Abstract
Catenulid turbellarians, common in shallow, tropical ponds, affect their rotifer prey via the production of toxins. There is, however, no quantitative information on their effect on the demography of their prey. Here, we test the impact of Stenostomum cf leucops on the population dynamics of the rotifers Euchlanis dilatata and Plationus patulus, and the cladoceran Moina macrocopa. Experiments were initiated with rotifers at 0.5 ind. ml−1 and the cladoceran at 0.2 ind. ml−1; growth patterns were compared in the absence and presence of worms (2 Stenostomum ind. per 50 ml). Results revealed that brachionids were most adversely affected: there was a lower growth rate of the rotifers in the presence of worms (P < 0.01, repeated measures ANOVA), although at the densities applied, the predator did not wipe out its prey. These littoral predators may therefore regulate rotifer prey in natural conditions. In Moina, the population evolved differently; initially, we found no difference between control and treatment, but after about 10 days, the population collapsed, irrespective of a direct or indirect contact with the predator. This delayed effect deserves more study, as it could represent flatworm toxin accumulation by the cladoceran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rotifers are subject to predation by vertebrates and invertebrates. In deep water bodies, fish larvae, juveniles and insect larvae exert the bulk of the predation pressure, while in shallow waters, the impact of amphibians and micro-invertebrates such as copepods, cladocerans, other rotifers or protozoans is usually higher (O’Brien, 1987; Gilbert, 1998). Since the life cycle of micro-invertebrate predators is often similar to that of their rotifer prey, they show increased consumption rates (functional response) as well as population numbers (numerical response) with increasing availability of rotifer prey (Murdoch & Bence, 1987).
Many pelagic rotifer taxa show defenses to avoid predation. These include the presence of large and small spines in Plationus macracanthus and Brachionus budapestinensis, respectively (Garza-Mouriño et al., 2005), darting behaviour in Filinia longiseta and Hexarthra mira (Iyer & Rao, 1996), small body size in Anuraeopsis fissa (Sarma & Nandini, 2007), epizoic behaviour in Brachionus rubens (Iyer & Rao, 1995) and colonial life or production of toxins as in Sinantherina semibullata (Felix et al., 1995). Little information is available on the defense strategies of littoral rotifers. Serrania-Soto & Sarma (2009) have shown that Lecane stokesii shows spine elongation in the presence of infochemicals from Asplanchnopus multiceps but the efficacy of this behaviour as a defense mechanism is yet to be proven. High population growth rates or retreat into the resting egg stage could also be an effective method to withstand predation pressure and could be the strategy that zooplankton species adopt to survive such stress (Nielsen et al., 2000).
While certain organisms that feed on rotifers, such as fish larvae, the predatory rotifer Asplanchna or notonectid insect larvae, have been well studied, little information is available on the effect of other invertebrate predators. These include turbellarians, frequently found in high densities (80 ind. l−1 and up to 27,000 ind. m−2; Kolasa, 2001) in shallow water and in the benthos of lakes and ponds. It has been suggested that turbellarian predation could structure zooplankton communities (Dumont & Carels, 1987; Blaustein & Dumont, 1990).
There are about 400 turbellarian species, several of them with a wide distribution. The genus Stenostomum (Family Catenulidae) has 50 species, 25 of which are found in South America (Noreña et al., 2005). They feed on detritus, bacteria and protozoans, and they consume rotifers as well. They produce infochemicals, in response to which protozoans such as Euplotes develop defensive ‘wings’ (Fyda et al., 2005; Altwegg et al., 2006). That they also hunt for littoral rotifers was claimed more than 70 years ago (Nuttycomb & Waters, 1935) but after that, little work on their impact on zooplankton populations was conducted. While Stenostomum predatorium bites parts of their prey, other Stenostomum sp. feed by suction (Kolasa, 2001; Kratina et al., 2009).
We found that our field-collected Stenostomum cf leucops measured 2.0–3.0 mm but could measure up to 5 mm when extended. In laboratory cultures, they had their guts filled with rotifers and their eggs (10–15 per worm). They fed on live and dead individuals by sucking them with their protrusible pharynx. Here, we present information on the impact of S. leucops on the population dynamics of Plationus patulus, Euchlanis dilatata and the cladoceran Moina macrocopa. The rotifer species are part of the aufwuchs community where the worms are also found; M. macrocopa, on the other hand, is found in both the plankton of deep water and in shallow ponds. We analysed the direct and indirect effects of Stenostomum sp. on its prey by studying the feeding behaviour and the population dynamics patterns.
Methods
We isolated Stenostomum cf leucops (hereafter Stenostomum) from a shallow, eutrophic pond in the State of Veracruz, Mexico. Cultures were established from a single worm and fed a mixture of Chlorella vulgaris, Scenedesmus acutus, Lecane bulla, Lecane quadridentata, Plationus patulus and Euchlanis dilatata. Prey were provided ad libitum, and their adequacy was confirmed by the fact that prey were present at densities between 1 and 2 ind. ml−1 when cultures were changed. The culture volume was 300 ml in 500-ml glass vessels at 23 ± 2°C, under diffuse fluorescent illumination. The worms reproduced asexually by paratomy; most were at the bottom of the culture vessel, often attached to the glass; a few were observed swimming. We maintained the cultures with around 200 ind. l−1. The medium was changed once a week, and fresh algae and rotifers were added. We selected uniformly sized worms which showed an active swimming movement for all the experiments.
For culturing prey, we cultured Chlorella vulgaris in transparent bottles using Bold’s basal medium (Borowitzka & Borowitzka, 1988). Algae in the log-phase of growth were harvested, centrifuged and resuspended in distilled water, from which, using a Neubauer haemocytometer we counted the number of cells and obtained an algal density of 1 × 106 cells ml−1 as the food level appropriate for mass culture of the brachionid rotifers. Zooplankton cultures were maintained at 23 ± 2°C, in diffuse fluorescent light. For routine maintenance of all zooplankton and Stenostomum sp. as well as the experiments, we used moderately hard water (EPA medium: Weber, 1993), prepared by dissolving 96 mg NaHCO3, 60 mg CaSO4, 60 mg MgSO4 and 4 mg KCl in 1 l of distilled water.
Plationus patulus, Euchlanis dilatata and Moina macrocopa were used as prey. All cultures were established from a single female and were maintained under laboratory conditions for more than 6 months prior to experimentation. All the prey taxa were isolated from Lake Xochimilco (Mexico City). Cultures were maintained separately in 1-l glass beakers. They were filtered every alternate day with a 50-μm mesh and transferred to a fresh medium with algae at the above mentioned concentration.
Population growth studies
The experiments were conducted in 100-ml transparent plastic vessels with 50 ml of medium in each. For experiments with the rotifers we used two treatments, controls (without Stenostomum sp.) and treatments in which Stenostomum sp. were introduced at a density of 0.08 ind. ml−1. Plationus patulus and Euchlanis dilatata were introduced at a density of 1 ind. ml−1. The experimental design consisted of 16 test jars: 2 prey species × 2 treatments (with and without Stenostomum sp.) × 4 replicates.
Following initiation of the experiment, we daily counted rotifers and eggs (loose and attached) in each replicate in the case of P. patulus. Next, all prey and predators were transferred to fresh medium in a new vessel. The predator population was culled to maintain it at a constant 0.08 ind. ml−1. Euchlanis dilatata laid their eggs on the walls of the beaker which were difficult to dislodge, therefore we only changed the medium and not the test vessel daily. In the case of Moina macrocopa, we set up a control and two treatments, one in which worms were introduced directly into the test vessels and one in which worms were separated from the cladocerans by a mesh (they were fed 20 individuals of B. patulus in the mesh per day). The cladoceran Moina macrocopa was introduced at a concentration of 0.2 ind. ml−1 and was counted and transferred to fresh medium daily. The experiments were terminated after day 15 when prey populations began to decline. Based on the data collected, we derived the rate of population increase (r) using the exponential growth equation (Krebs, 1985): r = (ln N t – ln N 0)/t, where N 0 is the initial population density, N t population density at time t and t time in days. For each replicate, we derived five values during the exponential phase of the population growth, by selecting different time periods, the mean of which gave the r per replicate. Population growth data were analysed by means of repeated measures ANOVA and population growth rates (r) compared by one way ANOVA (Sigma-Plot 11).
Results
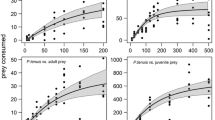
The population growth curves of Plationus patulus and Euchlanis dilatata in the presence and absence of Stenostomum sp. are shown in Fig. 1. Peak densities of P. patulus were three times higher in the absence of the catenulid worm, and the lag phase was also prolonged. This resulted in significant differences in growth pattern with or without the presence of worms (repeated measures ANOVA, P < 0.01). In E. dilatata, we also observed significantly lower population growth in the presence of worms (repeated measures ANOVA, P < 0.01), although the percent difference in the lag-phase and peak population density in the presence and absence of worms was less than in P. patulus.
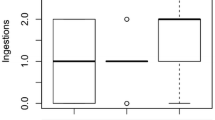
The egg ratio of P. patulus in the absence and presence of Stenostomum sp. is shown in Fig. 2. In the controls, egg-bearing P. patulus were abundant even at densities higher than 20 ind. ml−1; in the presence of Stenostomum sp., no egg bearing females were found at densities higher than 10 ind. ml−1. Egg ratios of E. dilatata were not calculated since several eggs were attached to the vessel and it was difficult to identify and count them.
Growth patterns of Moina macrocopa in direct or indirect contact with Stenostomum and in the controls are shown in Fig. 3. Population growth trends were similar in all three cases for the first 10 days; after that, the cladoceran population collapsed, irrespective of a direct or indirect contact with the worms.
Population growth rates of the rotifers and M. macrocopa in the presence and absence of Stenostomum sp. are shown in Figs. 4 and 5, respectively. The flatworm had a greater adverse impact on P. patulus than on E. dilatata and was able to lower the growth rate significantly (P < 0.002 and P < 0.03, respectively, One-way ANOVA, Table 1). On the other hand, considering only the exponential phase of growth of the population, no significant differences in the growth rates of M. macrocopa were observed (P > 0.05, One-way ANOVA). Nevertheless, after about 10–12 days of direct or indirect contact with the worms, the cladoceran population crashed.
Discussion
Population growth patterns of rotifers in nature are influenced, simultaneously, by several abiotic and biotic factors (Dumont, 1977; Wallace et al., 2006). Among the role of biotic factors, predation by micro-invertebrates has been well analysed (Kerfoot & Sih, 1987). Predators such as Asplanchna have a devastating effect due to two reasons: (i) they consume large numbers of prey (Sarma & Nandini, 2007) and (ii) they have short life cycles which allow a rapid increase in population numbers (Dumont & Sarma, 1995). We did observe some details of the consumption process of rotifer prey by Stenostomum sp. in this study and found that they sucked out prey from the bottom of the feeding vials using their eversible pharynx and occasionally captured free swimming animals. Others have documented their feeding on large numbers of protozoans in a short period of time (Kratina et al., 2009) and thus, it came as no surprise that Stenostomum sp. significantly reduced population densities of both Plationus patulus and Euchlanis dilatata after few days of exposure. That the worms did not drive their rotifer prey to extinction within the duration of our experiment, we ascribe to (1) the relatively short experimental time and (2) the low density of the worms. In shallow water with low oxygen and in the absence of vertebrates, higher densities of worms can be found (Kolasa, 2001) and in such cases, we expect them to structure their prey community.
The feeding behaviour of a few turbellarians has been studied in detail. Dumont & Carels (1987) have shown that Mesostoma ehrenbergi attacks its prey individually and sucks out its body contents. Other species, like Stenostomum predatorium, bite parts of their prey at each feeding attempt (Kolasa, 2001) while we observed Stenostomum leucops in this study to suck in prey using the pharynx. There have been few studies on the feeding behaviour of this flatworm; we observed that they capture and ingest whole rotifers, although not as voraciously as Asplanchna. They do not ‘chase’ their prey, but swim around and capture the ones that are in the appropriate zone. We analysed the stomach contents of several S. leucops and found more animal than algal matter in them. They do suction dead animals but also feed on live prey. In our test vessels, around 70% of the predator population was attached to the bottom or sides and 30% swimming freely in the column.
A different resistance to the sucking force of Stenostomum sp. could explain the differences in vulnerability of the two rotifer taxa studied. A larger proportion of the Euchlanis dilatata settled at the bottom or sides of the experimental containers, yet their population was relatively less affected by the worms than that of P. patulus, possibly reflecting the fact that the secretions of the pedal glands of E. dilatata are stronger than those of P. patulus (Koste, 1978). An alternative explanation is that the two rotifers differ in their tolerance to toxins produced by the flatworms. The release of such toxins by catenulids (they have been known in typhloplanids where they are used to sedate prey before consumption: Dumont & Carels, 1987) was suggested by the behaviour of the cladoceran Moina (see below) and by the rotifer egg ratios.
An aspect of toxin ecology appeared in our experiments with Moina macrocopa: whether prey were in direct or indirect contact with the predator, there was a delayed but precipitous decline after about 10 days. This suggests that the cladocerans may have accumulated toxin in their bodies until a lethal threshold was reached. However, this would require the flatworm toxin to remain active for a period of time longer than was suggested by Dumont and Carels (1987).
Egg ratio analysis is a useful tool in analyzing stress-related effects on rotifer populations (Sarma et al., 2005). In this study, we analysed the effect of Stenostomum sp. on the egg ratio of P. patulus and found that it declined significantly in the presence of the turbellarian. We consider that this inhibited egg production or decrease in population density is also consistent with the release of a toxin by the worms. It is also possible that the decline was due to the worms feeding on egg-bearing females. This has not been quantified in our study. Further studies will be needed to confirm the nature of these toxins and their ecological effects.
References
Altwegg, R., M. Eng, S. Caspersen & B. R. Anholt, 2006. Functional response and prey defence level in an experimental predator–prey system. Evolutionary Ecology Research 8: 115–128.
Blaustein, L. & H. J. Dumont, 1990. Typhloplanid flatworms (Mesostoma and related genera): mechanisms of predation and evidence that they structure aquatic invertebrate communities. Hydrobiologia 198: 61–77.
Borowitzka, M. A. & L. J. Borowitzka, 1988. Micro-Algal Biotechnology. Cambridge University Press, Cambridge: 480 pp.
Dumont, H. J., 1977. Biotic factors in the population dynamics of rotifers. Archiv für Hydrobiologie Beiheft 8: 98–122.
Dumont, H. J. & I. Carels, 1987. Flatworm predator (Mesostoma cf lingua) releases a toxin to catch planktonic prey (Daphnia magna). Limnology and Oceanography 32: 699–702.
Dumont, H. J. & S. S. S. Sarma, 1995. Demography and population growth of Asplanchna girodi (Rotifera) as a function of prey (Anuraeopsis fissa) density. Hydrobiologia 306: 97–107.
Felix, A., M. E. Stevens & R. L. Wallace, 1995. Unpalatability of a colonial rotifer, Sinantherina socialis, to small zooplanktivorous fishes. Invertebrate Biology 114: 139–144.
Fyda, J., A. Warren & J. Wolinska, 2005. An investigation of predator-induced defense responses in ciliated protozoa. Journal of Natural History 39: 1431–1442.
Garza-Mouriño, G., M. Silva-Briano, S. Nandini, S. S. S. Sarma & M. E. Castellanos-Páez, 2005. Morphological and morphometrical variations of selected rotifer species in response to predation: a seasonal study of selected brachionid species from Lake Xochimilco (Mexico). Hydrobiologia 546: 169–179.
Gilbert, J. J., 1998. Kairomone-induced morphological defenses in rotifers. In Tollrian, R. & C. D. Harvell (eds), The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton: 127–141.
Iyer, N. & T. R. Rao, 1995. The epizoic mode of life in Brachionus rubens Ehrenberg as a deterrent against predation by Asplanchna intermedia Hudson. Hydrobiologia 313–314: 377–380.
Iyer, N. & T. R. Rao, 1996. Responses of the predatory rotifer Asplanchna intermedia to prey species differing in vulnerability: laboratory and field studies. Freshwater Biology 36: 521–533.
Kerfoot, W. C. & A. Sih (eds), 1987. Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover, NH.
Kolasa, J., 2001. Flatworms: Turbellaria and Nemertea. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York: 145–172.
Koste, W., 1978. Rotatoria. Die Rädertiere Mitteleuropas. Ein Bestimmungswerk begründet von Max Voigt, Vol. 1, Textband: 673 pp; Vol. 2, Tafelband: 234 pp. Bornträger, Stuttgart.
Kratina, P., M. Vos, A. Bateman & B. R. Anholt, 2009. Functional responses modified by predator density. Oecologia 159: 425–433.
Krebs, C. J., 1985. Ecology: The Experimental Analysis of Distribution and Abundance, 3rd ed. Harper and Row, New York.
Murdoch, W. W. & J. Bence, 1987. General predators and unstable prey populations. In Kerfoot, W. C. & A. Sih (eds), Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover, NH: 17–30.
Nielsen, D. L., F. J. Smith, T. J. Hillman & R. J. Shiel, 2000. Impact of water regime and fish predation on zooplankton resting egg production and emergence. Journal of Plankton Research 22: 433–446.
Noreña, C., C. Damborenea & F. Brusa, 2005. A taxonomic revision of South American species of the genus Stenostomum O. Schmidt (Platyhelminthes: Catenulida) based on morphological characteristics. Zoological Journal of the Linnaean Society 144: 37–58.
Nuttycomb, J. W. & A. J. Waters, 1935. Feeding habits and pharyngeal structure in Stenostomum. Biological Bulletin 69: 439–446.
O’Brien, W. J., 1987. Planktivory by freshwater fish: thrust and parry in the pelagia. In Kerfoot, W. C. & A. Sih (eds), Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover, NH: 3–16.
Sarma, S. S. S. & S. Nandini, 2007. Small prey size offers immunity to predation: a case study on two species of Asplanchna and three brachionid prey (Rotifera). Hydrobiologia 593: 67–76.
Sarma, S. S. S., R. D. Gulati & S. Nandini, 2005. Factors affecting egg-ratio in planktonic rotifers. Hydrobiologia 546: 361–373.
Serrania-Soto, C. & S. S. S. Sarma, 2009. Morphometric changes in Lecane stokesii (Pell, 1890) (Rotifera: Lecanidae) induced by allelochemicals from the predator Asplanchnopus multiceps (Schrank, 1793). Allelopathy Journal 23: 215–222.
Wallace, R. L., T. W. Snell, C. Ricci & T. Nogrady, 2006. Rotifera Part 1: Biology, Ecology and Systematics. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. Kenobi Productions Gent/Backhuys, The Netherlands.
Weber, C. I., 1993 Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 4th ed. United States Environmental Protection Agency, Cincinnati, OH, EPA/600/4-90/027F, xv + 293 pp.
Acknowledgments
We thank Dr. J. Kolasa for identifying the flatworm. The first two authors thank National Autonomous University of Mexico (DGAPA–PASPA) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: N. Walz, R. Adrian, J.J. Gilbert, M.T. Monaghan, G. Weithoff & H. Zimmermann-Timm / Rotifera XII: New aspects in rotifer evolution, genetics, reproduction, ecology and biogeography
Rights and permissions
About this article
Cite this article
Nandini, S., Sarma, S.S.S. & Dumont, H.J. Predatory and toxic effects of the turbellarian (Stenostomum cf leucops) on the population dynamics of Euchlanis dilatata, Plationus patulus (Rotifera) and Moina macrocopa (Cladocera). Hydrobiologia 662, 171–177 (2011). https://doi.org/10.1007/s10750-010-0493-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0493-3