Abstract
We evaluated a biomanipulation program to test for short-term changes in water quality (chlorophyll a, Secchi depth, total phosphorus) and macrozooplankton biomass following partial removal of omnivorous gizzard shad Dorosoma cepedianum. The removal occurred at a eutrophic subtropical lake, and responses were compared to an unmanipulated control lake using a before-after-control-impact paired series analysis. The removal reduced the biomass of large (>300 mm) gizzard shad by 75% over 2 years via a subsidized commercial gill net fishery. However, the total population biomass of gizzard shad was reduced by approximately 32% from an average pre-manipulation biomass of 224 kg ha−1 due to the size selectivity of the gear, which did not effectively capture small fish (<300 mm). No significant short-term changes in chlorophyll a concentration, Secchi depth, total phosphorus concentration or macrozooplankton biomass were detected following biomanipulation. The partial removal may have fallen short of the biomass reduction required to cause ecosystem responses. Our results suggest that moderate omnivore removals (i.e., <40% biomass reduction) will have little short-term benefits to these lakes, and future manipulations should use a less size-selective gear to achieve a larger total biomass reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reversing eutrophication is a central goal of lake management. Reductions in point-source inputs have decreased phosphorus and phytoplankton concentrations in many lakes since the 1970s. However, many lakes experience persistent eutrophy due to non-point source phosphorus loading and internal nutrient recycling, which has resulted in alternative approaches to reverse eutrophication, including biomanipulation. Biomanipulation is the deliberate manipulation of biotic components of an aquatic ecosystem to reduce phytoplankton abundance (Shapiro et al., 1975). This approach has traditionally involved reduction of planktivorous fish populations to increase zooplankton abundance, resulting in increased grazing pressure of cladocerans on phytoplankton. However, the concept has more recently been applied to benthivorous fish removals to directly reduce nutrient cycling (Horppila et al., 1998), and manipulations of organisms other than fish (Galanti et al., 1990).
Ideally, biomanipulation seeks to shift lake systems from a eutrophic state to a more oligotrophic stable state that persists in the long-term (Scheffer et al., 1993). Fish biomanipulation in temperate lakes can reduce phytoplankton biomass over the short- (Shapiro & Wright, 1984; Søndergaard et al., 2008) or long-term (Benndorf, 1997; Drenner & Hambright, 1999; Søndergaard et al., 2008), but effects seem more readily apparent over the short-term where they occur (DeMelo et al., 1992; Drenner & Hambright, 1999; Meijer et al., 1999; Søndergaard et al., 2008). Compared to temperate systems, tropical and subtropical lakes may be less amenable to classical biomanipulation because top-down control of phytoplankton is weaker in these systems due to a lack of large herbivorous cladocerans, high fish predation, a high degree of omnivory, abundant invertebrate predators, and a predominance of blue-green algae (Jeppesen et al., 2005; Havens & Beaver, 2010). However, relatively few studies of biomanipulation have been conducted in tropical and subtropical lakes.
There is increasing evidence that omnivorous fishes can contribute substantially to internal nutrient recycling and primary productivity in aquatic ecosystems (Carpenter et al., 1987; Vanni, 2002). Omnivorous fish can affect phytoplankton productivity from the bottom-up by translocating nutrients from the benthos to the water column, and from the top-down via predation on zooplankton (Stein et al., 1995; Vanni, 1995; Dettmers & Stein, 1996). The gizzard shad Dorosoma cepedianum is an omnivorous clupeid found in eutrophic lakes in eastern North America (Stein et al., 1995; Bachmann et al., 1996; Allen et al., 2000). Gizzard shad are obligate zooplanktivores as larvae and juveniles, and can exert strong predation pressure on zooplankton (Dettmers & Stein, 1992; DeVries & Stein, 1992) due to their high densities. As juveniles, they undergo an ontogenetic diet shift to omnivory and as adults consume benthic organic detritus as well as zooplankton, when zooplankton are abundant (Yako et al., 1996; Schaus et al., 2002). Benthic detritivory by adult gizzard shad has important consequences for lake nutrient cycling because it translocates (i.e., via excretion) soluble forms of sediment-bound nutrients into the water column where they are highly available for phytoplankton growth (Vanni, 1995; Vanni, 2002; Vanni et al., 2006). This cross-habitat nutrient translocation can exceed watershed phosphorus loads (Schaus et al., 1997; Gido, 2002) and may account for up to 67% of total lake phosphorus demand by phytoplankton in productive lakes (Vanni et al., 2006).
In Florida, USA, the St. Johns River Water Management District (SJRWMD) has used gizzard shad removal in an attempt to reduce eutrophication at large eutrophic lakes within the Harris Chain of Lakes. Gizzard shad removals have been carried out at Lake Apopka (12,400 ha) since 1993 and Lake Griffin (3,815 ha) since 2002, with the goal of reducing phytoplankton abundance and improving water clarity. These are large-scale removals with the total annual gizzard shad biomass removed exceeding 400,000 kg in both lakes during some years. These biomanipulation efforts have typically used gill nets with a minimum mesh size restriction of 102 mm to protect recreational fisheries, such as black crappie Pomoxis nigromaculatus. As gill nets of this mesh size are highly selective for large gizzard shad (>300 mm), many smaller gizzard shad are invulnerable to gill net removal. Smaller fish have higher mass-specific nutrient excretion rates (Schaus et al., 1997; Schaus et al., 2010b), and thus it is not clear whether harvest of large gizzard shad could influence lake water chemistry and zooplankton biomass. No previous studies have evaluated the potential of the SJRWMD gizzard shad biomanipulation efforts to cause short-term improvements in water quality at eutrophic Florida lakes.
An experimental gizzard shad removal was conducted in 2005–2006 at Lake Dora, Florida, a previously unmanipulated system within the Harris Chain of Lakes, to evaluate the short-term effectiveness of SJRWMD gizzard shad removals at improving water quality and reducing gizzard shad populations. As part of this broader evaluation, this article focuses on the short-term effects of the removals on water quality. Specifically, our objective was to evaluate whether the biomanipulation could improve water clarity within a 1–2 year time frame at Lake Dora. We examined changes in water quality (chlorophyll a, Secchi depth, total phosphorus) and macrozooplankton biomass for 2 years before and 2 years after gizzard shad removal at Lake Dora and an unmanipulated control lake (Lake Harris). We hypothesized that if gizzard shad contribute substantially to lake nutrient loads, then gizzard shad removal would decrease phytoplankton abundance and total phosphorus concentrations, and increase Secchi depth. We also evaluated macrozooplankton abundance to assess whether any changes in phytoplankton resulted from changes in zooplankton grazing pressure or from changes in bottom-up nutrient loads.
Study sites
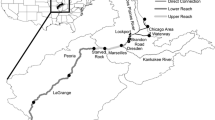
The study was conducted at Lake Dora (2,320 ha) and Lake Harris (5,580 ha), Lake County, FL, USA (Fig. 1, Table 1). Lake Dora was the impact lake where gizzard shad were removed, and Lake Harris served as an unmanipulated control system. Both are large, shallow eutrophic lakes (Table 1) with similar plankton and fish communities. The phytoplankton communities of these lakes consist primarily of blue-green algae, but chlorophytes, dinoflagellates, cryptophytes, chrysophytes, and euglenoids are also present. The lakes have very minimal littoral zone habitat. The littoral zone consists of a narrow band of emergent vegetation within 20 m of the shoreline. The lakes contain few submerged macrophytes due to rapidly increasing depth near the shore and high phytoplankton densities that lead to low transparency. Lake Dora is composed of two distinct basins: Lake Dora proper and the smaller Lake Beauclair basin (Fig. 1). The two basins are separated by a 300-m long by 80-m wide canal. Gizzard shad harvest occurred in both basins, so they were treated as one system for these analyses and will be referred to collectively as Lake Dora hereafter. Water quality and macrozooplankton were sampled in the deepest portion of the two main basins at each lake (Fig. 1).
Methods
Biomanipulation
Gizzard shad harvest at Lake Dora was achieved with a subsidized commercial gill net fishery by the SJRWMD. Prior to fish removal, gizzard shad populations in both lakes were unfished. Gizzard shad were removed from Lake Dora during March–April 2005 and again during January–April 2006 using gill nets with a minimum mesh size restriction of 102 mm (stretch mesh) to minimize bycatch of black crappie. Thus, the experimental removal selected for gizzard shad larger than approximately 300 mm total length. These removal methods were similar to previous biomanipulation efforts at nearby Lakes Apopka and Griffin. An average of five boats per lake set 3–5 sinking gill nets per boat per day, each net ranging in length from 75 to 600 m. The SJRWMD used onboard observers to obtain data on commercial catch-per-effort (CPE), catch composition and to monitor bycatch.
The annual exploitation rate (proportion of vulnerable-sized fish removed) on gizzard shad was estimated by dividing an annual estimate of the initial vulnerable biomass of gizzard shad by the observed total catch for that year. The initial vulnerable biomass was estimated with a Leslie depletion analysis, which is a linear regression of fishery CPE against cumulative catch (Van Den Avyle & Hayward, 1999). CPE was quantified as the daily biomass (kg) captured per 100 m of commercial net per hour and cumulative catch was the total biomass (kg) captured in the fishery as reported via mandatory daily catch reports from commercial fishers. Estimates of the annual exploitation rate were used in an age-structured model that used monthly time steps to estimate the total biomass reduction of gizzard shad each month relative to the unharvested biomass (Catalano & Allen, 2009). These estimates were conditional on estimates of natural mortality, growth, recruitment, and gear selectivity, which were estimated using a cohort analysis model fit to fishery-dependent and fishery independent gill net data as well as total harvested biomass (Catalano & Allen, 2010). These analyses showed that recruitment remained relatively constant across years despite a decrease in adult gizzard shad biomass (Catalano & Allen, 2010).
Water quality
Water quality data were obtained from a long-term monitoring program by the SJRWMD that has been ongoing since 1990. We obtained data for the time period that spanned 2 years before and 2 years after the initial gizzard shad removal in 2005 (April 2003–March 2007). Monthly water samples were collected by SJRWMD at two pelagic locations at each lake (Fig. 1). Two sample sites were adequate since preliminary analyses suggested that coefficients of variation between sites did not exceed about 10%. We focused our sampling in the pelagic zone because littoral habitats comprised such a small portion of the total lake surface area. Thus, pelagic sites were representative of the overall plankton communities and water characteristics of the lakes. Secchi depth was measured at each site to the nearest 0.1 m.
Water samples for chlorophyll a (CHLA) and total phosphorus (TP) analysis were collected once per month at each site at a depth of 0.5 m using a horizontal Van Dorn bottle. Brown et al. (1999) found that surface (0.5 m) CHLA and TP samples did not differ significantly from integrated samples in shallow Florida lakes. Therefore, our surface sampling procedure was likely adequate to capture ambient concentrations of CHLA and TP. Samples were analyzed for CHLA concentration and TP by the SJRWMD using standard operating procedures of the Florida Department of Environmental Protection (FDEP, 2004). Chlorophyll a samples were obtained by passing a known volume of water through a 0.7-μm Whatman (Maidstone, Kent, UK) glass fiber filter and the concentration (μg l−1) was measured with a spectrophotometer following acetone extraction (FDEP, 2004). Total phosphorus (μg l−1) was determined using an Alpkem Flow Solution 3000 analyzer (OI Analytical, College Station, TX, USA) after acid digestion in mercuric sulfate and potassium sulfate and reaction with molybdenum and antimony (FDEP, 2004).
Macrozooplankton
Macrozooplankton samples were collected monthly from April 2003 to March 2007 using a 13-cm diameter Wisconsin-style plankton net at two pelagic sites at each lake (Fig. 1). A mesh size of 75 μm was used in 2003–2004 (collected by SJRWMD) and a mesh size of 83 μm was used in 2005–2007 (collected by University of Florida). An integrated sample was collected at each site by towing the net vertically from ~0.25 m above the bottom to the surface and depth was recorded. Samples were preserved in 5% buffered formalin solution. Sample preparation and enumeration followed methods described in Tugend & Allen (2000). Macrozooplankton were identified to species level in 2003 and 2004, and to genus level after 2004. A minimum of 20 individuals of each genus were measured using an ocular micrometer. Macrozooplankton biomass estimates (dry weight; dw μg l−1) were calculated using published length–weight relationships (McCauley, 1984; Culver et al., 1985). Rotifers and nauplii were excluded from all calculations because of the large mesh size, which did not effectively capture these taxa. For analyses, macrozooplankton were divided into calanoid copepods, cladocerans, and cyclopoid copepods.
Statistical analyses
The effects of gizzard shad removal on phytoplankton biomass (as estimated from chlorophyll a concentration), water transparency (Secchi depth), total phosphorus, and macrozooplankton biomass were evaluated using a before-after-control-impact paired series (BACIPS) analysis. The analysis tests whether differences between a control and impact site change after an intervention (Stewart-Oaten et al., 1986). Data were divided into two time periods based on the timing of the initial shad removal. The pre-removal period was 24 months long and extended from April 2003 to March 2005. The post-removal period was also 24 months and extended from April 2005 to March 2007. The post-removal period began in April 2005 because 98% of the gizzard shad removed (by biomass) in 2005 had been harvested by that time. The post-manipulation period was begun just after the gizzard shad removal was complete because a review of 18 biomanipulation case studies by Meijer et al. (1999) found that substantial increases in water transparency usually occurred in spring just after fish removal. A single 24-month post-manipulation period was used instead of a partial (2005) and full (2006–2007) removal period because gizzard shad age structure analyses combined with depletion estimates (see Biomanipulation under Methods section, above) suggested that at least 3/4 of the total biomass reduction occurred during the first year of the study; reductions after the second year of harvest were weaker because of gizzard shad growth and recruitment.
The response variables CHLA, Secchi depth, TP, calanoid copepod biomass, and cyclopoid copepod biomass were analyzed using BACIPS analysis. Descriptions of the computational details of the analysis can be found in Stewart-Oaten et al. (1986). The BACIPS analysis uses a t-test to evaluate whether differences in a response variable between an impact lake and a control lake change after an intervention (Stewart-Oaten et al., 1986). First, we averaged the two values (sites) from each lake for a particular response variable. Then, we computed monthly differences between the average control and impact lake values. These differences were split into the two time periods as described above and a t-test was used to test for differences in the between-lake differences between time periods (i.e., before vs. after manipulation). Monthly between-lake differences were log-transformed to satisfy the additivity assumption. Cladoceran biomass was not statistically analyzed because of a lack of temporal variation in Lake Dora densities due to a high proportion of zero biomass values.
The effect size was calculated as the change in the average between-lake differences following gizzard shad removal. In addition, Cohen’s effect size (Cohen, 1988) was calculated as the effect size divided by the standard deviation of the between-lake differences to standardize the effect relative to the variability of the data. Generally speaking, a Cohen’s effect size of 0.2 is considered small, 0.5 medium, and 0.8 large. For each analysis, statistical power was estimated for a two-tailed t-test using methods found in Cohen (1988). In addition to these analyses, a standard BACIPS was carried out using only data from the summer months (June–September) to evaluate whether effects were seasonal. Statistical analyses were considered significant at α = 0.1.
Results
Biomanipulation
Commercial fishers removed 124,989 kg (54 kg ha−1) of gizzard shad in 2005 and 135,095 kg (58 kg ha−1) in 2006 (Table 2). Depletion analysis estimated an exploitation rate on vulnerable-sized fish of 0.71 (95% confidence interval 0.40–0.90) in 2005 and 0.65 (95% confidence interval 0.37–0.86) in 2006. Despite the higher exploitation rate in 2005 than 2006, more total biomass was removed in 2006 due to fish growth and because the above average 2003 year class became vulnerable to the fishery in 2006. An age-structured population model estimated that the percent reduction of vulnerable-sized (>300 mm) gizzard shad was 75% (95% confidence interval 59–90%) after both removals had occurred, relative to the average pre-removal abundance estimates. This corresponded to a total biomass (i.e., including all sizes of fish) reduction of 32% (95% confidence interval 24–41%) from an average pre-manipulation biomass of 224 kg ha−1 (Table 2). At least three-fourths of this total biomass reduction occurred during the first year of harvest (2005); the 2006 harvest did not contribute as much to the reduction because of fish growth and the above average 2003 year class. Gill net catch was dominated by gizzard shad (83%), with black crappie (7%), white catfish Ameiurus catus (2%), and channel catfish Ictalurus punctatus (1%) also captured.
Water quality
Lake Harris (control) had lower CHLA, total phosphorus, and higher Secchi depths than Lake Dora in nearly all months of the study (Fig. 2). Chlorophyll a concentrations ranged from 51.8 to 157.2 μg l−1 at Lake Dora and from 19.7 to 92.7 μg l−1 at Lake Harris (Fig. 2) and were generally more variable at Lake Dora than at Lake Harris both before and after the biomanipulation. Total phosphorus ranged from 36.5 to 88.8 μg l−1 at Lake Dora and from 21.0 to 67.5 μg l−1 at Lake Harris (Fig. 2) and was also more variable at Lake Dora than Lake Harris. Secchi depth showed the opposite trend and was more consistent at Lake Dora and ranged from 0.28 to 0.53 m at Lake Dora and from 0.4 to 1.5 m at Lake Harris (Fig. 2). The time series of water quality parameters frequently moved together for both lakes, particularly for CHLA and TP, suggesting similar environmental drivers at both lakes, and making Lake Harris an adequate control system.
Mean monthly chlorophyll a concentration (μg l−1; upper), Secchi depth (m; middle) and total phosphorus concentration (μg l−1; lower) at Lake Dora (impact lake; solid line) and Lake Harris (control lake; dashed line) from April 2003 to March 2007. Data represent average values from two sites per lake that were sampled once per month by the St. Johns Water Management District, Palatka, Florida, USA. Vertical dashed lines indicate the timing of gizzard shad removals
There were no detectable changes in water quality at Lake Dora relative to Lake Harris following biomanipulation, and effect sizes were small (Fig. 2). The mean difference in CHLA between Lake Dora and the Lake Harris did not differ significantly following biomanipulation (standard BACIPS, Welch’s t = −0.31, df = 38, P = 0.758; Table 3). Statistical power for this test was low (1 − β = 0.09). Therefore, measures of effect size were more informative than P values and indicated very little change in CHLA values following biomanipulation (Table 3). Although CHLA (Fig. 2) declined somewhat from April to August in 2005 and 2006 at Lake Dora after biomanipulation, a similar decline occurred in 2003, suggesting a seasonal effect unrelated to the manipulation (Fig. 2). The BACIPS analysis of mid-summer (June–September) CHLA indicated that there was no biomanipulation effect on differences in CHLA between lakes Dora and Harris (t = −0.73, df = 14, P = 0.48) and effect sizes were small (Cohen’s = 0.37, proportional effect = 0.11). Mean between-lake differences in Secchi depth and total phosphorus did not differ significantly after biomanipulation and effect sizes were small (Table 3). Although there was a decline in TP from April to August of 2005 and 2006 at Lake Dora, a similar decline appeared in 2004 suggesting a seasonal rather than a biomanipulation effect (Fig. 2). Thus, our results indicated no short-term changes in water quality at Lake Dora in response to the biomanipulation relative to the control lake.
Macrozooplankton
Calanoid copepod densities were higher at Lake Dora than Harris, whereas the opposite was true for cladocerans (Fig. 3). Cyclopoid copepod densities were similar between lakes and the two lakes tended to track one another over time (Fig. 3). Cladoceran densities were near zero at Lake Dora during both time periods but densities at Lake Harris exhibited typical seasonal cycles with peaks from February to May (Fig. 3).
Mean monthly biomass (dw μg l−1) of calanoid copepods (upper), cladocerans (middle), and cyclopoid copepods (lower) at Lake Dora (impact lake; solid line) and Lake Harris (control lake; dashed line) from April 2003 to March 2007. Data represent average values from two sites per lake that were sampled once per month by vertical tows with a 75 μm (2003–2004) and 83 μm (2005–2007) mesh plankton net. Vertical dashed lines indicate the timing of gizzard shad removals. Cladocerans were dominated by Eubosmina tubicen, Alona guttata, and Daphnia ambigua. Calanoid copepods were comprised entirely of Artcodiaptomus dorsalis. Cyclopoid copepods were dominated by Tropocylops prasinus and Acanthocyclops vernalis
Differences in macrozooplankton biomass between the impact and control lake showed no detectable changes following biomanipulation, and biomass of all three zooplankton groups was highly variable (Fig. 3). The BACIPS analyses indicated that there was no statistically significant biomanipulation effect for calanoid and cyclopoid copepods (Table 3). In general, effect sizes were small indicating that responses were not biologically meaningful. Statistical power was <0.5 for macrozooplankton analyses because of small effect sizes and high variability in the observations.
Discussion
We found no significant short-term (i.e., within 2 years) benefits to water clarity following the partial removal of gizzard shad at Lake Dora. Previous studies have found that gizzard shad contribute substantially to internal phosphorus loading at eutrophic lakes via bioturbation and translocation of sediment-bound nutrients to the water column (Schaus et al., 1997; Higgins et al., 2006; Vanni et al., 2006; Domine et al., 2010). However, no changes in phytoplankton, phosphorus concentration, and macrozooplankton biomass were detected following partial removal of large gizzard shad at Lake Dora. Most of the statistical tests resulted in very small effect sizes (i.e., negligible responses to biomanipulation), none of which were biologically meaningful or statistically significant.
The strength and duration of the biomanipulation should be a key consideration in biomanipulation efforts and may have influenced our results. In general, fish removals must exceed a 75% total biomass reduction to achieve measureable declines in phytoplankton biomass through cascading trophic interactions (Hansson et al., 1998; Meijer et al., 1999). In the current study, biomanipulation with a 102-mm gill net mesh size achieved a total biomass reduction of 32% over 2 years. The large difference between the percent reduction of vulnerable-sized fish (75%) and the total biomass reduction indicates that a large proportion of the gizzard shad population was invulnerable to harvest. Thus, the fishing gear restrictions imparted on the commercial fishers precluded capture of small gizzard shad, which may have contributed to the lack of water clarity improvements at this system. Our results were likely representative of other gizzard shad removal efforts in Florida because the Lake Dora removal was similar in removal method and magnitude (but not duration) of removal to the other lakes. For example, the gizzard shad removals at Lake Dora, on a per hectare basis (~56 kg ha−1), were similar to the removals at Lake Apopka (~39 kg ha−1) and Lake Griffin (~49 kg ha−1) from 2002 to 2007 (Schaus, 2007), suggesting that the degree of manipulation was comparable to previous biomanipulation efforts in Florida.
Many studies have documented reductions in phytoplankton biomass following fish removals (Drenner & Hambright, 1999; Meijer et al., 1999; Søndergaard et al., 2008) although considerable uncertainty remains regarding success rates, the generality of the approach, and its mechanisms (DeMelo et al., 1992). Biomanipulation effects on phytoplankton are usually attributed to cascading trophic interactions and subsequent effects on zooplankton grazing pressure. However, several studies have detected phytoplankton reductions without commensurate shifts in zooplankton biomass or species composition, suggesting that bottom-up effects of fish on nutrient cycling may be underappreciated. Horppila et al. (1998) reported substantial decreases in cyanobacteria biomass and phosphorus concentrations following a 78% total biomass reduction of roach Rutilus rutilus at Lake Vesijärvi, Finland. Starling et al. (2002) reported reduced total phosphorus and chlorophyll a following mass mortality of omnivorous Nile tilapia (Oreochromis niloticus, Linnaeus) at Lago Paranoá, Brazil. They did not find changes in zooplankton biomass, suggesting that Nile tilapia stimulated phytoplankton growth via phosphorus translocation and recycling.
However, biomanipulation is not always successful. Kim & DeVries (2000) evaluated a whole-lake partial piscicide treatment targeting gizzard shad at Walker County Lake, Alabama, a shallow eutrophic south-temperate lake. They detected no changes in phytoplankton biomass following biomanipulation despite drastically reduced larval gizzard shad densities and increased zooplankton biomass. Kim & DeVries (2000) concluded that the classic trophic cascade paradigm of strong zooplankton–phytoplankton linkages did not apply due to high lake productivity and the absence of large herbivorous daphnids. Although such mechanisms may have buffered effects of gizzard shad removal at Lake Dora, the most plausible explanation for the lack of an effect is that not enough gizzard shad were removed to elicit an ecosystem response given the constraints placed on the removal method to reduce bycatch of black crappie.
Few studies have evaluated reduction targets for benthivorous and omnivorous fish, particularly in the tropics and subtropics. Horppilla et al. (1998) suggested that biomass reduction thresholds for benthivores may be less than for planktivores because benthivores act as a bottom-up nutrient source that may affect phytoplankton biomass more directly than top-down effects mediated by zooplankton grazing. Conversely, Jeppesen et al. (2005) suggested that biomass reductions may need to be greater in tropical/subtropical than in temperate lakes because of weak top-down control of phytoplankton owing to a high degree of omnivory, abundant invertebrate predators such as Chaoborus sp. and a lack of large herbivorous cladocerans such as Daphnia sp. Few cladoceran grazers were observed in Lake Dora before and after gizzard shad removal, and those that were collected were dominated by small bosminids. Zooplankton communities in Florida and other subtropical lakes are typically dominated by small taxa and/or copepods (Blancher, 1984; Elmore et al., 1984; Beaver & Havens, 1996; Havens & Beaver, 2010) and top-down control on phytoplankton biomass appears weak relative to more northern lakes (Havens et al., 1996).
Another possible explanation for a lack of biomanipulation effect at Lake Dora is that internal phosphorus loading by gizzard shad may have been small relative to watershed and other inputs. Existing work examining the magnitude of phosphorus loading by gizzard shad has been conducted in reservoirs, where P excretion by gizzard shad is typically high relative to total phytoplankton demand (14–67%) especially with increasing trophic status (Vanni et al., 2006). It is clear that omnivores such as gizzard shad can be an important source of nutrients relative to watershed inputs in temperate reservoir ecosystems (Schaus et al., 1997). However, Schaus et al. (1997) examined effects of relatively small gizzard shad (mean 46 g wet mass, range 2–211 g) compared to those that were removed at Lake Dora (mean 444 g wet mass, range 196–1,387 g). Mass-specific phosphorus excretion rates are inversely related to fish mass (Schaus et al., 1997), and thus selective removal of large gizzard shad at Lake Dora may be less effective at reducing internal phosphorus loading than removal of smaller size classes.
To compare the nutrient effects of gizzard shad harvesting relative to external loads, Schaus et al. (2010a) measured excretion rates of large Lake Dora gizzard shad and combined them with the size structure of the catch to estimate the maximum annual phosphorus loading (assuming 100% benthivory) prevented by the fish removal. Schaus et al. (2010a) estimated that biomanipulation prevented the release of approximately 1,956 kg year−1 of phosphorus at Lake Dora in 2005 and 2,528 kg year−1 in 2006. The 2005 and 2006 estimates of watershed phosphorus loading into the Lake Dora basin (Lake Dora proper and Lake Beauclair) were approximately 13,350 and 2,886 kg (Fulton & Smith, 2008; SJRWMD unpublished data), respectively, with high variability due to large differences in rainfall. Therefore, the gizzard shad removed accounted for 15 and 87% of watershed inputs in each year. Thus, biomanipulation resulted in a substantial reduction in P loading relative to watershed inputs in 2006 but not in 2005. The magnitude of the load reduction from biomanipulation relative to external loads will likely influence the value of biomanipulation as a management tool. However, the duration of load reductions could also be important as it could take multiple years for improved water quality to be realized if in-water nutrient concentrations exhibit a multiple-year lag with loading, which has been observed at Lake Apopka (Coveney et al., 2005). Other sources of P loading such as benthic invertebrates, benthic-feeding fishes other than gizzard shad, wind re-suspension and sediment flux may contribute substantially to internal nutrient loads, which could further lessen the impact of biomanipulation.
This study represented one of only two peer-reviewed experimental evaluations of gizzard shad biomanipulation to date (Kim & DeVries, 2000) and the only such study conducted in Florida. Our results suggested that future gizzard shad biomanipulation efforts of similar magnitude, duration and size selectivity are unlikely to result in short-term reductions in phytoplankton abundance in shallow eutrophic Florida lakes such as Lake Dora. These results do not infer that biomanipulation targeting all sizes of gizzard shad and/or achieving a biomass reduction over longer time period would not reduce phytoplankton abundance. Additional modeling of food web interactions, nutrient cycling and alternative biomanipulation methods could help identify critical levels of gizzard shad harvest required to affect phytoplankton abundance.
References
Allen, M. S., M. V. Hoyer & D. E. Canfield Jr., 2000. Factors related to gizzard shad and threadfin shad occurrence and abundance in Florida lakes. Journal of Fish Biology 57: 291–302.
Bachmann, R. W., B. L. Jones, D. D. Fox, M. Hoyer, L. A. Bull & D. E. Canfield Jr., 1996. Relations between trophic state indicators and fish in Florida (USA) lakes. Canadian Journal of Fisheries and Aquatic Sciences 53: 842–855.
Beaver, J. R. & K. E. Havens, 1996. Seasonal and spatial variation in zooplankton community structure and their relation to possible controlling variables in Lake Okeechobee. Freshwater Biology 36: 45–56.
Benndorf, J., 1997. Possibilities and limits for controlling eutrophication by biomanipulation. International Review of Hydrobiology 80: 519–534.
Blancher, E. C., 1984. Zooplankton-trophic state relationships in some north and central Florida lakes. Hydrobiologia 109: 251–263.
Brown, C. D., D. E. Canfield Jr., R. W. Bachmann & M. V. Hoyer, 1999. Evaluation of surface sampling for estimates of chlorophyll, total phosphorus, and total nitrogen concentrations in shallow Florida lakes. Journal of Lake and Reservoir Management 15: 121–132.
Carpenter, S. R., J. F. Kitchell, J. R. Hodgson, P. A. Cochran, J. J. Elser, M. M. Elser, D. M. Lodge, D. Kretchmer, X. He & C. N. von Ende, 1987. Regulation of lake primary productivity by food web structure. Ecology 68: 1863–1876.
Catalano, M. J. & M. J. Allen, 2009. Assessing Effects of Gizzard Shad Removal on Gizzard Shad Population Dynamics in Florida Lakes. Final Report to the St. Johns River Water Management District: Contract #SI40613. University of Florida, Gainesville, FL.
Catalano, M. J. & M. S. Allen, 2010. A size- and age-structured model to estimate fish recruitment, growth, mortality, and gear selectivity. Fisheries Research 105: 38–45.
Cohen, J., 1988. Statistical Power Analysis for the Behavioral Sciences. Erlbaum Associates, Hillsdale, NJ.
Coveney, M. F., E. F. Lowe, L. E. Battoe, E. R. Marzolf & R. Conrow, 2005. Response of a eutrophic, shallow subtropical lake to reduced nutrient loading. Freshwater Biology 50: 1718–1730.
Culver, D. A., M. M. Boucherle, D. J. Bean & J. W. Fletcher, 1985. Biomass of freshwater crustacean zooplankton from length–weight regressions. Canadian Journal of Fisheries and Aquatic Sciences 42: 1380–1390.
DeMelo, R., R. France & D. J. McQueen, 1992. Biomanipulation: hit or myth. Limnology and Oceanography 37: 192–207.
Dettmers, J. M. & R. A. Stein, 1992. Food consumption by larval gizzard shad: zooplankton effects and implications for reservoir communities. Transactions of the American Fisheries Society 121: 494–507.
Dettmers, J. M. & R. A. Stein, 1996. Quantifying linkages among gizzard shad, zooplankton, and phytoplankton in reservoirs. Transactions of the American Fisheries Society 125: 27–41.
DeVries, D. R. & R. A. Stein, 1992. Complex interactions between fish and zooplankton: quantifying the role of an open-water planktivore. Canadian Journal of Fisheries and Aquatic Sciences 49: 1216–1227.
Domine, L. M., M. J. Vanni & W. H. Renwick, 2010. New and regenerated primary production in a productive reservoir ecosystem. Canadian Journal of Fisheries and Aquatic Sciences 67: 278–287.
Drenner, R. W. & K. D. Hambright, 1999. Review: biomanipulation of fish assemblages as a lake restoration technique. Archiv für Hydrobiologie 146: 129–165.
Elmore, J. L., B. C. Cowell & D. S. Vodopich, 1984. Biological communities of three subtropical Florida lakes of different trophic character. Archiv für Hydrobiologie 100: 455–478.
FDEP, 2004. Standard Operating Procedures for Laboratory Activities. DEP-SOP-002/01, Tallahassee, FL.
Fulton, R. S. I. & D. Smith, 2008. Development of phosphorus load reduction goals for seven lakes in the Upper Ocklawaha River Basin, Florida. Lake and Reservoir Management 24: 139–154.
Galanti, G., P. Guilizzoni & V. Libera, 1990. Biomanipulation of Lago di Candia (Northern Italy): a three-year experience of aquatic macrophyte management. Hydrobiologia 200(201): 409–417.
Gido, K. B., 2002. Interspecific comparisons and the potential importance of nutrient excretion by benthic fishes in a large reservoir. Transactions of the American Fisheries Society 131: 260–270.
Hansson, L.-A., H. Annadotter, E. Bergman, S. F. Hamrin, E. Jeppesen, T. Kairesalo, E. Luokkanen, P.-A. Nilsson, M. Søndergaard & J. Strand, 1998. Biomanipulation as an application of food-chain theory: constraints, synthesis, and recommendations for temperate lakes. Ecosystems 1: 558–574.
Havens, K. E. & J. R. Beaver, 2010. Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia. doi:10.1007/s10750-010-0386-5.
Havens, K. E., T. L. East & J. R. Beaver, 1996. Experimental studies of zooplankton–phytoplankton–nutrient interactions in a large subtropical lake (Lake Okeechobee, Florida, USA). Freshwater Biology 36: 579–597.
Higgins, K. A., M. J. Vanni & M. J. González, 2006. Detritivory and the stoichiometry of nutrient cycling by a dominant fish species in lakes of varying productivity. Oikos 114: 419–430.
Horppila, J., H. Peltonen, T. Malinen, E. Luokkanen & T. Kairesalo, 1998. Top-down or bottom-up effects by fish: issues of concern in biomanipulation of lakes. Restoration Ecology 6: 20–28.
Jeppesen, E., M. Sondergaard, N. Mazzeo, M. Meerhoff, C. C. Branco, V. Huszar & F. Scasso, 2005. Lake restoration and biomanipulation in temperate lakes: relevance for subtropical and tropical lakes. In Reddy, M. V. (ed.), Tropical Eutrophic Lakes: Their Restoration and Management. Science Publishers, Enfield, New Hampshire: 331–349.
Kim, G. W. & D. R. DeVries, 2000. Effects of a selectively reduced gizzard shad population on trophic interactions and age-0 fishes in Walker County Lake, Alabama. North American Journal of Fisheries Management 20: 860–872.
McCauley, E., 1984. The estimation of abundance and biomass of zooplankton in samples. In Downing, J. A. & F. H. Rigler (eds), A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters. Blackwell Scientific, Oxford: 228–265.
Meijer, M.-L., I. de Bois, M. Scheffer, R. Portielje & H. Hosper, 1999. Biomanipulation in shallow lakes in the Netherlands: an evaluation of 18 case studies. Hydrobiologia 408(409): 13–30.
Schaus, M. H., 2007. Effects of Biomanipulation on Nutrient Cycles in Central Florida Lakes via Nutrient Excretion and Bioturbation by Gizzard Shad. Project #SK933AA, Final Report. St. Johns River Water Management District, Palatka, FL.
Schaus, M. H., M. J. Vanni, T. E. Wissing, M. T. Bremigan, J. E. Garvey & R. A. Stein, 1997. Nitrogen and phosphorus excretion by detritivorous gizzard shad in a reservoir ecosystem. Limnology and Oceanography 42: 1386–1397.
Schaus, M. H., M. J. Vanni & T. E. Wissing, 2002. Biomass-dependent diet shifts in omnivorous gizzard shad: implications for growth, food web, and ecosystem effects. Transactions of the American Fisheries Society 131: 40–54.
Schaus, M. H., W. W. Morris & A. Ford, 2010a. Quantifying the Role of an Omnivorous Fish in Central Florida Lakes: Diet Analyses and Simulation Modeling. Project #25244, Final Report. St. Johns River Water Management District, Palatka, FL.
Schaus, M. H., W. Godwin, L. Battoe, M. Coveney, E. Lowe, R. Roth, C. Selecky, M. Vindigni, C. Weinberg & A. Zimmerman, 2010b. Impact of the removal of Gizzard Shad (Dorosoma cepedianum) on nutrient cycles in Lake Apopka, Florida. Freshwater Biology. doi:10.1111/j.1365-2427.2010.02440.x.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Shapiro, J. & D. I. Wright, 1984. Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshwater Biology 14: 371–383.
Shapiro, J., V. Lamarra & M. Lynch, 1975. Biomanipulation: an ecosystem approach to lake restoration. In Brezonik, P. L. & J. L. Fox (eds), Proceedings of a Symposium on Water Quality Management through Biological Control. University of Florida, Gainesville, FL: 85–86.
Søndergaard, M., L. Liboriussen, A. R. Pedersen & E. Jeppesen, 2008. Lake restoration by fish removal: short- and long-term effects in 36 Danish lakes. Ecosystems 11: 1291–1305.
Starling, F., X. Lazzaro, C. Cavalcanti & R. Moriera, 2002. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: evidence from a fish kill. Freshwater Biology 47: 2443–2452.
Stein, R. A., D. R. DeVries & J. M. Dettmers, 1995. Food-web regulation by a planktivore: exploring the generality of the trophic cascade hypothesis. Canadian Journal of Fisheries and Aquatic Sciences 52: 2518–2526.
Stewart-Oaten, A., W. W. Murdoch & K. R. Parker, 1986. Environmental impact assessment: “pseudoreplication” in time? Ecology 67: 929–940.
Tugend, K. I. & M. S. Allen, 2000. Temporal dynamics of zooplankton community composition and mean size at Lake Wauberg, Florida. Florida Scientist 63: 142–154.
Van Den Avyle, M. J. & R. S. Hayward, 1999. Dynamics of exploited fish populations. In Kohler, C. C. & W. A. Hubert (eds), Inland Fisheries Management in North America. American Fisheries Society, Bethesda, MD: 127–166.
Vanni, M. J., 1995. Nutrient transport and recycling by consumers in lake food webs: implications for algal communities. In Polis, G. A. & K. O. Winemiller (eds), Food Webs: Integration of Patterns and Dynamics. Chapman and Hall, New York: 81–95.
Vanni, M. J., 2002. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics 33: 341–370.
Vanni, M. J., A. M. Bowling, E. M. Dickman, R. S. Hale, K. A. Higgins, M. J. Horgan, L. B. Knoll, W. H. Renwick & R. A. Stein, 2006. Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 87: 1696–1709.
Yako, L. A., J. M. Dettmers & R. A. Stein, 1996. Feeding preferences of omnivorous gizzard shad as influenced by fish size and zooplankton density. Transactions of the American Fisheries Society 125: 753–759.
Acknowledgments
Many thanks to B. Baker, C. Barrientos, G. Binion, A. Bunch, M. Bunch, J. Dotson, P. Hall, G. Kaufman, E. Thompson, and A. Watts for assistance with sample collection and taxonomic identification of zooplankton. Walt Godwin and Brian Sparks of the SJRWMD were helpful with planning logistics for field work and providing portions of the data set presented here. The authors are grateful to the SJRWMD staff and two anonymous reviewers for helpful comments on drafts of the manuscript. M. Catalano was supported by a University of Florida Alumni Doctoral Fellowship. The research presented here was funded in part by the St. John’s River Water Management District, Palatka, Florida.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luiz Carlos Gomes
Rights and permissions
About this article
Cite this article
Catalano, M.J., Allen, M.S., Schaus, M.H. et al. Evaluating short-term effects of omnivorous fish removal on water quality and zooplankton at a subtropical lake. Hydrobiologia 655, 159–169 (2010). https://doi.org/10.1007/s10750-010-0418-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0418-1