Abstract
The rotifer Brachionus plicatilis shows a typical sigmoid growth curve, where calorie restriction (CR) and hypoxia are thought to be introduced at high population density in the stationary phase. CR may induce a shift from aerobic to anaerobic metabolism in this stationary phase, possibly contributing to an increased hypoxia tolerance. This study was undertaken to investigate the effect of CR on hypoxia tolerance at the molecular level. When rotifers were cultured under CR (fed every second day) or fed ad libitum (AL), and subsequently exposed to hypoxia, those in the CR group had a higher survival rate than their AL counterparts. We then cloned cDNAs encoding three glycolytic enzymes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), enolase (ENO), and phosphoglycerate mutase (PGM) and compared their accumulated mRNA levels between CR and AL rotifers at ages of 1–8 days by quantitative real-time PCR. The CR group showed significantly higher mRNA levels of GAPDH and ENO than their AL counterparts. Furthermore, rotifers in the stationary phase showed higher mRNA levels of these enzymes than those in the exponential growth phase. These results suggest that CR induces anaerobic metabolism, which possibly contributes to population stability under hypoxia in the stationary phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rotifer Brachionus plicatilis, a zooplankton species of approximately 0.3 mm inhabiting brackish water, is highly appreciated as a food organism for fish larvae in aquaculture. Several advantages of this species as a food organism, such as high population growth rate and simple culture procedure, have made the rotifer also useful as a laboratory model organism. In addition, their asexual reproduction produces a genetically identical population which decreases the individual variation in experimental data. Thus, B. plicatilis has been used in various research fields such as aging, ecotoxicology and population dynamics (Enesco, 1993; Yoshinaga et al., 2001, 2003; Marcial et al., 2005). Moreover, recent expressed sequence tag (EST) analyses have provided a catalog of highly expressed transcripts, enabling us to investigate B. plicatilis by a molecular biological approach (Suga et al., 2007; Denekamp et al., 2009).
The population dynamics of B. plicatilis is strongly affected by food availability. Each individual that takes enough food produces 3–5 eggs daily at an early period of batch culture, resulting in period of rapid population growth known as the exponential growth phase (Yoshinaga et al., 2001). When the population density reaches a high level, available food resources become limited. Under such conditions, B. plicatilis suppresses reproduction and in turn increases lifespan, leading the population to be stably maintained, a period known as the stationary phase. Such lifespan extension by food shortage is usually associated with enhanced stress resistance, and is considered as a strategy to cope with adverse environments (Yu & Chung, 2001). Although little is known about the effect of food shortage on stress resistance in B. plicatilis, up-regulation of MnSOD, an anti-oxidant enzyme, has been reported (Kaneko et al., 2005).
Hypoxia is an environmental stress typically observed with rotifers under artificial and natural conditions. It is also known to affect population dynamics in high density mass cultures. For example, enough oxygen supply (>2 mg/ml) is necessary to obtain high density cultures in the mass production of the related rotifer B. calyciflorus (Park et al., 2001). Various rotifer species are able to inhabit hypoxic environments in nature (Miracle & Vicente, 1983; Kizito & Nauwerck, 1995). Therefore, it is assumed that rotifers possess the ability to resist against hypoxic stresses.
In this study, we investigated the effect of food shortage on hypoxia tolerance. The shortage in food availability is often mimicked by calorie restriction (CR) in laboratories, which is known to increase viability in wide variety of species. We therefore exposed CR rotifers to hypoxia and analyzed their survival. The accumulated mRNA levels of glycolytic enzymes were also determined in CR rotifers, as well as in the population at the stationary phase in relation to the hypoxia tolerance.
Materials and methods
Rotifers and culture conditions
We used the rotifer B. plicatilis Ishikawa strain originally collected from a Japanese eel culture pond several decades ago (Yoshinaga et al., 2004), which is reported to lack sexual reproduction for more than a decade (Yoshinaga et al., 1999). This strain was originally provided by Professor A. Hagiwara and has been maintained at least 5 years in our laboratory only by parthenogenesis, under conditions described below. Rotifers were cultured in 0.5× sterilized Brujewicz artificial seawater (16.5 ppt salinity, 227 mM NaCl, 5 mM KCl, 5 mM CaCl2, 13.5 mM MgSO4, 13 mM MgCl2, 1.2 mM NaHCO3, 0.4 mM NaBr) (culture media) at 25°C under total darkness except during observations. Feeding was conducted by suspending the microalgae Nannochloropsis oculata (Nikkai Center, Tokyo, Japan) in the culture media. The same production lot of the algae was used throughout the experiments. The algae were rinsed twice with the culture media and kept at 4°C until feeding to the rotifers.
Rotifers bearing 2 eggs were randomly selected from a population in the exponential growth phase. The first neonates hatched from these individuals were transferred to newly prepared culture media and grown with continuous feeding. This step was repeated twice to standardize the age and environmental conditions for the testing animals, which may otherwise affect their metabolism and gene expression, and the third generation rotifers were used for the experiments described below.
For the hypoxia treatment, a series of samples containing 30 neonates from one cohort were prepared and cultured in 3 ml of the culture media using 6-well culture plates (Nunc, Rochester, NY, USA) under either ad libitum (AL) or CR feeding conditions. The AL group was daily transferred to a newly prepared culture media containing microalgae, and the CR group was alternately placed in a culture media with and without the microalgae (Fig. 1A). The concentration of the microalgae in case of feeding was approximately 7 × 106 cells/ml for both AL and CR groups. For the AL group, two sets each containing three batches were fed every day; one set was reared for 3 days and the other for 4 days. For the CR group, two sets each containing three batches were fed every 2 days with two rearing periods as the case for the AL group. Neonates produced during the culture were removed everyday. Thirty individuals each from the three batches in AL and CR groups were collected on day 3 from one set and day 4 from the other set and exposed to hypoxia (less than 0.1% oxygen in the container) using an anaerobic culture kit (Cosmo Bio, Tokyo, Japan). Briefly, rotifers were placed in a 2 cm Petri dish containing 300 μl of the culture media and packed in a container together with an oxygen absorber, a carbon dioxide generating agent and an oxygen indicator provided in the kit. Oxygen level was determined as less than 0.1% based on the color alteration of the oxygen indicator (which changes into pink under oxygen concentration less than 0.1%) and this was confirmed approximately 3 h after packing. Rotifers were kept in the container for 11 and 7.5 h for day 3- and day 4-set, respectively. These time points were determined based on the reduction in the movement of rotifers in the container. The survival rate was calculated after the recovery for 16 h under normoxia.
Survival rate after exposure to hypoxia of rotifers fed ad libitum or calorie-restricted. Experimental designs are shown in (A) with arrowheads indicating ages (days) of rotifers subjected to hypoxia. Means ± standard errors are represented (n = 3) for 3-days-old (B) and 4-days-old rotifers (C) and significant differences between the fed ad libitum (AL) and calorie-restricted (CR) groups are shown at the level of P < 0.005 (**) and P < 0.05 (*)
A series of samples containing eight neonates from one cohort were prepared, cultured in 1 ml of the culture media using 12 well culture plates (Nunc) for 1–8 days under AL or CR condition as described above, and subjected to quantitative real-time PCR to determine the accumulated mRNAs encoding glycolytic enzymes (Fig. 2A). Neonates produced during the culture were removed every day. Eight individuals were collected every day from each group (n = 3) prior to the change of the medium, and subjected to quantitative real-time PCR.
The accumulated mRNA levels of glycolytic enzymes in rotifers fed ad libitum or calorie-restricted. Experimental schedules are shown in A with arrowheads indicating the time point for rotifers subjected to analysis of the relative mRNA levels of GAPDH (B), ENO (C) and PGM (D) by quantitative real-time PCR, using the mRNA levels of EF-1α as the control. Means ± standard errors are represented (n = 3) and differences between the fed ad libitum (AL) and calorie-restricted (CR) groups are shown at the level of P < 0.05 (*)
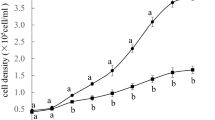
In the last experiment, 100 individuals each bearing 2–3 eggs were randomly collected from the cohort in the exponential growth phase and placed into a beaker containing 100 ml of the culture media. One milliliter of the culture media was taken and numbers of individuals were counted five times on days 4, 6, 8, 10, 12, 14 and one time on the other days to prevent the retardation of the population growth. An equal volume of newly prepared culture media containing approximately 14 × 107 cells/ml algae was added to the beaker after every counting. Approximately 160 and 250 individuals thus randomly collected from the population on day 6 (exponential growth phase) and day 14 (stationary phase) (Fig. 3A) (n = 5), respectively, were subjected to real-time PCR analysis. Days 6 and 14 were selected according to a preliminary culture conducted under similar conditions (data not shown).
The accumulated mRNA levels of glycolytic enzymes in rotifer population at the exponential growth and stationary phases. Sampling schedules are shown in (A) with arrowheads indicating days at which rotifers were collected (day 6 at the exponential growth phase and day 14 at the stationary phase). The relative mRNA levels of GAPDH (B), ENO (C) and PGM (D) were determined by quantitative real-time PCR, using the mRNA levels of EF-1α. Means ± standard errors are represented (n = 5) and significant differences between day 6 and day 14 are shown at the levels of P < 0.05 (*)
Cloning of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), enolase (ENO) and phosphoglycerate mutase (PGM) cDNA
Three hundred milliliter of culture media containing approximately 200 individuals/ml of rotifers was filtered through a 60-μm mesh filter. Total RNA was extracted from the collected individuals with ISOGEN (Nippon Gene, Tokyo, Japan) and subjected to first strand cDNA synthesis using transcriptor reverse transcriptase (Roche, Basel, Switzerland) with either the 3′ rapid amplification of cDNA ends (RACE) adapter primer (Invitrogen, Carlsbad, CA, USA) or the GeneRacer oligo dT primer (Invitrogen) followed by the degradation of RNA templates with RNase H (Invitrogen). First strand cDNA thus obtained was subjected to PCR and 3′ and 5′ RACEs. PCR and RACE products were subcloned into pGEM-T vector (Promega, Madison, WI, USA) and sequenced with an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA). DNA nucleotide sequences of primers used for first strand cDNA synthesis and for PCR and RACE are shown in Table 1.
GAPDH
Primers GAPDH_F1, GAPDH_F2, and GAPDH_R were designed with reference to amino acid sequences of GAPDH from fission yeast Schizosaccharomyces pombe (NP_595236), nematode Caenorhabditis elegans (NP_508535), fruit fly Drosophila melanogaster (Q01597), zebrafish Danio rerio (XP_684297), African clawed frog Xenopus laevis (AAH43972), chicken Gallus gallus (NP_989636), mouse Mus musculus (NP_032110), and human Homo sapiens (NP_002037). The reaction mixture for PCR in 20 μl contained approximately 1 μg of first strand cDNA, 10 pmol of GAPDH_F1 and GAPDH_R primers, 1.6 μl of 2.5 mM dNTP mixture, 2.0 μl of 10× Ex Taq buffer and 0.2 μl of 5 U/μl Ex Taq DNA polymerase (Takara, Otsu, Japan). The reaction mixture was treated at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 51°C for 30 s and extension at 72°C for 30 s. The final extension step was performed at 72°C for 1 min. The PCR products were diluted 10 times with sterilized distilled water and used as a template for nested PCR. Nested PCR was performed by the same method as described above except primer GAPDH_F2 instead of GAPDH_F1. 3′ RACE was performed with primers AUAP and GAPDH_3RACE using 3′ RACE system for Rapid Amplification of cDNAs Ends (Invitrogen). Primer GAPDH_3RACE was constructed referring to the nucleotide sequence determined in the first PCR. PCR was performed under the same conditions as described above except for annealing at 57°C for 30 s. The reaction mixture contained the same constituents as described above except for 5 pmol of primers AUAP and GAPDH_3RACE.
5′ RACE was performed with GeneRacer kit (Invitrogen) using primer GAPDH_5RACE designed from the partial nucleotide sequence determined by the nested PCR. PCR was conducted under the same conditions described in the first PCR except for annealing at 56.5°C for 30 s. The reaction mixture contained the same constituents as in the first PCR except for 5 pmol of primers GAPDH_5RACE and GeneRacer 5′, the later being provided by the kit.
ENO
Primers ENO_5RACE1 and ENO_5RACE2 were designed with reference to the amino acid sequences of ENO from budding yeast Saccharomyces cerevisiae (NP_011770, NP_012044), nematode (NP_001022349), fruit fly (NP_722721), zebrafish (NP_997887, NP_001003848, NP_999888) and mouse (NP_001020559, NP_038537, NP_031959). cDNA fragments containing the 5′ end of rotifer ENO gene were amplified using GeneRacer kit with primers ENO_5RACE1 and ENO_5RACE2. The first PCR was performed under the same conditions as described for GAPDH except for the initial denaturation at 94°C for 7 min, 35 cycles and annealing at 55°C for 30 s. The 20 μl reaction mixtures contained the same constituents as described for GAPDH except for 10 pmol of ENO_5RACE1 and GeneRacer 5′ primers. The PCR product was diluted 10-fold and used for nested PCR which was conducted as described above using primers ENO_5RACE2 and GeneRacer5′_nested with annealing temperature at 50°C.
3′ RACE was performed using GeneRacer kit with gene-specific primers ENO _fl3RACE1 and ENO_fl3RACE2, designed from the partial nucleotide sequence of the rotifer ENO gene determined as above. PCR was performed as described above except annealing at 58°C for 30 s. The reaction mixtures contained the same constituents as described above except primers ENO_fl3RACE1 and GeneRacer 3′. Nested PCR was performed with the PCR product diluted 100-fold with primers ENO fl3RACE2 and GeneRacer 3′_nested.
PGM
Primers PGM_5RACE1 and PGM_5RACE2 were designed with reference to cDNA nucleotide sequences of PGM from budding yeast (X06408), fruit fly (NM_079822), zebrafish (NM_201024), African clawed frog (BC054230), mouse (NM_018870), and human (NM_000290). cDNA fragments containing the 5′ end of the rotifer PGM gene was amplified using GeneRacer kit with primers PGM_5RACE1 and PGM_5RACE2. PCR was performed under the same conditions as described for ENO except for annealing at 58°C for 30 s and extension at 72°C for 1 min. The reaction mixture contained the same constituents as for ENO except for primer PGM_5RACE1. PCR products were diluted 10-fold with sterilized distilled water and used as a template for nested PCR using primer PGM_5RACE2. 3′ RACE was performed using GeneRacer kit with gene-specific primers PGM_fl3RACE1 and PGM_fl3RACE2, designed from the partial nucleotide sequence of rotifer PGM gene determined as above. PCR was performed as described above except for annealing at 56°C for 30 s and extension at 72°C for 30 s. The reaction mixture contained the same constituents as described above except primer PGM_fl_3RACE_1. The PCR product was diluted 100-fold and used for nested PCR with primer PGM_fl3RACE2.
Quantitative real-time PCR
Primers for quantitative real-time PCR were designed with Primer Express ver. 1.0 (Applied Biosystems) using DNA nucleotide sequences determined in the present study (GAPDH, ENO and PGM) or registered in the database [elongation factor (EF)-1α (AB513493)] (Table 2). RNA extraction and first strand cDNA synthesis from rotifer samples were performed as described in the section on “cDNA cloning”. The extracted RNA was treated with DNase I (Takara) prior to first strand cDNA synthesis. Quantitative real-time PCR was carried out with an ABI PRISM 7300 real-time PCR system (Applied Biosystems). The 20 μl reaction mixture contained 1 μl of 50-fold diluted cDNA, 10 pmol of forward and reverse primers, 10 μl of 2× SYBR Premix Ex Taq (Takara), and 0.3 μl of 50× ROX reference dye (Takara). The reaction mixture was treated at 50°C for 2 min and 95°C for 10 min, and the subsequent PCR consisted of 40 cycles of 95°C for 15 s and 65°C for 1 min. The absence of non-specific PCR products was confirmed by running the dissociation protocol (95°C 15 s, 60°C 30 s, and 95°C 15 s). Data were analyzed by the comparative Ct method using the mRNA levels of EF-1α as the internal control.
Statistical analysis
Two-way analysis of variance (ANOVA) was carried out to analyze possible significant effects of age (1–8 days) and feeding conditions (AL or CR) on the mRNA levels. Differences between CR and AL groups were evaluated using a Student’s t test at all ages examined. All statistical analyses were carried out by StatView 5.0 J (SAS Institute Inc., Cary, NC, USA).
Results
Hypoxia treatment
The survival rate of 3-day-old individuals in the CR group was 96% and significantly higher than that of the AL group, 76% (P < 0.005) (Fig. 1B). The rate of 4-day-old individuals in the former group was 91% and significantly higher than in the latter group, 60% (P < 0.05) (Fig. 1C).
cDNA cloning
GAPDH
A cDNA fragment amplified by nested PCR consisted of 367 nt; 3′ and 5′ RACE yielded cDNA fragments consisting of 253 and 617 nt, respectively. The full-length GAPDH cDNA amplified by a single PCR using primers designed from 5′ and 3′ non-coding regions consisted of 1,237 nt encoding 334 amino acids. The full-length deduced amino acid sequence showed 69–71% identity with those of yeast, nematode, fruit fly, zebrafish, mouse, and human. The DNA sequence of the rotifer GAPDH gene has been registered to the DDBJ/EMBL/GenBank databases with accession number AB492236.
ENO
The cDNA fragments amplified by 5′ and 3′ RACE were 1,077 and 420 nt, respectively; the full-length ENO cDNA thus amplified consisted of 1,497 nt encoding 435 amino acids. The full-length deduced amino acid sequence showed 60–74% identity with those from the organisms described above. The DNA sequence of the rotifer ENO gene has been registered to the databases with accession number AB492237.
PGM
A cDNA fragment amplified by 5′ RACE consisted of 601 nt; 3′ RACE yielded an additional 403 nt not determined by 5′ RACE. The full-length PGM cDNA thus amplified consisted of 1,004 nt encoding 251 amino acids. The full-length deduced amino acid sequence showed 52–60% identity with those from the organisms described above. The DNA sequence of the rotifer PGM gene has been registered to the databases with accession number AB492238.
Quantitative real-time PCR analysis
Individual culture system
The culture plates showed no apparent changes in algae concentrations during feeding periods. Moreover, the green algae were observed in the stomachs of rotifers in the AL group under a stereoscopic microscope consistently throughout the experiments.
The relative mRNA levels of GAPDH from the CR group were 1.7–3.6 times higher than those from the AL group at all ages examined (Fig. 2B). The relative mRNA levels of ENO from the CR group were 1.5–3.0 times higher than those from the AL group except on day 5 (0.9 times) (Fig. 2C). Two-way ANOVA revealed significant effects of CR on the mRNA levels of GAPDH (P < 0.0001) and ENO (P < 0.05). Two-way ANOVA also revealed significant effects of age (P < 0.05) and significant interaction between CR and age (P < 0.05) on the mRNA levels of GAPDH, but not for ENO. A Student’s t test showed significant differences in the mRNA levels of GAPDH in 2-, 3-, 4-, and 5-day-old rotifers between the CR and AL groups (P < 0.05), whereas those of ENO were significantly different in 2 and 4-days-old rotifers between the two groups (P < 0.05). The relative mRNA levels of PGM were 1.5–4.5 times higher in the CR than AL group except on days 3 and 8 (0.5 and 1.0 times, respectively), although the differences were statistically not significant (two-way ANOVA and Student’s t test, P > 0.05) (Fig. 2D).
Batch culture
The relative mRNA levels of GAPDH, ENO, and PGM were 1.5, 2.7, and 2.9 times higher, respectively, in the rotifers at the stationary phase (day 14) than those at the exponential growth phase (day 6). A Student’s t test showed significant differences in the mRNA levels of ENO (P < 0.05) (Fig. 3C), although the differences in GAPDH and PGM were not statistically significant (P = 0.18 and 0.06, respectively) (Fig. 3B, D).
Discussion
Various rotifer species are able to inhabit a hypoxic environment in the natural habitat, such as at depths where oxygen level is less than 2 mg/l and sometimes where practically no oxygen is available in a meromictic lake (Miracle & Vicente, 1983; Kizito & Nauwerck, 1995). The resistance to extreme hypoxia is also confirmed in the laboratory using the rotifer B. plicatilis, which maintains relatively high population density for more than 50 days at oxygen concentrations lower than 1 mg/l (Esparcia et al., 1989). The shift from aerobic to anaerobic metabolism associated with activation of lactate and glucose–succinate pathways is considered to compensate for this hypoxic condition (Esparcia et al., 1992). The up-regulation of genes related to glycolysis is observed under anaerobic conditions in diverse animals (Semenza, 1999; Gracey et al., 2001; Ton et al., 2003; Shen et al., 2005). In the present study, CR rotifers showed higher capability to withstand acute hypoxia (O2 consentration < 0.1% in the container) with a concomitant elevation of the mRNA levels of glycolytic enzymes. It is thus likely that CR triggered a metabolic shift from aerobic to anaerobic metabolism.
The lifespan extension by CR has been found in a wide variety of species (Koubova & Guarente, 2003). Although CR is conducted by limiting the daily food supply in certain cases, the feeding regimen for CR in the present study (fed every other day) is often applied in rodents and has been shown to have similar beneficial effects (Goodrick et al., 1990; Castello et al., 2005; Donati et al., 2008). In B. plicatilis, lifespan has been reported to increase more than 2-fold by feeding 3 h daily followed by starvation for the remaining time (Yoshinaga et al., 2000). We also observed that rotifers in the CR group extended their lifespan up to 150% compared with those in the AL group (Kaneko et al., in preparation), suggesting that the present feeding regimen properly triggered the well-known CR response.
CR often exerts beneficial effects on not only lifespan but also stress resistance (Yu & Chung, 2001). Regarding to the short lifespan of B. plicatilis compared to that of rodents, a starvation time period for 1 day may cause severe stress in B. plicatilis. Therefore, knowing the general stress levels of rotifers in response to the feeding every other day, the levels of stress proteins such as heat shock proteins (HSPs) would provide a useful information. However, Kaneko et al. (2002) reported that the mRNA levels of HSP70 and GRP94 were significantly higher in the exponential growth than stationary phase, suggesting that food shortage in the stationary phase does not directly increase the expression of these stress responsive genes. Thus, it is unlikely that the up-regulation of glycolytic enzyme in the CR group was caused by stress response.
The shift from aerobic to anaerobic metabolism by CR could also explain CR-induced longevity of B. plicatilis. Accumulated oxidative damage caused by reactive oxygen species (ROS), which are mostly produced in the process of aerobic metabolism, is considered to be the major cause of aging (Finkel & Holbrook, 2000). Therefore, the elimination of such harmful ROS is thought to lead to the CR-induced longevity. It has been reported that CR up-regulates anti-oxidant enzymes that eliminate ROS (Semsei et al., 1989; Rao et al., 1990; Houthoofd et al., 2004; Kaneko et al., 2005). The reduction of ROS production by decreasing metabolic rate is another hypothetical reason for the lifespan extension (Sohal & Weindruch, 1996). The reduction of body temperature and heat production by CR (Koizumi et al., 1992; Gonzales-Pacheco et al., 1993) provides supportive evidence for this hypothesis, although conflicting results are available (Masoro et al., 1982; Houthoofd et al., 2002; Hulbert et al., 2004). In this context, the enhancement of glycolytic flux prevents cellular senescence together with suppression of oxidative damage in cultured cells (Kondoh et al., 2005, 2007). We have chosen GAPDH and ENO for the present investigation in reference to their possible role in longevity (Murphy et al., 2003; Lee et al., 1999) as well as PGM because of its contribution to above-mentioned protection from ROS. In agreement with our real-time PCR results, transcript-profiling studies have shown the increase in the transcripts of glycolytic enzymes in CR mice (Lee et al., 1999). Furthermore, two types of nematode C. elegans, long-lived mutants and developmentally arrested dauer larvae that show no aging and dramatic resistance to environmental stresses, also show the increased mRNA levels of glycolytic enzymes (Holt & Riddle, 2003; Murphy et al., 2003). These changes possibly reflect a shift from aerobic to anaerobic metabolism, resulting in the extension of lifespan (Rea & Johnson, 2003).
The regulation of the levels of mRNAs encoding glycolytic enzymes through hypoxia inducible factor 1 (HIF-1) is highly conserved among Caenorhabditis elegans, Drosophila melanogaster, fish and mammals (Semenza, 1999; Gorr et al., 2006). HIF-1 without hydroxylation of its subunit under oxygen-limited conditions activates the transcription of glycolytic enzymes. In mammals, however, PGM and glucose phosphate isomerase (GPI), another glycolytic enzyme, are not regulated by HIF-1 and thus exceptional among 15 proteins involved in glycolysis (Iyer et al., 1998). Thus, no significant changes in the mRNA levels of PGM by CR in this study with rotifers, in contrast to significant changes in those of other two glycolytic enzymes, suggest the involvement of HIF-1 in CR responses. In fact, we found cDNA clones showing homology with the HIF-1α subunit gene from other animals in the EST databases of B. plicatilis (Denekamp et al., 2009) (data not shown).
Brachionus plicatilis at the stationary phase showed higher mRNA levels of glycolytic enzymes compared with those at the exponential growth phase but only the difference in ENO mRNA was statistically significant between the two phases. We speculate that this is due to the difference in age distribution of the two populations. It is known that immature rotifers are more abundant at the exponential growth than the stationary phase (Yoshinaga et al., 2001). Since 1-day-old rotifers showed higher mRNA levels of GAPDH (see Fig. 2B), a high ratio of immature rotifers would have led to increased levels of GAPDH transcripts at the exponential growth phase. In addition, the up-regulation of GAPDH mRNA was lower in old than young rotifers. Therefore, a population at the stationary phase consisting mainly of old rotifers could have resulted in relatively low levels of GAPDH even when the rotifers were under CR. Meanwhile, CR is assumed to be responsible for the increased mRNA levels of glycolytic enzymes since rotifers at the stationary phase are considered to be under CR conditions due to high population density. It is highly possible that the resulting shift from aerobic to anaerobic metabolism is accompanied with the increase in hypoxia tolerance, stabilizing population at the stationary phase.
In conclusion, the mRNA levels of GAPDH and ENO were increased in B. plicatilis by CR, suggesting a shift from aerobic to anaerobic metabolism. CR also provided the rotifer with hypoxia tolerance. Thus, CR with anaerobic metabolism seems to stabilize B. plicatilis populations at the stationary phase, although further investigations, including those on signaling pathways connecting CR and glycolysis, are required to substantiate the mechanisms involved.
References
Castello, L., T. Froio, G. Cavallini, F. Biasi, A. Sapino, G. Leonarduzzi, E. Bergamini, G. Poli & E. Chiarpotto, 2005. Calorie restriction protects against age-related rat aorta sclerosis. FASEB Journal 19: 1863–1865.
Denekamp, N. Y., M. A. S. Thorne, M. S. Clark, M. Kube, R. Reinhardt & E. Lubzens, 2009. Discovering genes associated with dormancy in the monogonont rotifer Brachionus plicatilis. BMC Genomics 10: 108.
Donati, A., G. Recchia, G. Cavallini & E. Bergamini, 2008. Effect of aging and anti-aging caloric restriction on the endocrine regulation of rat liver autophagy. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 63: 550–555.
Enesco, H. E., 1993. Rotifers in aging research: use of rotifers to test various theories of aging. Hydrobiologia 255: 59–70.
Esparcia, A., M. R. Miracle & M. Serra, 1989. Brachionus plicatilis tolerance to low oxygen concentrations. Hydrobiologia 186: 331–337.
Esparcia, A., M. Serra & M. R. Miracle, 1992. Relationships between oxygen concentration and patterns of energy metabolism in the rotifer Brachionus plicatilis. Comparative Biochemistry and Physiology B – Biochemistry and Molecular Biology 103: 357–362.
Finkel, T. & N. J. Holbrook, 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247.
Gonzales-Pacheco, D. M., W. C. Buss, K. M. Koehler, W. F. Woodside & S. S. Alpert, 1993. Energy restriction reduces metabolic rate in adult male Fisher-344 rats. Journal of Nutrition 123: 90–97.
Goodrick, C. L., D. K. Ingram, M. A. Reynolds, J. R. Freeman & N. Cider, 1990. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mechanisms of Ageing and Development 55: 69–87.
Gorr, T. A., M. Gassmann & P. Wappner, 2006. Sensing and responding to hypoxia via HIF in model invertebrates. Journal of Insect Physiology 52: 349–364.
Gracey, A. Y., J. V. Troll, & G. N. Somero, 2001. Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proceedings of the National Academy of Sciences of the United States of America 98: 1993-1998.
Holt, S. J. & D. L. Riddle, 2003. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Mechanisms of Ageing and Development 124: 779–800.
Houthoofd, K., B. P. Braeckman, I. Lenaerts, K. Brys, A. De Vreese, S. Van Eygn & J. R. Vanfleteren, 2002. No reduction of metabolic rate in food restricted Caenorhabditis elegans. Experimental Gerontology 37: 1359–1369.
Houthoofd, K., B. P. Braeckman, A. De Vreese, S. Van Eygen, I. Lenaerts, K. Brys, F. Matthijssens & J. R. Vanfleteren, 2004. Caloric restriction, Ins/IGF-1 signalling and longevity in the nematode Caenorhabditis elegans. Belgian Journal of Zoology 134: 79–84.
Hulbert, A. J., D. J. Clancy, W. Mair, B. P. Braeckman, D. Gems & L. Partridge, 2004. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Experimental Gerontology 39: 1137–1143.
Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu & G. L. Semenza, 1998. Cellular and developmental control of O-2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & Development 12: 149–162.
Kaneko, G., S. Kinoshita, T. Yoshinaga, K. Tsukamoto & S. Watabe, 2002. Changes in expression patterns of stress protein genes during population growth of the rotifer Brachionus plicatilis. Fisheries Science 68: 1317–1323.
Kaneko, G., T. Yoshinaga, Y. Yanagawa, S. Kinoshita, K. Tsukamoto & S. Watabe, 2005. Molecular characterization of Mn-superoxide dismutase and gene expression studies in dietary restricted Brachionus plicatilis rotifers. Hydrobiologia 546: 117–123.
Kizito, Y. S. & A. Nauwerck, 1995. Temporal and vertical distribution of planktonic rotifers in a meromictic crater lake, Lake Nyahirya (western Uganda). Hydrobiologia 313: 303–312.
Koizumi, A., M. Tsukada, Y. Wada, H. Masuda & R. Weindruch, 1992. Mitotic activity in mice is suppressed by energy restriction-induced torpor. Journal of Nutrition 122: 1446–1453.
Kondoh, H., M. E. Lleonart, J. Gil, J. Wang, P. Degan, G. Peters, D. Martinez, A. Carnero & D. Beach, 2005. Glycolytic enzymes can modulate cellular life span. Cancer Research 65: 177–185.
Kondoh, H., M. E. Lleonart, D. Bernard & J. Gil, 2007. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histology and Histopathology 22: 85–90.
Koubova, J. & L. Guarente, 2003. How does calorie restriction work? Genes & Development 17: 313–321.
Lee, C. K., R. G. Klopp, R. Weindruch & T. A. Prolla, 1999. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393.
Marcial, H. S., A. Hagiwara & T. W. Snell, 2005. Effect of some pesticides on reproduction of rotifer Brachionus plicatilis Müller. Hydrobiologia 546: 569–575.
Masoro, E. J., B. P. Yu & H. A. Bertrand, 1982. Action of food restriction in delaying the aging process. Proceedings of the National Academy of Sciences of the United States of America 79: 4239–4241.
Miracle, M. R. & E. Vicente, 1983. Vertical distribution and rotifer concentrations in the chemocline of meromictic lakes. Hydrobiologia 104: 259–267.
Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath, J. Ahringer, H. Li & C. Kenyon, 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–284.
Park, H. G., K. W. Lee, S. H. Cho, H. S. Kim & M. M. Jung, 2001. High density culture of the freshwater rotifer, Brachionus calyciflorus. Hydrobiologia 446: 369–374.
Rao, G., E. N. Xia, M. J. Nadakavukaren & A. Richardson, 1990. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. Journal of Nutrition 120: 602–609.
Rea, S. & T. E. Johnson, 2003. A metabolic model for life span determination in Caenorhabditis elegans. Developmental Cell 5: 197–203.
Semenza, G. L., 1999. Regulation of mammalian O-2 homeostasis by hypoxia-inducible factor 1. Annual Review of Cell and Developmental Biology 15: 551–578.
Semsei, I., G. Rao & A. Richardson, 1989. Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochemical and Biophysical Research Communications 164: 620–625.
Shen, C., D. Nettleton, M. Jiang, S. K. Kim & J. A. Powell-Coffman, 2005. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. The Journal of Biological Chemistry 280: 20580–20588.
Sohal, R. S. & R. Weindruch, 1996. Oxidative stress, caloric restriction, and aging. Science 273: 59–63.
Suga, K., D. Mark Welch, Y. Tanaka, Y. Sakakura & A. Hagiwara, 2007. Analysis of expressed sequence tags of the cyclically parthenogenetic rotifer Brachionus plicatilis. PLoS ONE 2: e671.
Ton, C., D. Stamatiou & C. Liew, 2003. Gene expression profile of zebrafish exposed to hypoxia during development. Physiological Genomics 13: 97–106.
Yoshinaga, T., A. Hagiwara & K. Tsukamoto, 1999. Effect of conditioned media on the asexual reproduction of the monogonont rotifer Brachionus plicatilis O. F. Müller. Hydrobiologia 412: 103–110.
Yoshinaga, T., A. Hagiwara & K. Tsukamoto, 2000. Effect of periodical starvation on the life history of Brachionus plicatilis O.F. Müller (Rotifera): a possible strategy for population stability. Journal of Experimental Marine Biology and Ecology 253: 253–260.
Yoshinaga, T., A. Hagiwara & K. Tsukamoto, 2001. Why do rotifer populations present a typical sigmoid growth curve? Hydrobiologia 446: 99–105.
Yoshinaga, T., G. Kaneko, S. Kinoshita, K. Tsukamoto & S. Watabe, 2003. The molecular mechanisms of life history alterations in a rotifer: a novel approach in population dynamics. Comparative Biochemistry and Physiology B-Biochemistry and Molecular Biology 136: 715–722.
Yoshinaga, T., Y. Minegishi, I. F. M. Rumengan, G. Kaneko, S. Furukawa, Y. Yanagawa, K. Tsukamoto & S. Watabe, 2004. Molecular phylogeny of the rotifers with two Indonesian Brachionus lineages. Coastal Marine Science 29: 45–56.
Yu, B. P. & H. Y. Chung, 2001. Stress resistance by caloric restriction for longevity. Annals of the New York Academy of Sciences 928: 39–47.
Acknowledgments
We are grateful to Professor A. Hagiwara, Graduate School of Science and Technology, Nagasaki University, Japan for providing Brachionus plicatilis Ishikawa strain. This work was partly supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y. O. was supported by Research Fellowships for Young Scientist from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Darcy J. Lonsdale
Yori Ozaki and Gen Kaneko contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ozaki, Y., Kaneko, G., Yanagawa, Y. et al. Calorie restriction in the rotifer Brachionus plicatilis enhances hypoxia tolerance in association with the increased mRNA levels of glycolytic enzymes. Hydrobiologia 649, 267–277 (2010). https://doi.org/10.1007/s10750-010-0269-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0269-9