Abstract
The East River (Dong Jiang), a major tributary of the Pearl River (Zhu Jiang, the second largest river in China by discharge), is situated in southern China, which has the highest rates of urbanization and development on Earth. The East River also provides 80% of Hong Kong’s water supply. However, there have been no ecological studies to examine the combined impacts of changes in land use and water quality degradation on this river ecosystem. We tested the hypothesis that land-use disturbance and water quality degradation would significantly reduce benthic biodiversity in the East River by investigating macroinvertebrate community composition and relating it to data on water quality and catchment condition. The percentage of total impervious area within each catchment (%TIA—an indicator of land-use disturbance) was negatively related to a composite water quality index—the ERWQI—we developed for the East River. Modeling by partial least squares projection to latent structures (PLS) showed that family richness and relative abundance index (RAI) of macroinvertebrates were strongly influenced by both %TIA and ERWQI. Multi-response permutation procedure (MRPP) tests showed highly significant differences in family richness composition and RAI of macroinvertebrates among sites in the upper, middle, and lower course of the East River. MRPP also revealed differences in the family richness composition of nighttime drift samples between upper and middle site groups. Abundance (individuals m−3) and total family richness of drifting macroinvertebrates at each site were positively related to %TIA (range: 1.0–8.5%), while drift biomass was negatively related to dissolved oxygen and positively related to total suspended solids. Thus, human disturbances associated with land-use changes (increasing %TIA) and nutrient inputs severely degraded ecosystem integrity and the water quality of the East River and thereby reduced aquatic biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities affect freshwater ecosystems through changes in land use and habitat degradation posing severe threats to lotic biodiversity (Allan & Flecker, 1993; Harding et al., 1998; Malmqvist & Rundle, 2002; Dudgeon et al., 2006). Such impacts have become especially severe in the developing economies of Southeast Asia and, particularly in China (Dudgeon, 1992, 2000). Land conversion for agricultural and urban development impacts stream and river ecosystem dynamics by changing hydrological regimes and increasing sediment and pollution loads. These human disturbances are especially widespread and intense in many regions of China, reflecting the environmental degradation associated with its large population and rapid economic growth. For instance, 40% of urban wastewater is discharged into streams, rivers, and lakes without treatment (Fu, 2008). Such untreated industrial and municipal wastewater discharges, along with diffuse runoff of fertilizers and pesticides from agricultural land have given rise to poor water quality in most Chinese rivers (Liu & Diamond, 2005). Among the 197 large rivers monitored in China, 50% of them are rated as heavily polluted (Fu, 2008).
The East River (Dong Jiang), a tributary of the Pearl River, which is China’s second largest river by discharge, mainly drains Guangdong Province (90% of the basin area). The East River not only supplies water to this fastest-growing region—China’s so-called “factory of the world” (Escobar, 2005)—but also supplies 80% of domestic water consumption to neighbouring Hong Kong (Water Supplies Department, HKSAR, 2005). Economic development and migration from other parts of China into the Pearl River Delta region in recent years have led to impacts on the East River from a variety of human activities, including agriculture, logging, sand dredging, industrial and domestic sewage discharges, and flow regulation. In the East River Basin, organic nitrogen has been considered as a major pollutant (Yuan, 1999).
Running water ecosystems integrate biogeochemical processes from the scale of the catchment to local spatial scales, which can be altered extensively by human land-use activities (Huryn et al., 2002; Townsend et al., 2004). Ecological responses of benthic invertebrate communities, in terms of taxon richness and abundance, in streams and rivers reflect land-use and the physicochemical conditions at each study site (Allan, 2004). However, information on freshwater taxa and ecological conditions in the East River is scant and benthic communities in the East River basin are very poorly understood, as is the case for rivers in much of China and elsewhere in monsoonal Asia (Dudgeon, 2003). Such lacking data is a barrier for planning for integrated river basin management strategies and the assessment of river health.
The objectives of this research were to investigate changes in benthic macroinvertebrate assemblage composition along the East River and to explore any relationships with water quality and drainage basin characteristics. We surveyed 11 study sites with different human impacts from upstream tributaries to the lower course of the East River. We used the percentage of total urban and rural areas (cities vs. villages) in a catchment as a representation of the total impervious area (%TIA) within a catchment to indicate land-use disturbance (Morse et al., 2003; Chadwick et al., 2006). We collected environmental data for examining human disturbance impacts on macroinvertebrate communities, and combined sampling of benthic and drifting macroinvertebrates in order to provide comprehensive information on the structure of stream macroinvertebrate communities (Pringle & Ramírez, 1998; Dudgeon, 2006). We hypothesized that land-use disturbance and water quality degradation would reduce the richness and abundance of macroinvertebrates along the East River.
Materials and methods
Study area
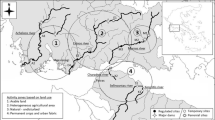
The East River (Dong Jiang, N22°45′–25°20′ and E113°30′–116°45′), the third largest tributary of the Pearl River, is located on the northern margins of the tropics in a region dominated by a monsoonal climate. Its main channel (562 km) drains an area of 35,340 km2. The average annual runoff of the East River is 32.4 billion m3, of which 80% occurs in the wet season and only 20% between October and March (Lee et al., 2007). Annual sediment transport load at Boluo (a gauging station in the lower course: Fig. 1) is 0.11 kg m−3. The headwaters of the East River originate in Jiangxi Province. The length of the upstream section from the headwaters to Longchuan is 138 km with an average slope of 0.22%, the middle section to Boluo is 232 km with a slope of 0.03%, and the lower section to the river mouth has a slope of 0.02% (Fig. 1).
Site descriptions and sampling
Fieldwork was carried out during stable flow conditions immediately prior to the onset of the wet summer monsoon in April 2006. Eleven study sites distributed from upstream tributaries to the lower course: four sites were positioned in the headwaters, four in the middle course, and three in the downstream reach. Site characteristics are listed in Table 1. We obtained land-use data of the East River Basin with 1 km2 spatial resolution (2000) from the Institute of Geographic Sciences and Natural Resources Research, the Chinese Academy of Science (Liu et al., 2003). Land use was generalized into the following categories: woodland, grassland, agriculture, urban, rural, and water bodies.
Land-use disturbance level within a catchment was indicated by the amount of the total impervious area (TIA), which is a percentage of the total urban and rural areas within each catchment within the basin (Walsh et al., 2001, 2005; Roy & Shuster, 2009). Since nutrient inputs to streams and rivers often occur as a consequence of changes in land use, we predicted that in-stream nutrient concentrations should increase with intensifying land use (i.e., %TIA) in the basin.
In-stream habitat variables were measured by visual estimates of the percentage cover of particle classes, which were sand/silt (diameter 0.1–2 mm), gravel (2–16 mm), pebble (16–64 mm), cobble (64–256 mm), and boulder (>256 mm). The substrate types represented at each study site were coded in order of coarseness as follows: 1 = sand/silt; 2 = gravel; 3 = pebble; 4 = cobble; and 5 = boulder. The mean value of the substrate codes (six counts) at each site was used to express substrate coarseness. In situ measurements of water temperature, conductivity, dissolved oxygen, and salinity were carried out using a YSI Model 55 DO meter; pH was measured by Smartest Model pHScan 2 on site. Water samples (collected in 1.2 l polyethylene bottles) were preserved in the field by acidification with concentrated H2SO4 and kept in cool box until they could be refrigerated in the laboratory prior to chemical analysis. Concentrations of NH4, NO2, NO3, PO4, and total suspended solids (TSS) were analyzed according to Standard Methods for the Examination of Water and Wastewater (1999). Concentrations of heavy metals, i.e., vanadium, chromium, copper, zinc, molybdenum, barium, thallium, and lead, were determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
We took kick samples at all sites (50–100 m reach) using D-frame nets (mesh size 500 μm). For each sample, the substrates in a variety of microhabitats were disturbed by kicking for 5 min and all materials were collected in the net. Six kick samples at each site were collected from upper, middle, and downstream reaches (two samples were collected from each reach). Macroinvertebrates visible to the naked eye were hand-picked within 8 h of collection, stored, and preserved with 70% ethanol. Macroinvertebrates were indentified to family level in the laboratory, based on identification keys for Asian stream macroinvertebrates (Dudgeon, 1999). Since species diversity of macroinvertebrates has not been studied thoroughly and the majority of benthic species are undescribed for most rivers in China, taxon identification at family level was used, which can provide a representative and persistent pattern of macroinvertebrate distribution in response to pollution and land-use change (e.g., Bournaud et al., 1996; Metzeling et al., 2002; Dudgeon, 2006; Pond et al., 2008). We rated the relative abundance of each family in each sample according to an abundance index comprising three classes: 0 = not found, 1 = one specimen, and 2 = more than one specimen. The total index score for all families in a sample was used to calculate a relative abundance index (RAI) for that sample, and the sum of the abundance indices of the six samples taken at each site was used to represent the relative abundance at that site (Englund et al., 1997). Such categorization incorporated robust transformation of abundance that down-weighted the effect of single samples with high abundance. Family richness was represented by the total number of families encountered in the six kick samples at each site.
We collected night drift samples at five study sites (Up 1, 4, and Mid 1, 2, 4) for monitoring drift of macroinvertebrates. At each site we collected six drift samples simultaneously using six drift nets (1-m long, mesh size 500 μm, opening size 30 × 40 cm). The drift nets were immersed in the water column in mid-channel for 2 h after dark (19:00–21:00 h). For each drift sample, we measured current velocity in front of the drift net to the nearest 0.01 m s−1 with handheld ADV (SonTek FlowTracker Handheld ADV). The current velocity was used to calculate the volume of water passing through the drift net. The macroinvertebrates in each drift net were preserved in 70% ethanol, and identified to family in the laboratory. In the absence of such data from China, we used published length–mass relationships from North America (Benke et al., 1999) to estimate the biomass of macroinvertebrate families in drift samples.
East river water quality index
Water quality conditions at each study site were assessed using a water quality index (WQI) we developed for the East River (the ERWQI), which transformed a large quantity of complex data into a convenient index (0–100) (see Smith, 1990). Calculation of the index involved two steps. First, the concentration of each parameter is transformed by a rating curve as a score at 0–100% range from low to high water quality for that parameter. Second, the scores of parameters are weighed by different factors whose sum equals one. The index is the sum of the weighted scores of different parameters derived from the following equation (Štambuk-Giljanović, 2003).
where W i is the weighting factor of parameter i, ∑W i = 1 and Q i is the water quality score value of parameters on a continuous scale from 0 to 100. Environments with better water quality have higher WQI scores as follows: Excellent: (WQI Value 90–100); Good: (70–90); Medium: (50–70); Bad: (25–50); and Very bad: (0–25). We calculated of the ERWQI for each site in the East River using the following parameters: dissolved oxygen (DO), pH, conductivity, ammonia plus nitrate (NH4 + NO3), phosphate (PO4), and total suspended solids (TSS). The weighed factors for these parameters were obtained from Štambuk-Giljanović (2003) and Liou et al. (2004): i.e., 0.2 for DO, 0.25 for ammonia plus nitrate, 0.16 for PO4, 0.11 for TSS, 0.11 for pH, and 0.1 for conductivity. As organic nitrogen has been considered to be the major pollutant in the East River Basin (Yuan, 1999), the nitrogen-loading parameter was assigned the highest weighting factor of 0.25.
Data analyses
To explore relationships between land use and both water quality and benthic community data, partial least squares projection to latent structures (PLS) was used to construct a descriptive model (Zhang et al., 1998). PLS analysis can be viewed as a generalized multiple regression that copes with colinearity, thus adequately taking into account the auto-correlations among the physicochemical variables for finding the linear or polynomial relations between a set of predictor variables and a set of response variables (Eriksson et al., 1999). The PLS method has advantages in analyzing a large array of environmental, non-independent variables collected from limited study sites and describing response variables (biological response variables) simultaneously. These data conditions are not suitable for classical regression approaches (Carrascal et al., 2009). The values of VIP (variable importance in the projection) reflect the importance of a given variable in the model with respect to both the benthic communities and the environmental variables. Eriksson et al. (1999) suggested that the variables with a VIP value >0.7 represent important predictors. PLS analyses were carried out using software SIMCA-P (version 11.0, Umetri AB, Umeå, Sweden).
Additionally, we used principal components analysis (PCA) to summarize environmental physical, chemical variables and habitat characteristics. By reducing the multidimensional data set with multiple variables to a few uncorrelated composite variables, PCA provided a visual diagram of similarities and dissimilarities among stream sites.
Analysis of variance (ANOVA) was used to explore differences in benthic community summary variables between upper, middle and lower courses of the East River. Differences in the total RAI of macroinvertebrates at each site (accumulated abundance index from six samples at a site) and total family richness (total number of present families at a site) were analyzed between the three site groups by using study sites as the relevant unit of replication (within upper, middle, and lower sections of the river course). ANOVAs and regression analyses were performed using SPSS version 15. Where necessary, data were logarithmically transformed to improve normality prior to analysis.
Multi-response permutation procedures (MRPP) provide non-parametric test statistics to analyze multivariate data in order to detect significant differences between two or more groups of sampling units. MRPP are based on the weighted averages of symmetric distance functions over all possible pairwise combinations among site groups identified by PCA. We used MRPP to test the null hypothesis of no difference in macroinvertebrate community structure among those site groups (up-, mid-, and lower-section). As a non-parametric procedure, MRPP based on Sorensen’s (Bray-Curtis) coefficient of computed inter-sample compositional dissimilarities and produced statistic A [A = 1 − (observed difference/expected difference)], which describes observed within-group homogeneity, compared to that randomly expected (McCune & Grace, 2002). If all community assemblages are identical within defined groups (within-group homogeneity), A = 1, which is the maximum value. If within-group heterogeneity equals to that expected by chance, A = 0; and if within-group heterogeneity is more than that expected by chance, A < 0. MRPP was based on percentage data of RAI and presence/absence data of macroinvertebrate families at each site. MRPPs were run using PC-ORD ver. 5.0 (McCune & Mefford, 2002).
Results
Land use and water quality
Land use varied from the headwaters to the lower course and included woodland, farm land, and urban areas (Table 2). Study sites with high proportion of woodland cover had relatively low farmland and low levels of urbanization, but urban area became important in the catchment around the most downstream sites. TIA varied from 1.3 to 28.0%. Average TIA of the studies catchments were 1.6 ± 0.1% (1 SE) for up-stream sites, 6.0 ± 1.6% for mid-section sites, and 15.1 ± 7.4% for low-section sites.
There was high variability in physicochemical parameters, including water quality, among sites (Table 3). Upstream sites were slightly acidic, and had high DO and low conductivity. Middle-course sites had higher TSS, which reflected intensive in-channel disturbance by sand dredging (Table 3; Fig. 2A; Appendix 2A in supplementary material). Low-course sites were characterized by farmland and urban land (Table 2; Appendix 2B in supplementary material). Nitrate concentrations varied from undetectable (<0.001 mg l−1) to 1.217 mg l−1 among all sites. ANOVA revealed significant differences among upper-, middle-, and lower-course in TSS (F 2,8 = 5.91, P < 0.05) and NO2 (F 2,8 = 9.49, P < 0.01) with higher concentrations in the middle course (Fig. 2A). There were no significant differences among three site groups in NH4 and NO3 (all P > 0.05) (Table 3). Total N was low in upstream site groups (0.49 ± 0.21 mg l−1), and high in mid-section (1.15 ± 0.10 mg l−1) and low-sections (1.04 ± 0.22 mg l−1), with marginal significant difference among three site groups (ANOVA, F 2,8 = 4.47, P = 0.05).
East River Water Quality Index scores indicated that upstream sites in general had better water quality. The site UP3 had the highest ERWQI (98). The four middle-section sites had ERWQI from 65 to 50, with a trend of deterioration when moving downstream from site to site. LOW3 had the highest NH4 and PO4 concentrations and the lowest DO, and thus the ERWQI was the lowest among the 11 sites (Table 2). These results reflect impacts of catchment land-use patterns. There was a significant negative relationship between %TIA and ERWQI across study sites (Fig. 2B). The sum of %TIA and Farmland (%) also was also negatively related to ERWQI (Simple linear regression: abundance, R 2 = 0.47, F 1,10 = 8.08, P < 0.05). %TIA was significantly negatively related to DO (R 2 = 0.69, F 1,10 = 20.16, P < 0.01) and substrate particle size (R 2 = 0.51, F 1,10 = 9.19, P < 0.05) (Fig. 3), but positively related to conductivity (R 2 = 0.49, F 1,10 = 8.55, P < 0.05), NH4 (R 2 = 0.77, F 1,10 = 30.00, P < 0.001), and PO4 (R 2 = 0.46, F 1,10 = 7.64, P < 0.05) (Table 3). Total nitrogen also had a significant positive relationship with %TIA (R 2 = 0.52, F 1,10 = 9.73, P < 0.05), suggesting high nutrient inputs from urban areas.
PCA of 19 environmental variables generated 3 components with eigenvalues larger than 1.0 and that collectively explained 76.6% of variation in the original variables. Principal component (PC) 1 explained 48% of variance was influenced by TIA and ERWQI, and the additional 17% of variance was explained by the second component that was also influenced by water quality. Ordination of PC 1 and 2 on habitat variables separated the 11 sites into 3 distinct groups: (1) upper, (2) middle, and (3) lower course sites (Fig. 4).
Differences in benthic community taxonomic composition
A total of 41 benthic macroinvertebrate families were collected from the 11 study sites. In general, communities were dominated by Diptera (22% of total family richness), Coleoptera (20%), and Ephemeroptera (17%), with Odonata (12%) and Trichoptera (10%) ranking fourth and fifth, respectively. The range of average family richness in six kick samples at each site was between two and 25 families per sample across 11 sites. The range of average RAI in the six kick samples at each site was two and 44 per sample. At upstream sites, 34 insect families were found; 26 families were collected from the middle-course sites and 20 from the lower-course sites. Family richness in all sites except LOW3 was dominated by Diptera (mean = 25% of all families present; range 4–48%), Ephemeroptera (16%; 4–27%), and Coleoptera (11%; 7–17%; Fig. 5; Appendix 1 in supplementary material). Total RAI of benthic macroinvertebrates in all 11 sites was dominated by Diptera, including Chironominae (7.1% of total for all taxa from 11 sites), Orthocladiinae (4.9%), Tipulidae (3.2%), Tanypodinae (2.2%), Simuliidae (1.6%), and Stratiomyidae (1.1%). Coleoptera mainly comprised Elmidae (4.3%) and Psephenidae (2.3%). At LOW3, which was highly disturbed, the entire assemblage was made up of non-insect groups, including corbiculid bivalves, decapod shrimps (Palaemonidae), and water mites (Hydrachnidiae) (Fig. 5).
Modeling by partial least squares projection to latent structures (PLS) showed that benthic diversity was strongly influenced by drainage basin disturbance (Fig. 3). In the PLS analysis of family richness data, %TIA, farmland, NO2, NH4, conductivity, PO4, NO3, TSS, salinity, chromium, and vanadium was negatively associated with total family richness along PLS component 1 that explained 41.9% of variance. Substrate coarseness, longitude, woodland, ERWQI, and DO were positive associated with total family richness along component 1 (Fig. 3; Table 4A). The PLS analysis of total RAI data for macroinvertebrates showed a similar pattern of association of environmental variables with PLS component 1 (Table 4B).
Macroinvertebrate community structure differed significantly between upstream, middle- and lower-course sites (ANOVA, mean family richness per sample: F 2,8 = 5.66, P < 0.05, U > M = D; total RAI: F 2,8 = 7.47, P < 0.05T, U > M = D, Fig. 6). The highest benthic diversity (total family richness and total RAI at a site) was seen at two relatively pristine upstream sites (UP3 and 4), while diversity was least at LOW3 which had the lowest ERWQI, high turbidity, and silt substrate. UP3 had the highest ERWQI (98), the highest family richness per site (35) and the highest total RAI (263); vice versa, the low-section site 3 had the lowest numbers in ERWQI (39), family richness (7), and total RAI (12) (Fig. 7). The ERWQI was significantly positively related to total family richness at each site (R 2 = 0.59, F 1,10 = 13.30, P < 0.01). Total RAI per site increased with ERWQI (R 2 = 0.70, F 1,10 = 20.53, P < 0.01).
The MRPP test showed highly significant among-group differences in the family composition of different macroinvertebrate orders (A = 0.125, P < 0.05), which confirmed that macroinvertebrate community composition of the three site groups was distinctly different (Fig. 5). MRPP tests also indicated significant differences in RIA among site groups (A = 0.148, P < 0.05).
Differences in drift macroinvertebrate composition
A total of 37 benthic macroinvertebrate families were collected by drift nets from the 5 sites (UP1, 4, MID1, 2, 4). Dominant taxa in total abundance in drift samples from all sites were planktonic Copepoda (53.9%), plus benthic Elmidae (11.2%, Coleoptera), Baetidae (6.3%, Ephemeroptera), Caenidae (4.1%, Ephemeroptera), Gomphidae (3.2%, Odonata), Simuliidae (2.9%, Diptera) and Leptophlebiidae (2.8%, Ephemeroptera). Dominant taxa (in terms of total dry mass in all samples combined) were Gomphidae (63.5%), Elmidae (17.0%), Leptophlebiidae (5.7%), Hydropsychidae (3.3%, Trichoptera), Baetidae (2.1%), and Copepoda (0.5%).
The nighttime average drift rates (density and biomass) were 15.7 individuals 100 m−3 and 10.7 mg 100 m−3 at UP1 and 7.6 individuals 100 m−3 and 13.5 mg 100 m−3 at UP4. They were 26.4 individuals 100 m−3 and 0.8 mg 100 m−3 at MID1, 324.7 individuals 100 m−3 and 48.8 mg 100 m−3 at MID2, and 13.7 individuals 100 m−3 and 80.2 mg 100 m−3 at MID4. The MRPP test indicated significant differences between upper and middle-course sites with respect to drifting family number among different orders (A = 0.1, P < 0.001). The family composition of drift samples was also distinctly different between site groups (Fig. 8), but MRPP tests failed to find any difference in biomass of any orders between site groups (A = −0.23, P = 1.0).
Mean drift abundance (individuals m−3) and total family richness in drift samples at each site were positively related to %TIA (the range of %TIA at five study sites: 1.0–8.5%) (abundance, R 2 = 0.91, F 1,4 = 31.81, P < 0.05; richness, R 2 = 0.84, F 1,4 = 16.25, P < 0.05), but this was not evident for biomass data (%TIA-biomass, R 2 = 0.08, F 1,4 = 0.25, P = 0.64). The abundance, total family richness, and biomass of drifting macroinvertebrates at each site were not significantly related to ERWQI (R 2 ≤ 0.69, P ≥ 0.08). Drift biomass (but not abundance or family richness) showed a negative relationship with DO (R 2 = 0.81, F 1,4 = 12.46, P < 0.05), and positive relationships with TSS (R 2 = 0.88, F 1,4 = 21.55, P < 0.05), vanadium (R 2 = 0.92, F 1,4 = 35.93, P < 0.01), and chromium (R 2 = 0.89, F 1,4 = 23.96, P < 0.05).
Discussion
Land use and water quality are closely related (Zampella et al., 2007; Broussard & Turner, 2009), and land use disturbance in terrestrial ecosystems impacts local habitat features and biological diversity in the rivers and streams that drain them (Allan, 2004; Dudgeon et al., 2006). Agriculture, urban development, and industrial activities are associated with drainage basin degradation (Foley et al., 2005), and increasing nitrate loads (Peierls et al., 1991), as well as sedimentation and other sources of pollution, which combine to impact ecological integrity by lowering water quality, degrading natural habitat, and reducing biodiversity (Allan et al., 1997; Sponseller et al., 2001). The results of this study lend weight to this conclusion since they demonstrate that land-use and water quality degradation reduced the diversity of benthic macroinvertebrates in the East River. Nutrient concentrations and fine sediments increased with greater land-use disturbance, and the family richness and abundance of benthic macroinvertebrate assemblages declined from up- to downstream in association with an increase in TIA.
There has been a growing interest in advancing knowledge on landscape effects of land use on community structure and river ecosystem processes (Allan, 2004; Raymond et al., 2008). Experimental manipulations at large spatial scales have some constraints (Carpenter et al., 1995), since they are often too expensive and difficult to implement. Accordingly, correlations of environmental variables with biological responses have been considered as a practicable alternative approach to large-scale ecosystem manipulative investigations of the consequences of land-use change (e.g., Johnson & Gage, 1997; Davies & Jackson, 2006). By taking advantage of comparing relatively unimpaired natural systems (the putative reference condition) and human-disturbed systems, the impacts of human land-use disturbance on streams can be detected and potential mechanisms to link landscape processes to local patterns can be identified (Malmqvist, 2002; Zhang et al., 2009), and the results used to inform management at the landscape level (Stoddard et al., 2006).
This study revealed a negative relationship between TIA and ERWQI: ERWQI was positively related to family richness and total RAI of macroinvertebrates, while TIA had a negative relationship with these community parameters, and hence both were good indicators of ecological responses of macroinvertebrates to human disturbances. Our results also show that sediment particle size was influenced by land use practices by way of an inverse relation to TIA; and TSS and nutrients were positively related to % agriculture land within each catchment, which suggest geomorphic change related to land use disturbance (Nilsson et al., 2003). Land-use disturbance is frequently associated with hydrological change, in terms of altered flood regimes, and increased sediment input to the channel, arising from such land-use practices as agriculture, forest logging, road construction, and conversion for urban development (Waters, 1995; Foster et al., 2003). We did not find a significant relationship between %TIA and TSS perhaps because the effect of land-use disturbance on TSS may be masked by instream disturbance events at a local scale. For instance, sand dredging for construction was widespread in the middle sections of the East River causing elevated TSS concentrations at MID2, 3, and 4 (Fig. 2A; Appendix 2A in supplementary material).
Land-use disturbance associated with urban development and agriculture activity caused water quality deterioration in the lower course of the East River (Table 2; Appendix 2B in supplementary material), resulting in decreased aquatic biodiversity. This pattern accords with previous studies demonstrating that urbanization and agriculture lead to increased nutrient loadings (Paul & Meyer, 2001; Turner & Rabalais, 2003). Higher inputs of phosphorous and nitrogen are related to wastewater from urban areas and fertilizers from farmland catchments (Osborne & Wiley, 1988; Broussard & Turner, 2009), which impact steam benthic macroinvertebrates (Townsend et al., 2008). Ecosystem degradation of streams and rivers draining urbanized landscapes gives rise to urban stream syndrome, which describes flashier hydrographs, elevated nutrient loads and contaminant concentrations, altered stream morphology, and reduced biodiversity (Meyer et al., 2005; Walsh et al., 2005). Interestingly, this is rather similar to the overall pattern of degradation we noted in the East River, suggesting that the phenomenon of urbanization has a globally coherent “fingerprint” and patterns of degradation associated with such land-use change will be easily recognizable regardless of geographic location.
PLS analysis revealed significant relationships among land use disturbance, water quality and benthic family richness and relative abundance. Benthic diversity was strongly negatively influenced by increased TIA and deteriorating water quality. Given the wide range of spatial and physicochemical variables (19 parameters, Fig. 3), PLS models showed the importance of geographical (longitude), catchment, water quality, and habitat variables (sediment particle size) in explaining among-site differences in benthic family richness and relative abundance. PLS is an alternative to current regression methods used in environmental science and ecology, which can analyze a large array of related predictor variables when the number of environmental variables is higher than the number of observations, and predictor variables are not truly independent. Along with previous works (Englund et al., 1997; Zhang et al., 1998), the present study indicated that PLS is a powerful method for examining human impacts on running water ecosystems through modeling, monitoring, and prediction (see also Zhang et al., 2009).
There was surprising positive relationship between TIA and drift family richness, and this was the converse of the effects of TIA on benthic diversity in the East River. Such positive relationship of TIA and drift richness may be limited within a relatively narrow disturbance range (at five sites for drift samples collected, TIA: 1.0–8.5%), but may not occur in a wide disturbance range, such as TIA up to 28%. Land-use effects on drift have been little studied and information on how land-use disturbance within catchment influences macroinvertebrate drift is limited (Edwards & Huryn, 1996; Collier & Quinn, 2003). Catastrophic or passive drift of macroinvertebrates is often related to changes in physicochemical characteristics (O’Hop & Wallace, 1983), pollution (Lauridsen & Friberg, 2005) and disturbance (Malmqvist & Sjöström, 1987), while sediment inputs and bed movement have been shown to increase drift (Walton, 1978; Culp et al., 1986). Our results showed that the drift biomass was negatively related to DO and positively related to TSS, vanadium, and chromium, suggesting that low water quality increased the drift of benthic insects that made up 90% of total drift biomass. High TSS reflected the high intensity of sand dredging in the middle section of the East River. Drift composition can offer a useful indication of human disturbance and land-use alteration on stream and river ecosystems (e.g., Pringle & Ramírez, 1998; Dudgeon, 2006), but our results show that the direction of change (increase or decrease in diversity of drifting macroinvertebrates) might not be that expected a priori. Comparison of drift assemblages at impacted and reference sites will be essential to the more widespread adoption of drift sampling as an indicator of disturbance or in-stream degradation.
This study is the first for the East River in southern China to illustrate the impacts of human disturbances from upstream tributaries to the lower course on benthic macroinvertebrate assemblages. Changing landscape cover, especially urbanization and agriculture, degraded river ecosystem integrity and reduced aquatic biodiversity. We detected a significant negative relationship between percentage of catchment disturbance and water quality (as captured in a system-specific index, the ERWQI), suggesting that urbanization (as %TIA) can be a useful predictor of water quality and river health since TIA and ERWQI were both related to benthic community richness and abundance. Thus our study accords with the view that land-use change, water quality, and the condition of physical habitats and biological communities are highly intercorrelated. Our results also show that family-level data sets can be used for detecting relationships between environmental variables and benthic community assemblages (see also Bournaud et al., 1996; Bowman & Bailey, 1998; Dudgeon, 2006). This approach, together with the statistical tools used by this study (e.g., PLS analysis), can provide aquatic ecologists and environmental managers with a means of diagnosing of the causes of ecosystem impairment.
References
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology and Systematics 35: 257–284.
Allan, J. D. & A. S. Flecker, 1993. Biodiversity conservation in running waters. BioScience 43: 32–43.
Allan, J. D., D. L. Erickson & J. Fay, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology 37: 149–161.
Benke, A. C., A. D. Huryn, L. A. Smock & B. J. Wallace, 1999. Length–mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 18: 308–343.
Bournaud, M., B. Cellot, P. Richoux & A. Berrahou, 1996. Macroinvertebrate community structure and environmental characteristics along a large river: congruity of patterns for identification to species or family. Journal of the North American Benthological Society 15: 232–253.
Bowman, M. F. & R. C. Bailey, 1998. Does taxonomic resolution affect the multivariate description of the structure of freshwater benthic macroinvertebrate communities? Canadian Journal of Fisheries and Aquatic Sciences 54: 1802–1807.
Broussard, W. & R. E. Turner, 2009. A century of changing land-use and water-quality relationships in the continental US. Frontiers in Ecology and the Environment 7: 302–307.
Carpenter, S. R., S. W. Chisholm, C. J. Krebs, D. W. Schindler & R. F. Wright, 1995. Ecosystem experiments. Science 269: 324–327.
Carrascal, L. M., I. Galván & O. Gordo, 2009. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118: 681–690.
Chadwick, M. A., D. R. Dobberfuhl, A. C. Benke, A. D. Huryn, K. Suberkropp & J. E. Thiele, 2006. Urbanization affects stream ecosystem function by altering hydrology, chemistry, and biotic richness. Ecological Applications 16: 1796–1807.
Collier, K. J. & J. M. Quinn, 2003. Land-use influences macroinvertebrate community response following a pulse disturbance. Freshwater Biology 48: 1462–1481.
Culp, J. M., F. J. Wrona & R. W. Davies, 1986. Response of stream benthos and drift to fine sediment deposition versus transport. Canadian Journal of Zoology 64: 1345–1351.
Davies, S. P. & S. K. Jackson, 2006. The biological condition gradient: a descriptive model for interpreting change in aquatic ecosystems. Ecological Applications 16: 1251–1266.
Dudgeon, D., 1992. Endangered ecosystems: a review of the conservation status of tropical Asian rivers. Hydrobiologia 248: 167–191.
Dudgeon, D., 1999. The future now: prospects for the conservation of riverine biodiversity in Asia. Aquatic Conservation: Marine and Freshwater Ecosystems 9: 497–501.
Dudgeon, D., 2000. The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annual Review of Ecology & Systematics 31: 239–263.
Dudgeon, D., 2003. The contribution of scientific information to the conservation and management of freshwater biodiversity in tropical Asia. Hydrobiologia 500: 295–314.
Dudgeon, D., 2006. The impacts of human disturbance on stream benthic invertebrates and their drift in North Sulawesi, Indonesia. Freshwater Biology 51: 1710–1729.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. Kawabata, D. Knowler, C. Lévêque, R. J. Naiman, A. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Edwards, E. D. & A. D. Huryn, 1996. Effect of riparian land use on contributions of terrestrial invertebrates to streams. Hydrobiologia 337: 151–159.
Englund, G., B. Malmqvist & Y. X. Zhang, 1997. Using predictive models to estimate effects of flow regulation on net-spinning caddis larvae in North Swedish rivers. Freshwater Biology 37: 687–697.
Eriksson, L., E. Johansson, N. Kettaneh-Wold & S. Wold, 1999. Introduction to Multi- and Megavariate Data Analysis Using Projection Methods (PCA and PLS). Umetrics, Umeå, Sweden.
Escobar, P., 2005. Guangdong, the unstoppable ‘world’s factory’. Asia Times Online Ltd. http://www.atimes.com/atimes/China/GA25Ad05.html.
Foley, J. A., R. DeFries, G. P. Asner, C. Barford, G. Bonan, S. R. Carpenter, F. S. Chapin, M. T. Coe, G. C. Daily, H. K. Gibbs, J. H. Helkowski, T. Holloway, E. A. Howard, C. J. Kucharik, C. Monfreda, J. A. Patz, I. C. Prentice, N. Ramankutty & P. K. Snyder, 2005. Global consequences of land use. Science 309: 570–574.
Foster, D., F. Swanson, J. Aber, I. Burke, N. Brokaw, D. Tilman & A. Knapp, 2003. The importance of land-use legacies to ecology and conservation. BioScience 53: 77–88.
Fu, B. J., 2008. Blue skies for China. Science 321: 611.
Harding, J. S., E. F. Benfield, P. V. Bolstad, G. S. Helfman & E. B. D. Jones III, 1998. Stream biodiversity: the ghost of land use past. Proceedings of the National Academy of Sciences (USA) 95: 14843–14847.
Huryn, A. D., V. M. B. Huryn, C. J. Arbuckle & L. Tsomides, 2002. Catchment land-use, macroinvertebrates and detritus processing in headwater streams: taxonomic richness versus function. Freshwater Biology 47: 401–415.
Johnson, L. B. & S. H. Gage, 1997. Landscape approaches to the analysis of aquatic ecosystems. Freshwater Biology 37: 113–132.
Lauridsen, R. B. & N. Friberg, 2005. Stream macroinvertebrate drift response to pulsed exposure of the synthetic pyrethroid lambda-cyhalothrin. Environmental Toxicology 20: 513–521.
Lee, J. H. W., Z. Y. Wang, W. Thoe & D. S. Cheng, 2007. Integrated physical and ecological management of the East River. Water Science & Technology: Water Supply 7: 81–91.
Liou, S. M., S. L. Lo & S. H. Wang, 2004. A generalized water quality index for Taiwan. Environmental Monitoring and Assessment 96: 35–52.
Liu, J. G. & J. Diamond, 2005. China’s environment in a globalizing world – how China and the rest of the world affect each other. Nature 435: 1179–1186.
Liu, J. Y., D. F. Zhuang, D. Luo & X. Xiao, 2003. Land-cover classification of China: integrated analysis of AVHRR imagery and geophysical data. International Journal of Remote Sensing 24: 2485–2500.
Malmqvist, B., 2002. Aquatic invertebrates in riverine landscapes. Freshwater Biology 47: 679–694.
Malmqvist, B. & S. D. Rundle, 2002. Threats to the running water ecosystems of the world. Environmental Conservation 29: 134–153.
Malmqvist, B. & P. Sjöström, 1987. Stream drift as a consequence of disturbance by invertebrate predators: field and laboratory experiments. Oecologia 74: 396–403.
McCune, B. & J. B. Grace (with D. L. Urban), 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, OR.
McCune, B. & M. J. Mefford, 2002. PC-ORD. Multivariate Analysis of Ecological Data, Version 5.0. MJM Software Design, Gleneden Beach, OR.
Metzeling, L., D. Robinson, S. Perriss & R. Marchant, 2002. Temporal persistence of benthic invertebrate communities in south-eastern Australian streams: taxonomic resolution and implications for the use of predictive models. Marine and Freshwater Research 53: 1223–1234.
Meyer, J. L., M. J. Paul & W. K. Taulbee, 2005. Stream ecosystem function in urbanizing landscapes. Journal of the North American Benthological Society 24: 602–612.
Morse, C. C., A. D. Huryn & C. Cronan, 2003. Impervious surface area as a predictor of the effects of urbanization on stream insect communities in Maine, USA. Environmental Monitoring and Assessment 89: 95–127.
Nilsson, C., J. E. Pizzuto, G. E. Moglen, M. A. Palmer, E. H. Stanley, N. E. Bockstael & L. C. Thompson, 2003. Ecological forecasting and the urbanization of stream ecosystems: Challenges for economists, hydrologists, geomorphologists and ecologists. Ecosystems 6: 659–674.
O’Hop, J. & J. B. Wallace, 1983. Invertebrate drift, discharge, and sediment relations in a southern Appalachian headwater stream. Hydrobiologia 98: 72–84.
Osborne, L. L. & M. J. Wiley, 1988. Empirical relationships between land use/cover and stream water quality in an agricultural watershed. Journal of Environmental Management 26: 9–27.
Paul, M. J. & J. L. Meyer, 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32: 333–365.
Peierls, B. L., N. F. Caraco, M. L. Pace & J. J. Cole, 1991. Human influence on river nitrogen. Nature 350: 386–387.
Pond, G. J., M. E. Passmore, F. A. Borsuk, L. Reynolds & C. J. Rose, 2008. Downstream effects of mountaintop coal mining: comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. Journal of the North American Benthological Society 27: 717–737.
Pringle, C. M. & A. Ramírez, 1998. Use of both benthic and drift sampling techniques to assess tropical stream invertebrate communities along an altitudinal gradient, Costa Rica. Freshwater Biology 39: 359–375.
Raymond, P. A., N. H. Oh, R. E. Turner & W. Broussard, 2008. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature 451: 449–452.
Roy, A. H. & W. D. Shuster, 2009. Assessing impervious surface connectivity and applications for watershed management. Journal of the American Water Resources Association 45: 198–209.
Smith, D. G., 1990. A better water quality indexing system for rivers and streams. Water Research 24: 1237–1244.
Sponseller, R. A., E. F. Benfield & H. M. Valett, 2001. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshwater Biology 46: 1409–1424.
Štambuk-Giljanović, N., 2003. Comparison of Dalmatian water evaluation indices. Water Environmental Research 75: 388–405.
Standard Methods for the Examination of Water and Wastewater, 1999. 20th edition. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC, USA.
Stoddard, J. L., D. P. Larsen, C. P. Hawkins, R. K. Johnson & R. H. Norris, 2006. Setting expectations for the ecological condition of streams: the concept of reference condition. Ecological Applications 16: 1267–1276.
Townsend, C. R., B. J. Downes, K. Peacock & C. J. Arbuckle, 2004. Scale and the detection of land-use effects on morphology, vegetation and macroinvertebrate communities of grassland streams. Freshwater Biology 49: 448–462.
Townsend, C. R., S. S. Uhlmann & C. D. Matthaei, 2008. Individual and combined responses of stream ecosystems to multiple stressors. Journal of Applied Ecology 45: 1810–1819.
Turner, R. E. & N. N. Rabalais, 2003. Linking landscape and water quality in the Mississippi river basin for 200 years. Bioscience 52: 563–572.
Walsh, C. J., A. K. Sharpe, P. F. Breen & J. A. Sonneman, 2001. Effects of urbanization on streams of the Melbourne region, Victoria, Australia. I. Benthic macroinvertebrate communities. Freshwater Biology 46: 535–551.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottingham, P. M. Groffman & R. P. Morgan, 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723.
Walton, O. E., 1978. Substrate attachment by drifting aquatic insect larvae. Ecology 59: 1023–1030.
Water Supplies Department, 2005. The Hong Kong Special Administrative Region (HKSAR) of the People’s Republic of China, Water Supplies Department Annual Report 2004–2005.
Waters, T. F., 1995. Sediment in Streams: Sources, Biological Effects and Control. American Fisheries Society, Bethesda, MD.
Yuan, G. S., 1999. A counter measures research on the water pollution and aquatic environmental protection in Dongjiang Basin (in Chinese). Journal of Huizhou University (Natural Science) 19: 101–104.
Zampella, R. A., N. A. Procopio, R. G. Lathrop & C. L. Dow, 2007. Relationship of land-use/land-cover patterns and surface-water quality in the Mullica River basin. Journal of the American Water Resources Association 43: 594–604.
Zhang, Y. X., B. Malmqvist & G. Englund, 1998. Ecological processes affecting community structure of blackfly larvae in regulated and unregulated rivers: a regional study. Journal of Applied Ecology 35: 673–686.
Zhang, Y. X., J. S. Richardson & X. Pinto, 2009. Catchment-scale effects of forestry practices on benthic invertebrate communities in Pacific coastal streams. Journal of Applied Ecology 46: 1292–1303.
Acknowledgments
This study was supported by a National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) joint research project (50838003 and HKU747/03). We thank the assistance of Lily Chiu Yee Ng, Xuehua Duan, Huaixiang Liu, Fu-Yee Wong, Man-Yee Lam, and many others in the field and in the laboratory. We also deeply appreciate the support of the Pearl River Water Resources Commission during field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: N. R. Bond
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Dudgeon, D., Cheng, D. et al. Impacts of land use and water quality on macroinvertebrate communities in the Pearl River drainage basin, China. Hydrobiologia 652, 71–88 (2010). https://doi.org/10.1007/s10750-010-0320-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0320-x