, Leningrad. (in Russian).
Sivonen, K. & G. Jones, 1999. Cyanobacterial toxins. In Chorus, I. & J. Bartram (eds), Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management. E&FN Spon, London: 41–111.
Sivonen, K., M. Namikoshi, W. R. Evans, W. W. Carmichael, F. Sun, L. Rouhiainen, R. Luukkainen & K. L. Rinehart, 1992. Isolation and characterization of a variety of microcystins from seven strains of the cyanobacterial genus Anabaena. Applied and Environmental Microbiology 58: 2495–2500.
Smith, P. T., 2000. Freshwater neurotoxins: mechanisms of action, pharmacology, toxicology, and impacts on aquaculture. In Botana, L. M. (ed.), Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection. Marcel Dekker, New York: 583–602.
Tamura, K., J. Dudley, M. Nei & S. Kumar, 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Molecular Biology and Evolution 24: 1596–1599.
Tikhonova, I. V., O. I. Byelykh, G. V. Pomazkina & A. S. Gladkikh, 2006. Analysis of cyanobacteria from Lake Baikal and the Ust-Ilim Reservoir for the gene responsible for microcystin synthesis. Doklady Biological Sciences 409: 425–427.
Tikhonova, I. V., A. S. Gladkikh, O. I. Belykh & E. G. Sorokovikova, 2007. Detection of potentially toxic cyanobacteria in Lake Baikal and reservoirs of Baikal region by molecular-biology methods. Ecology. ScienceBG Publishing, Bulgaria.
Vaitomaa, J., A. Rantala, K. Halinen, L. Rouhiainen, P. Tallberg, L. Mokelke & K. Sivonen, 2003. Quantitative real-time PCR for determination of microcystin synthetase E copy numbers for Microcystis and Anabaena in lakes. Applied and Environmental Microbiology 69: 7289–7297.
Vollenweider, R. A. & J. Kerekes, 1982. Eutrophication of Waters. Monitoring Assessment and Control. Organization for Economic Co-Operation and Development (OECD), Paris.
Welker, M. & H. Von Döhren, 2006. Cyanobacterial peptide—nature’s own combinatorial biosynthesis. FEMS Microbiology Reviews 30: 530–563.
World Health Organization WHO, 1998. Guidelines for Drinking-Water Quality. Addendum to Volume 2. Health Criteria and Other Supporting Information, 2nd ed. World Health Organisation, Geneva.
Yasuno, M., Y. Sugaya, K. Kaya & M. M. Watanabe, 1998. Variations in the toxicity of Microcystis species to Moina macrocopa. Phycological Research 46: 31–36.
Acknowledgments
We would like to express our gratitude to Prof. Mikhail Grachev (Limnology Institute, SB RAS) for assistance in data processing and useful remarks. Two anonymous referees whose comments contributed to the improvement of this manuscript also deserve recognition. This work was supported by the Russian Foundation for Basic Research, Project No. 10-004-01613a, 09-04-90420-Ukr_f_a, and MK-1239.2010.4, Project No. VI.51.1.9 (Limnological Institute SB RAS), and Grant “Complex Ecological-Biological Expedition…” of SB RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Belykh, O.I., Sorokovikova, E.G., Fedorova, G.A. et al. Presence and genetic diversity of microcystin-producing cyanobacteria (Anabaena and Microcystis) in Lake Kotokel (Russia, Lake Baikal Region). Hydrobiologia 671, 241–252 (2011). https://doi.org/10.1007/s10750-011-0724-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0724-2