Abstract
Gammarus lacustris and Hyalella azteca (hereafter G. lacustris and H. azteca, respectively) are important components of secondary production in wetlands and shallow lakes of the upper Midwest, USA. Within the past 50 years, amphipod densities have decreased while occurrences of fish and intensity of agricultural land use have increased markedly across this landscape. We investigated influences of fish, sedimentation, and submerged aquatic vegetation (SAV) on densities of G. lacustris and H. azteca in semipermanent and permanent wetlands and shallow lakes (n = 283) throughout seven eco-physiographic regions of Iowa, Minnesota, and North Dakota during 2004–2005. G. lacustris and H. azteca densities were positively correlated with densities of SAV (P < 0.001 and P < 0.001, respectively). Both species were negatively correlated with densities of large fish (non-Cyprinidae; P = 0.01 and P = 0.013, respectively) and with high densities of fathead minnows (Pimephales promelas; P < 0.001 and P = 0.033, respectively). H. azteca densities also were negatively correlated with densities of small fish (e.g., other minnows [Cyprinidae] and sticklebacks [Gasterosteidae]; P = 0.048) and common carp (Cyprinus spp.; P = 0.022). G. lacustris densities were negatively correlated with high levels of suspended solids (an index for sedimentation; P < 0.001). H. azteca densities were positively correlated with the width of upland-vegetation buffers (P = 0.004). Our results indicate that sedimentation and fish reduce amphipod densities and may contribute to the current low densities of amphipods in the upper Midwest. Thus, removing/excluding fish, and providing a thick buffer of upland vegetation around wetlands may help restore amphipod densities and wetland and water quality within this landscape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gammarus lacustris and Hyalella azteca (hereafter G. lacustris and H. azteca, respectively) are the most abundant amphipod species in semipermanent and permanent wetlands of the upper Midwest, USA (Kantrud et al., 1989). Both species are capable of producing multiple broods when breeding seasons are relatively long and often are observed at high densities (>1000 ind./m−2; Covich & Thorp, 1991; Wen, 1992; Pickard & Benke, 1996). Amphipods are an important component of secondary production in prairie wetland food webs; they shred coarse particulate organic matter and consume epibenthic algae and microbial bacteria (e.g., Wen, 1992; Murkin & Ross, 2000).
Amphipods are important prey of fish and wildlife in prairie wetlands and shallow lakes of the upper Midwest. Species that consume amphipods include fish ranging from fathead minnows (Pimephales promelas) to large northern pike (Esox lucius; >50 cm; Peterka, 1989; MacNeil et al., 1999; Anteau, 2006), tiger salamanders (Ambystoma tigrinum; Olenick & Gee, 1981; Kantrud et al., 1989; MacNeil et al., 1999), migrating and breeding diving ducks (Aythya spp.), especially lesser scaup (Aythya affinis; Afton & Hier, 1991; Anteau & Afton, 2006, 2008a, 2009a), ruddy ducks (Oxyura jamaicensis), common goldeneye (Bucephala clangula), and several species of dabbling ducks during brood rearing (Anas spp.; Kantrud et al., 1989; Swanson & Duebbert, 1989; Krapu & Reinecke, 1992; Eadie et al., 1995).
G.lacustris and H. azteca densities currently are relatively low in wetlands and shallow lakes throughout the upper Midwest (Anteau & Afton, 2008b), which has been linked with an apparent degradation of wetland habitats and losses of ecosystem services in the upper Midwest, including water quality, biodiversity, and recreational opportunities (Anteau & Afton, 2009b, 2011). Anteau & Afton (2004) hypothesized two potential mechanisms for a decline in amphipod populations across the upper Midwest, increases in (1) abundance or foraging effectiveness of fish and (2) anthropogenic loading of sediment. These mechanisms lead to the following predictions: (1) fish and/or sedimentation, respectively, have increased in wetlands of the upper Midwest; and (2) fish densities and/or sedimentation have negative effects upon amphipod densities. Anteau & Afton (2008b) presented evidence consistent increases in fish occurrence and sedimentation, but factors that influence amphipod density in wetlands of the upper Midwest require further study.
Fish may cause marked decreases in abundance, biomass, and size of amphipods through predation and indirectly through changes in the trophic structure of wetlands (Wellborn, 1994; Bouffard & Hanson, 1997; Duffy, 1998; Zimmer et al., 2001, 2002). Agricultural tilling can accelerate wind and water facilitated erosion, and subsequently increase loading of sediment into prairie wetlands, especially when snowmelt water flows over bare fields in spring (Martin & Hartman, 1987; Dieter, 1991; Gleason & Euliss, 1998; Gleason et al., 2003). Excessive loading of sediment and associated nutrification can influence wetland food webs, reduce wetland depth, modify hydrology, disrupt invertebrate hatching and germination of submerged-aquatic vegetation (SAV), and promote monotypic stands of cattail (Typha spp.; Scheffer et al., 1993; Gleason & Euliss, 1998; Gleason et al., 2003). Other important factors that may influence spring amphipod densities include SAV density, water chemistry, and over-winter survival of adult amphipods.
We examined two predictions of a hypothesis for the decline of amphipods in semipermanent and permanent wetlands and shallow lakes throughout the upper Midwest. The predictions state that relative densities of fish or indices of sedimentation have negative effects upon G. lacustris and H. azteca densities. We also explored influences of depth, water chemistry, and SAV as factors regulating densities of G. lacustris and H. azteca.
Methods
Study area
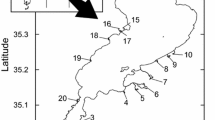
We sampled semipermanent and permanent wetlands and shallow lakes within six eco-physiographic regions (hereafter regions) of Iowa, Minnesota, and North Dakota (Fig. 1). Regions were delineated based on watershed and groundwater hydrology, geology, and plant communities (Kantrud et al., 1989; Minnesota Department of Natural Resources, unpublished data).
We used a constrained-random, clustered sampling approach to select wetlands and to minimize travel time between wetlands in areas with few wetlands. We first estimated numbers of townships (subdivision of a county consisting of a 6-by-6-mile block; 9,324 ha) within each region that had at least 200 ha of semipermanent wetlands (candidate townships for random selection; Table 1) based on converged basin (Johnson & Higgins, 1997) or comparable National Wetland Inventory data (see Anteau, 2006). In Iowa, we broadened the constraint to include townships with 200 ha of semipermanent or permanent wetlands. Constraining township selection helped insure that there were enough wetlands to sample within each township.
We allocated numbers of sampling clusters among the six regions (3–6 clusters per region; Table 1) based on region size and numbers of candidate townships available (Table 1; Fig. 1). Each region was divided into sub-regions based on latitude, so that there was one sampling cluster per sub-region each year, except in North Dakota Glaciated Plains (NDGP). In NDGP, we assigned two sampling clusters annually for each sub-region because of the larger width of this region (east to west) relative to other regions (Fig. 1). Sampling clusters comprised of three randomly selected 36-square-mile townships (27,972 ha total). The centrums of the second and third townships selected were constrained to be within 50 km of the center of the first randomly selected township. We randomly selected three semipermanent or permanent wetlands (>4 ha) in each township for amphipod and wetland sampling. We randomly selected new townships and wetlands annually within each sub-region from the list of candidate townships to maximize representation of spatial and landscape variability in amphipod densities. Wetlands ranged in size from 4 to 6,000 ha; however, 95% of our sample ranged from 4 to 213 ha (based on converged basin or comparable National Wetland Inventory data). Accordingly, our sample comprises semipermanent and permanent wetlands, many of which were large enough (>20 ha) to be considered “shallow lakes” (see Scheffer et al., 1993); hereafter we simply refer to them as wetlands. We also included 16 non-randomly selected wetlands from across Minnesota: seven in NWMN and nine from the other regions in Minnesota. The former were surveyed because of their inclusion in related studies (Anteau & Afton, 2004, 2006, 2008a, 2009b) and the latter were selected because they appeared to have relatively high amphipod densities. Preliminary analyses indicated that our modeling would be improved if more wetlands with high amphipod densities were included in Minnesota. We sampled a total of 283 wetlands in 2004 and 2005 (Table 1).
Wetland sampling
Variation in water depth, total area, topographic position, or surrounding land use of a wetland may influence water temperature and the timing of reproductive events for amphipods. Accordingly, we timed wetland sampling to occur prior to the first seasonal reproductive event of amphipods. We sampled wetlands during 3 April to 16 May in 2004 and 30 March to 3 May in 2005; we completed sampling well before mid to late June when the first amphipod recruits are evident (Wen, 1992). Three crews of technicians sampled simultaneously; all crews started in the southern portion of the study area and moved north, according to the timing of spring migrating scaup (see Anteau & Afton, 2009a). This approach ensured that (1) sampling occurred at similar times relative to phenology of spring (Anteau & Afton, 2009a), (2) problems with sampling in dense SAV (that develop later in the season) were minimized, and (3) variability in densities due to timing of reproductive events was eliminated. Indeed, neither young-of-the-year amphipods nor egg masses on females were evident in our samples.
Amphipod density
We established five transects within each wetland by randomly selecting five bearings (0–359); transects radiated out from the center to the bank of the wetland. One transect was used for fish sampling (see below), and the other four transects had two sampling stations. The first station was 10 m past the inner ring of the emergent vegetation and the second was 50 m further away from the first station toward the wetland center. However, we limited station locations to depths between 0.5 and 3 m for ease of sampling. Thus, in some instances (<5% of transects), station locations were adjusted along transects to accommodate depth requirements.
At each station, we recorded water depth and sampled amphipod density with a D-shaped sweep net (1,200 μm mesh, 0.072 m2 opening, WARD’s Natural Science, Rochester, New York). Each amphipod sample consisted of a bottom sweep (skimming the net along the bottom for a distance equal to the depth at the sampling station) and an upward sweep through the water column. This pattern ensured equal representation of bottom and water column in each sample. When SAV or other debris was brought up with the sweep net, it was included in the sample, unless greater than 50% of the object hung outside the net. Although SAV was present, it was never so dense that it impaired our ability to effectively sample amphipods with sweep nets. In the field, sweep-net samples (n = 8 per wetland) were combined into a single composite sample per wetland, preserved in a 95% ethanol solution, and then transported to the laboratory for processing.
In the laboratory, each composite sample was stained with Rose Bengal (Sigma # R3877, Sigma-Aldrich Corp., St. Louis, Missouri), sieved (500 μm mesh), floated with sugar and water solution in a large dissecting tray, and all plant and benthic material was searched. We sorted and counted all amphipods using a lighted magnifying glass and a stereomicroscope. We calculated the total water volume swept (VS; m3) for each wetland with the equation:
where D i = depth (m) at each sampling station, and SN = sweep-net opening (0.072 m2).
Submerged aquatic vegetation
We sampled SAV at each station after amphipod sampling was complete to avoid disturbing the distribution of amphipods. We used grab sampling to estimate relative abundance of SAV along a sub-transect starting at each sampling station and running parallel to the bank. Each sub-transect had 10 grabs (1/meter) using a 36-tine Lake Rake® (Ben Meadows Co., Janesville, Wisconsin) gently dug into the bottom and pulled straight up (Nyman & Chabreck, 1996). For each grab, presence/absence of vegetation (rhizomes and shoots were included) was recorded. Relative abundance of SAV was estimated for each wetland by the proportion of grabs with SAV present (n/80) and indexed the proportion of SAV coverage the previous growing season.
Fish abundance
We indexed abundance of various fish species with an experimental gill net (21 × 2 m with 7–3 m panels ranging in mesh size from 1.9 to 7.6 cm) set on the fifth transect. The end of the net with the smaller mesh sized panels was set about 10 m from the open water-emergent vegetation interface and extended along the transect toward the center of the wetland. We also set five minnow traps (Gee style; 44 cm long, 23 cm diameter cylinder with 22 mm inward funnel openings on both ends and made of 6.4 mm galvanized mesh) in each wetland; one minnow trap per transect was placed at the transition between emergent vegetation and open water. Gill nets and minnow traps were set and retrieved for the same time period within each wetland, 14–24 h (85% of sets were over 18 h), always including an overnight sampling period.
Fish caught in all five minnow traps and in the gill net were enumerated by species. We classified fish species into 1 of 4 categories: (1) fathead minnows, (2) large Cypriniformes (carp, buffalo [Ictiobus sp.], and suckers [Catostomus sp.]), (3) small fish (other minnows [Cyprinidae], darters [Anhingidae], sticklebacks [Gasterosteidae], and mudminnows [Umbra sp.]), and (4) other large fish (e.g., northern pike, walleye [Sander vitreus], perch [Perca sp.], sunfish [Lepomis spp.], bullhead and catfish [Ictaluridae]). All fathead minnows and small fish were caught with minnow traps and all large Cypriniformes and most large fish were caught in gill nets; however, occasionally small sunfish, perch, or bullhead were caught in minnow traps and were pooled with the sample of those caught in gill nets. We calculated catch per unit effort (relative abundances) by dividing total numbers of each fish in each class by the hours that the gill net and five minnow traps were in the water.
Surrounding land use
We categorized agricultural impacts surrounding each wetland as “low” (1), “moderate” (2), or “severe” (3) using criteria established in Tangen et al. (2003). We estimated the width of the ring of upland vegetation (buffer/filter strip; hereafter upland vegetation) immediately surrounding the wetland (0, 1–4, 5–10, 11–50, >50 m) and assigned “dummy” variables (0–4) to each level for our analysis.
Water depth and quality
We measured turbidity (±1 nephelometric turbidity unit [NTU]), chlorophyll a (±1 mg/l), and specific conductance (±1 μS/cm) with portable water quality meters (YSI 6600 sonde with optical chlorophyll [YSI 6025] and turbidity [YSI 6136] probes; YSI Inc., Yellow Springs, Ohio) at the first four transects near the center of the wetland. All water quality measurements were averaged for each wetland. We used maximum depth (deepest measurement of eight amphipod-sampling stations) to index the depth of each wetland.
Turbidity measurements taken in spring provide a good index of sedimentation in wetlands because this is a period with great spring runoff, often over bare agricultural soils (Dieter, 1991). The amount of phytoplankton (chlorophyll a) during summer is assumed to be indicative of the trophic structure of wetlands (Scheffer et al., 1993). Unfortunately, measurements of chlorophyll a in early spring probably do not predict those later in the summer (see Wen, 1992). However, chlorophyll a is a component of our turbidity measurements, and it adds unwanted variability into an index of sedimentation into wetlands. Accordingly, we estimated organic or mineral suspended solids (OMSS) by removing the chlorophyll a component from our turbidity measurements.
Statistical analyses
We estimated OMSS with a regression on the natural logs (ln) of turbidity (dependent) and chlorophyll a (independent). Turbidity and chlorophyll a were positively correlated (Y = 0.32 + 0.66[X]; P < 0.001), chlorophyll a accounted for 38% of the variation in turbidity. We calculated OMSS by adding the residuals of the regression to the overall mean of ln turbidity (\(\bar{\text{X}}\) = 2.45).
We examined covariates used in our subsequent analysis for collinearity (PROC CORR; SAS Institute, 2002). All correlations among covariates were low (|ρ| < 0.47, 75% of correlations were <0.20), indicating low potential for problems with collinearity among covariates in our following models. We presented summaries of covariate values in Table 2.
We separately modeled factors that potentially influenced G. lacustris and H. azteca densities with generalized linear regressions (PROC GENMOD; SAS Institute, 2002) because each species may respond differently to various environmental factors. In both models, counts of amphipods (G. lacustris or H. azteca) were used as the dependent variable and the full models included region as a class effect, width of upland vegetation and agricultural impacts as ordinal classes, relative densities of fish (fathead minnows, large Cypriniformes, large fish, and small fish), amount of SAV, specific conductivity, maximum depth, and OMSS as covariates. We used generalized linear models with a negative binomial distribution, a ln link function, and ln of VS was used as an offset variable (controls counts for the amount of water swept). We included quadratic effects for fathead minnows and small fish because amphipods and these fish may have similar tolerances to water quality and over-winter survival requirements. We included a quadratic effect for maximum depth because amphipods must survive during winter, but if wetlands are too deep then they might be less productive for amphipods due to light penetration to SAV. We also included a quadratic term for OMSS because wetlands with high amphipod densities often had moderate turbidity levels. We included interactions of agricultural impacts-by-width of upland vegetation and large fish-by-SAV in our analyses. We ln-transformed all covariates to improve model fit (based on ln likelihood), except OMSS, which was already in ln form. However, we excluded data from the Iowa Prairie Pothole (IAPP) region from the G. lacustris analysis because none were found in samples there. We selected final models for G. lacustris and H. azteca using backwards elimination procedures on the type III likelihood ratio statistic with α 0.05 (Zar, 1996).
Results
Our final model for predicting densities of G. lacustris fit the data well (deviance/DF = 0.55); deviance/DF below or near 1 indicates a good model fit. G. lacustris densities varied among regions and were positively correlated with the relative density of SAV, but negatively correlated with relative density of large fish (Table 3). G. lacustris densities were positively correlated with OMSS up to 3.37, and then negatively correlated at higher levels of OMSS (Table 3; holding all other variables constant). OMSS of 3.37 is roughly equal to turbidity of 30 or 40 NTU with none or mean levels of chlorophyll a, respectively. G. lacustris densities were positively correlated with relative density of fathead minnows up to 1.6 fatheads caught per-trap-per-hour, and then negatively correlated at higher relative densities (Table 3; holding all other variables constant).
Our final model for predicting H. azteca densities also fit the data well (deviance/DF = 1.03). H. azteca densities varied among regions and were positively correlated with SAV and width of upland vegetation; however, they were negatively correlated with relative densities of small fish large Cypriniformes, and large fish (Table 4). H. azteca densities were positively correlated with relative density of fathead minnows up to 0.8 fatheads caught per-trap-per-hour, and then negatively correlated at higher relative densities (Table 4; holding all other variables constant).
Discussion
Fish
Fish can reduce amphipod density directly (through predation) and indirectly through trophic cascades (Swanson & Nelson, 1970; Scheffer et al., 1993; Bouffard & Hanson, 1997). Percent occurrence of fish has increased markedly from historic estimates in wetlands throughout the upper Midwest, but especially in Minnesota and Iowa (Anteau & Afton, 2008b). We found strong negative correlations between relative fish densities and G. lacustris and H. azteca densities, consistent with the hypothesis that an increase in fish occurrences or densities has contributed to a recent decline in amphipod densities in the upper Midwest (Anteau & Afton, 2004).
Fathead minnows are the most commonly occurring and abundant fish species found in wetlands of the upper Midwest (Anteau & Afton, 2008b). Fathead minnows may reduce G. lacustris and H. azteca densities through both direct and indirect mechanisms. Fathead minnows typically consume zooplankton, amphipods, and other macroinvertebrates when those foods are abundant in wetlands (Held & Peterka, 1974; Price et al., 1991; Duffy, 1998). However, unlike other small fishes, fathead minnows consume detritus and periphyton when abundances of macroinvertebrates and zooplankton are low (Zimmer et al., 2002). Therefore, fathead minnows can compete with G. lacustris and H. azteca for the same foods. Moreover, alternate equilibria theory (Scheffer et al., 1993) predicts that because fathead minnows sustain their densities by eating detritus, they (1) can continually depress zooplankton abundance and (2) reduce SAV abundances through increases in turbidity, both of which may cause eventual shifts to a phytoplankton dominated trophic structure (Scheffer et al., 1993, 2001; Zimmer et al., 2002), which ultimately would result in less food and escape cover for amphipods.
The quadratic relationship between fathead minnows and both G. lacustris and H. azteca indicate that when amphipod densities were low or zero, fathead minnow densities also were low or zero. This suggests that amphipods and fathead minnows share similar requirements and tolerances that limit which wetlands they can occur. Like fishes, spring populations of amphipods in wetlands are dependent upon over-winter survival of adults (Peterka, 1989; Arts et al., 1995; Lindeman & Clark, 1999; MacNeil et al., 1999). Alternatively, lower densities of fish may remove grazer competition for amphipods allowing amphipod densities to increase (Batzer, 1998; McParland & Paszkowski, 2006). In either case, our results indicate that once a threshold density of fathead minnows is reached a strong affect on amphipod densities can be observed, suggesting either strong competition or direct predation of amphipods by fathead minnows.
Large Cypriniformes can increase turbidity through the consumption of detritus and SAV, and by mechanically resuspending sediment (Bouffard & Hanson, 1997). Large Cypriniformes and amphipods rarely occurred in the same wetlands. We found that relative density of large Cypriniformes was negatively correlated with H. azteca densities, but our model for G. lacustris did not fit a slope for large Cypriniformes, likely because only one wetland in our sample had both large Cypriniformes and G. lacustris. Accordingly, we argue that large Cypriniformes negatively influence occurrence and density of amphipods despite our inability to fit a linear relationship for G. lacustris.
Sedimentation
Within the past 50 years the intensity and extent of agriculture has increased markedly in the upper Midwest. Upland disturbances such as agricultural tilling increase runoff of sediments into wetlands (Gleason & Euliss, 1998). Sedimentation of wetlands can reduce amphipod densities through reduction of SAV seed emergence and by covering the coarse particulate organic matter, epibenthic algae, and periphyton upon which amphipods feed (Gleason & Euliss, 1998; Gleason et al., 2003). Additionally, sediment can transport contaminants and nutrients into wetlands, decrease wetland depth, and modify hydrology and emergent plant communities (Martin & Hartman, 1987; Gleason & Euliss, 1998). However, a buffer of undisturbed upland vegetation around wetlands reduces the amount of sedimentation of wetlands (Castelle et al., 1994). We argue that OMSS provides a good index of sedimentation in wetlands because measurements were made soon after spring runoff, often over bare agricultural soils (Dieter, 1991; Anteau & Afton, 2008b). The observed positive relationship between H. azteca and width of upland vegetation, coupled with the negative correlation between high levels of OMSS and G. lacustris densities, are consistent with the hypothesis that an increase in sedimentation has contributed to a decline in amphipod densities in the upper Midwest (c.f., Anteau & Afton, 2004). However, sedimentation could be influencing amphipods through a number of mechanisms (e.g., contaminants or nutrients transported by sediments, sediments influences upon SAV, depth, or turbidity, or hydrology) and further research is needed to understand better these mechanisms.
We expected that our agricultural impacts variable would index the amount of sedimentation into wetlands, but the correlation between agricultural impacts and amphipod densities was not significant. Tangen et al. (2003) found a weak relationship between macroinvertebrate assemblages and an identical classification of agricultural impacts. They speculated that high spatial variability was a potential reason for the lack of response in their tests. However, we suspect that their classification was not detailed or accurate enough for the large basins that we sampled because the landscape that drains into these wetlands likely is larger than can be evaluated by observations made at or immediately surrounding the wetland, particularly because wetlands appear to be increasingly inter-connected in many parts of the upper Midwest (Anteau & Afton, 2008b).
The observed quadratic relationship between OMSS and G. lacustris suggests that G. lacustris is sensitive to high levels of sedimentation. The positive correlation observed at lower levels of OMSS could be related to a number of factors. For example, (1) G. lacustris may be less susceptible to predation in more turbid water, (2) G. lacustris may benefit from low amounts of nutrients transported into wetlands by sediment, or (3) high densities of G. lacustris (e.g., hundreds or thousands m−3) in wetlands may increase OMSS by their consumption of coarse particulate organic matter and release of fine particulate organic matter (Murkin & Ross, 2000).
Submerged aquatic vegetation
Others documented that reductions in SAV were correlated with declines in invertebrate abundance or productivity (Krull, 1970; Euliss & Grodhaus, 1987; Neill & Cornwell, 1992; Schriver et al., 1995; Gleason & Euliss, 1998). Our results are consistent with those findings. The amount of SAV in a wetland likely is positively correlated with the amount of food for amphipods because SAV produces coarse particulate organic matter and provides structure on which periphyton grow (Covich & Thorp, 1991; Hentges & Stewart, 2010). Although we did not detect an interaction between large fish and SAV, physical vegetative structure in wetlands also may make amphipods less susceptible to predation by fishes (Covich & Thorp, 1991; MacNeil et al., 1999) and concomitantly may allow amphipods to maintain high activity levels in the presence of predators (Wooster, 1998).
Wetland depth
We expected that abundance of amphipods would be correlated with wetland depth because amphipods must survive over-winter as adults to be present in wetlands the following spring, and typically are associated with deeper wetlands (Arts et al., 1995; Lindeman & Clark, 1999; MacNeil et al., 1999). We tested linear and non-linear relationships for depth because we also expected that the deepest wetlands may be less productive for amphipods or have more populous or diverse communities of predators. However, we found that maximum depth was not correlated to amphipod density. We suspect that by selecting only semipermanent and permanent wetlands and shallow lakes over 4 ha in size that most were deep enough to over-winter amphipods. If smaller wetlands with less permanent hydroperiods were considered, depth probably would be an important factor influencing the density or occurrence of amphipods in spring.
Historically, productivity and abundance of aquatic invertebrates likely was driven by inter-annual hydrological dynamics (i.e., wet–dry cycles; Euliss et al., 1999). The drying of wetlands or portions of wetlands allows for nutrient cycling and a subsequent pulse of productivity when wet conditions return. Some regions in our study area were encompassed by a regime of relatively high and stable water in 2004 and 2005, so amphipod numbers within our study may have experienced little inter-annual variation. A long-term study is needed to differentiate effects of inter-annual variation in hydrological dynamics from those that we examined here. Furthermore, to understand the complete impacts of land use upon ecosystem services of prairie wetlands’ there is a need to determine whether or not interactions exist between land use, climate, and hydrological dynamics.
Conclusions
We suspected that G. lacustris and H. azteca densities would respond differently to the factors we examined because of potential for differences in susceptibility to predation or tolerances to sedimentation. Surprisingly, both species responded quite similarly to fish, SAV, and sedimentation, although different indices of sedimentation were important for each species. However, H. azteca densities declined in response to lower fathead minnow densities than did G. lacustris, suggesting that H. azteca are more sensitive to fathead minnows. Perhaps the smaller size of H. azteca may make them more vulnerable than G. lacustris to direct fathead minnow predation due to gape-size limitations of small fishes.
Our results are consistent with predictions of two mechanisms explaining an apparent decline in amphipod abundance (Anteau & Afton, 2004), namely that fish densities and sedimentation into wetlands appear to have negative influences upon densities of amphipods. However, further research and experimentation is needed to more clearly understand specific mechanisms driving the observed correlations between amphipod densities and fish communities, indices of sedimentation, and SAV.
Amphipods are relatively sensitive to water quality and anthropogenic chemicals (Grue et al., 1988; Tome et al., 1995; Murkin & Ross, 2000; Besser et al., 2004), and given our results, amphipod density likely is a good indicator of both wetland and water quality. Accordingly, several lines of evidence suggest that there has been a landscape-wide decrease in wetland and water quality in the upper Midwest: (1) current low densities of G. lacustris and H. azteca, (2) current high percent occurrence of fish, and (3) relatively high current turbidity levels in wetlands (Anteau & Afton, 2008b). Lastly, our results suggest that conservation efforts to restore wetland and water quality on this landscape should focus on: (1) re-isolating wetlands and removing fish communities, (2) providing a thick (>50 m) buffer of upland vegetation between wetlands and upland disturbances, and (3) promoting abundant SAV.
References
Afton, A. D. & R. H. Hier, 1991. Diets of lesser scaup breeding in Manitoba. Journal of Field Ornithology 62: 325–334.
Anteau, M. J., 2006. Ecology of lesser scaup and amphipods in the upper-Midwest: scope and mechanisms of the Spring Condition Hypothesis and implications for migration habitat conservation. Ph.D. Dissertation. Louisiana State University, Baton Rouge, Louisiana. http://etd.lsu.edu/docs/available/etd-01242006-093828.
Anteau, M. J. & A. D. Afton, 2004. Nutrient reserves of lesser scaup during spring migration in the Mississippi Flyway: a test of the Spring Condition Hypothesis. Auk 121: 917–929.
Anteau, M. J. & A. D. Afton, 2006. Diet shifts of lesser scaup are consistent with the spring condition hypothesis. Canadian Journal of Zoology 84: 779–786.
Anteau, M. J. & A. D. Afton, 2008a. Diets of lesser scaup during spring migration throughout the upper-Midwest are consistent with the spring condition hypothesis. Waterbirds 31: 97–106.
Anteau, M. J. & A. D. Afton, 2008b. Amphipod densities and indices of wetland quality across the upper-Midwest, USA. Wetlands 28: 184–196.
Anteau, M. J. & A. D. Afton, 2009a. Wetland use and feeding by lesser scaup during spring migration across the upper Midwest, USA. Wetlands 29: 704–712.
Anteau, M. J. & A. D. Afton, 2009b. Lipid reserves of lesser scaup (Aythya affinis) migrating across a large landscape are consistent with the “spring condition” hypothesis. Auk 126: 873–883.
Anteau, M. J. & A. D. Afton, 2011. Lipid catabolism of invertebrate predator indicates widespread wetland ecosystem degradation. PLoS ONE 6: e16029.
Arts, M. T., M. E. Ferguson, N. E. Glozier, R. D. Robarts & D. B. Donald, 1995. Spatial and temporal variability in lipid dynamics of common amphipods: assessing the potential for uptake of lipophilic contaminants. Ecotoxicology 4: 91–113.
Batzer, D. P., 1998. Trophic interactions among detritus, benthic midges, and predatory fish in a freshwater marsh. Ecology 79: 1688–1698.
Besser, J. M., W. G. Brumbaugh, N. E. Kemble, T. W. May & C. G. Ingersoil, 2004. Effects of sediment characteristics on the toxicity of chromium (III) and Chromium (IV) to the amphipod Hyalella azteca. Environmental Science and Technology 38: 6210–6216.
Bouffard, S. H. & M. A. Hanson, 1997. Fish in waterfowl marshes: waterfowl managers’ perspective. Wildlife Society Bulletin 25: 146–157.
Castelle, A. J., A. W. Johnson & C. Conolly, 1994. Wetland and stream buffer size requirements—a review. Journal of Environmental Quality 23: 878–882.
Covich, A. P. & J. H. Thorp, 1991. Crustacea: introduction and peracarida. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classifications of North American Freshwater Invertebrates. Academic Press, San Diego, CA: 665–689.
Dieter, C. D., 1991. Water turbidity in tilled and untilled prairie wetlands. Journal of Freshwater Ecology 6: 185–189.
Duffy, W. G., 1998. Population dynamics, production, and prey consumption of fathead minnows (Pimephales promelas) in prairie wetlands: a bioenergetics approach. Canadian Journal of Fisheries and Aquatic Sciences 54: 15–27.
Eadie, J. M., M. L. Mallory & H. G. Lumsden, 1995. Common Goldeneye (Bucephala clangula). Number 170. In Pool, A. & F. Gill (eds), The Birds of North America. The American Ornithologists’ Union, the Academy of Natural Sciences, Washington, DC, Philadelphia, PA.
Euliss N. H. Jr., & G. Grodhaus, 1987. Management of midges and other invertebrates for waterfowl wintering in California. California Fish and Game 73: 238–243.
Euliss N. H. Jr., D. A. Wrubleski & D. M. Mushet, 1999. Wetlands of the prairie pothole region: invertebrate species composition, ecology, and management. In Batzer, D. P., R. B. Rader & S. A. Wissinger (eds), Invertebrates in Freshwater Wetlands of North America: Ecology and Management. Wiley, New York, NY: 471–514.
Gleason, R. A. & N. H. Euliss Jr., 1998. Sedimentation of prairie wetlands. Great Plains Research 8: 97–112.
Gleason, R. A., N. E. Euliss Jr., D. E. Hubbard & W. G. Duffy, 2003. Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil egg and seed banks. Wetlands 23: 26–34.
Grue, C. E., M. W. Tome, G. A. Swanson, S. M. Borthwick & L. R. DeWeese, 1988. Agricultural chemicals and the quality of prairie-pothole wetlands for adult and juvenile waterfowl—what are the concerns? In Stuber, P. J. (ed.), Proceedings National Symposium of Protection of Wetlands from Agricultural Impacts. U.S. Department of Interior, U.S. Fish and Wildlife Service, Biological Report 88(16): 55–64.
Held, J. W. & J. J. Peterka, 1974. Age, growth, and food habits of the fathead minnow, Pimephales promelas, in North Dakota saline lakes. Transactions of the American Fisheries Society 4: 743–756.
Hentges, V. A. & T. W. Stewart, 2010. Macroinvertebrate assemblages in Iowa prairie pothole wetlands and relation to environmental features. Wetlands 30: 501–511.
Johnson, R. R. & K. F. Higgins, 1997. Wetland resources of eastern South Dakota. South Dakota State University, Brookings, South Dakota. http://www.npwrc.usgs.gov/resource/wetlands/sdwet/sdwet.htm.
Kantrud, H. A., G. L. Krapu & G. A. Swanson, 1989. Prairie basin wetlands of North Dakota: a community profile. Biological report 85(7.28). U.S. Department of the Interior, Fish and Wildlife Service, Research and Development, Washington, DC.
Krapu, G. L. & K. J. Reinecke, 1992. Foraging ecology and nutrition. In Batt, B. D. J., A. D. Afton, M. G. Anderson, C. D. Ankney, D. H. Johnson, J. A. Kadlec & G. L. Krapu (eds), Ecology and Management of Breeding Waterfowl. University of Minnesota Press, Minneapolis, MN: 1–29.
Krull, J. H., 1970. Aquatic plant-macroinvertebrate associations and waterfowl. Journal of Wildlife Management 34: 707–718.
Lindeman, D. H. & R. G. Clark, 1999. Amphipods, land-use impacts, and lesser scaup (Aythya affinis) distribution in Saskatchewan wetlands. Wetlands 19: 627–638.
MacNeil, C., J. T. A. Dick & R. W. Elwood, 1999. The dynamics of predation on G. lacustris spp. (Crustacea: Amphipoda). Biological Reviews of the Cambridge Philosophical Society 74: 375–395.
Martin, D. B. & W. A. Hartman, 1987. The effect of cultivation on sediment composition and deposition in prairie pothole wetlands. Water, Air, and Soil Pollution 34: 45–53.
McParland, C. E. & C. A. Paszkowski, 2006. Effects of small-bodied fish on invertebrate prey and foraging patterns of waterbirds in Aspen Parkland wetlands. Hydrobiologia 567: 43–55.
Murkin, H. R. & L. C. M. Ross, 2000. Invertebrates in prairie wetlands. In Murkin, H. R., A. G. van der Valk & W. R. Clark (eds), Prairie Wetland Ecology: The Contribution of the Marsh Ecology Research Program. Iowa State University Press, Ames, IA: 201–248.
Neill, C. & J. C. Cornwell, 1992. Stable carbon, nitrogen, and sulfur isotopes in a prairie marsh food web. Wetlands 12: 217–224.
Nyman, J. A. & R. H. Chabreck, 1996. Some effects of 30 years of weir-management on coastal marsh aquatic vegetation and implications for waterfowl management. Gulf of Mexico Science 1: 16–25.
Olenick, R. J. & J. H. Gee, 1981. Tiger salamanders (Ambystoma tigrinum) and stocked rainbow trout (Salmo gairdneri): potential competitors for food in Manitoba prairie pothole lakes. Canadian Field-Naturalist 95: 129–132.
Peterka, J. J., 1989. Fishes in northern prairie wetlands. In van der Valk, A. G. (ed.), Northern Prairie Wetlands. Iowa State University Press, Ames, IA: 302–315.
Pickard, D. P. & A. C. Benke, 1996. Production dynamics of Hyalella azteca (amphipoda) among different habitats in a small wetland in the southeastern USA. Journal of the North American Benthological Society 15: 537–550.
Price, C. J., W. M. Tonn & C. A. Paszkowski, 1991. Intraspecific patterns of resource use by fathead minnows in a small boreal lake. Canadian Journal of Zoology 69: 2109–2115.
SAS Institute, 2002. SAS/STAT user’s guide, version 9. SAS Institute, Cary, NC.
Scheffer, M., S. Carpenter, J. A. Foley, C. Folke & B. Walker, 2001. Catastrophic shifts in ecosystems. Nature 413: 591–596.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schriver, P., J. Bogestrand, E. Jeppesen & M. Sondergaard, 1995. Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33: 255–270.
Swanson, G. A. & H. F. Duebbert, 1989. Wetland habitats of waterfowl in the prairie pothole region. In van der Valk, A. G. (ed.), Northern Prairie Wetlands. Iowa State University Press, Ames, IA: 228–267.
Swanson, G. A. & H. Nelson, 1970. Potential influence of fish rearing programs on waterfowl breeding habitat. In Schneberger, E. (ed.), Symposium on the Management of Midwestern Winterkill Lakes. North Central Division of the American Fisheries Society, Winnipeg, Manitoba: 65–71.
Tangen, B. A., M. G. Butler & M. J. Ell, 2003. Weak correspondence between macroinvertebrate assemblages and land use in prairie pothole region wetlands, USA. Wetlands 23: 104–115.
Tome, M. W., C. E. Grue & M. G. Henry, 1995. Case studies: effects of agricultural pesticides on waterfowl and prairie pothole wetlands. In Hoffman, D. J., B. A. Rattner, G. A. Burton Jr. & J. Cairns Jr. (eds), Handbook of Ecotoxicology. Lewis Publishers, CRC Press, Boca Raton, FL: 565–576.
Wellborn, G. A., 1994. Size-biased predation and prey life-histories—a comparative-study of fresh-water amphipod populations. Ecology 75: 2104–2117.
Wen, Y. H., 1992. Life history of Hyalella azteca (Crustacea: Amphipoda) in a hypereutrophic prairie pond in southern Alberta. Canadian Journal of Zoology 70: 1417–1424.
Wooster, D. E., 1998. Amphipod (Gammarus lacustris minus) responses to predators and predator impact on amphipod density. Oecologia 115: 253–259.
Zar, J. H., 1996. Biostatisical analysis. Prentice-Hall, Upper Saddle River, NJ.
Zimmer, K. D., M. A. Hanson & M. G. Butler, 2001. Effects of fathead minnow colonization and removal on a prairie wetland ecosystem. Ecosystems 4: 346–357.
Zimmer, K. D., M. A. Hanson & M. G. Butler, 2002. Effects of fathead minnows and restoration on prairie wetland ecosystems. Freshwater Biology 47: 2071–2086.
Acknowledgments
We dedicate this manuscript to the memory of our colleague Barry Moser, who is dearly missed. We thank numerous technicians and professionals identified in a previous publication (Anteau & Afton, 2008b) for assistance with wetland sampling, logistics, and laboratory analyses. We acknowledge numerous landowners that allowed us to work on their property. We thank the Illinois Department of Natural Resources, Iowa Department of Natural Resources, Kibbe Field Station, Missouri Department of Conservation, Minnesota Department of Natural Resources, USFWS Regions 3 and 6 HAPET offices, USGS-National Wetland Research Center, USGS-Northern Prairie Wildlife Research Center, Western Illinois University, and Federal and Winchester Cartridge Companies for in-kind support. We thank the following organizations for financial support: Ducks Unlimited Inc. USA, IWWR of Ducks Unlimited Canada, IWWR of Ducks Unlimited Canada through the Bonnycastle Fellowship, Louisiana Department of Wildlife and Fisheries, Louisiana State University-through the Bosch Fellowship, Minnesota Department of Natural Resources, Minnesota Waterfowl Association, North Dakota Game and Fish Department, Prairie Pothole Joint Venture, Upper Mississippi River and Great Lakes Region Joint Venture, USGS-Louisiana Cooperative Fish and Wildlife Research Unit, and USGS-Northern Prairie Wildlife Research Center. We thank Steve Chipps, Mark Clark, and anonymous reviewers for helpful comments to the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handing editor: Sonja Stendera
E. Barry Moser is deceased.
Rights and permissions
About this article
Cite this article
Anteau, M.J., Afton, A.D., Anteau, A.C.E. et al. Fish and land use influence Gammarus lacustris and Hyalella azteca (Amphipoda) densities in large wetlands across the upper Midwest. Hydrobiologia 664, 69–80 (2011). https://doi.org/10.1007/s10750-010-0583-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0583-2