Abstract
To demonstrate the sensitivity of aquatic ecosystems to forecasted increases in nitrogen deposition along the eastern ranges of the Canadian Rocky Mountains, we conducted midsummer limnological surveys of 29 remote alpine lakes and ponds via helicopter in 2007. Chemical analysis of water and in vitro nitrogen-enrichment bioassays of phytoplankton collected from each site were performed to estimate nutrient limitation. Use of a common chemical index for nutrient limitation (total dissolved inorganic nitrogen: total phosphorus; DIN:TP) together with supportive experimental evidence revealed nitrogen limitation in only 14% of the cases. Shallow (≤1 m maximum depth) ponds were more likely to be nitrogen-limited than lakes, especially as the former exhibited a significantly lower mean DIN:TP ratio of 7.4 during the late summer. Chemical and bioassay-based inferences of nitrogen limitation agreed in 74.5% of the cases, owing mainly to evidence of phosphorus limitation of the surveyed lake ecosystems (mean DIN:TP = 18) being supported by nonsignificant responses of their phytoplankton to nitrogen amendment. Our findings reveal that increased nitrogen deposition should not result in immediate widespread eutrophication of the Canadian Rockies; however, certain alpine ponds appeared nitrogen-limited, making them sensitive early indicators of the potential effects of anthropogenic nitrogen deposition in remote mountainous regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic nitrogen deposition is a key stressor defining the impacts of global change on aquatic ecosystems (Sala et al., 2000; Bergström & Jansson, 2006). While pronounced levels of nitrogen deposition (e.g., 25 kg N ha−1 year−1) often occur near urban centers, rates are also increasing in remote areas, such the California Sierra Nevada and Colorado Front Range (Fenn et al., 2003). Although nitrogen deposition rates along the eastern front ranges of the Canadian Rockies have varied from 5 to 10 kg N ha−1 year−1 over the past decade (Alberta Environment, unpublished data), these are expected to similarly increase as a consequence of expanding human development (Schindler & Smol, 2006; Schindler et al., 2006).
Several lines of evidence suggest that mountain lake ecosystems in certain regions of North America are limited by the availability of nitrogen, making them highly sensitive to increased nitrogen deposition. Chemical analysis of water collected from mountain lakes has provided indirect evidence of nitrogen limitation. Specifically, a ratio of dissolved inorganic nitrogen (DIN) to total phosphorus (TP) below a value of one has been used to infer nitrogen limitation of phytoplankton in Colorado mountain lakes (Morris & Lewis, 1988), Sierra Nevada lakes (Sickman et al., 2003), and Canadian alpine ponds (McMaster & Schindler, 2005). Paleolimnological studies have also inferred that recent increases in nitrogen deposition best explain shifts in algal and diatom communities of mountain lakes (Saros et al., 2003; Das et al., 2005). Further, in situ and in vitro experiments have demonstrated nitrogen limitation of phytoplankton in high-elevation lakes of Colorado (Morris & Lewis, 1988), Alaska (Levine & Whalen, 2001), and Wyoming (Lafrancois et al., 2003; Nydick et al., 2004). The positive effect of nitrogen on phytoplankton abundance often depends on phosphorus availability (Schindler, 1977), which can vary across mountain lakes and ponds (McMaster & Schindler, 2005). However, a meta-analysis of the literature indicated that nitrogen limitation is relatively rare compared to co-limitation by both nitrogen and phosphorus in freshwater ecosystems based on evidence from in vitro nutrient-amendment bioassays (Elser et al., 2007).
The main aim of our study was to determine the potential sensitivity of phytoplankton to nitrogen deposition across alpine lakes and ponds of the eastern front range of the Canadian Rockies, using two indices of nitrogen limitation. We generated DIN:TP ratios for 29 water bodies surveyed during summer 2007 to infer the nature of their nutrient limitation (i.e., nitrogen-, phosphorus-, or co-limitation). Also, we conducted in vitro nitrogen-enrichment experiments using water and extant phytoplankton from each of the surveyed sites to corroborate our chemically based inferences of nutrient limitation. We hypothesized that inferred nitrogen limitation would be more pronounced in ponds than in lakes. The rationale for our hypothesis was based on an earlier report by McMaster & Schindler (2005), which indicated that ponds along the eastern front ranges of the Canadian Rockies often contain undetectable concentrations of dissolved inorganic nitrogen, while phosphorus levels are relatively elevated owing to internal loading of phosphorus from the sediments.
Materials and methods
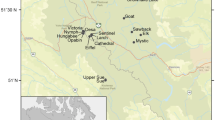
The survey spanned 29 water bodies located across Banff and Yoho National parks (Fig. 1). All sites were fishless, non-glacial, and situated above tree line (>2,100 m asl). At each site, sampling was performed from its deepest point from an inflatable one-person boat during early July and again in late August 2007. Lake depth was measured using a depth sounder. Air temperature was measured using a mercury thermometer, while underwater temperatures were recorded at 1-m depth intervals using a YSI temperature probe to 1 m above sediments in lakes, and to the water–sediment interface in ponds. The penetration of light was measured using a Secchi disk, which was deployed from the shaded side of the boat. We defined the depth of euphotic zone at each site by a doubling of the Secchi depth (Wetzel & Likens, 2000), which was truncated in the surveyed ponds (≤1 m maximum depth) where it surpassed the maximum depth of the sediments.
The maximum depth of both the sampled water body and its euphotic zone influenced how we sampled for water. Water was collected usually from the top, middle, and bottom of each euphotic zone using a 2-l Van Dorn bottle, and pooled into an acid-washed 10-l cubitainer. If the euphotic zone extended to the bottom of a lake, then water was collected from the top, middle, and 1 m above the sediments. In the sampled ponds, surface water collections were performed. At each site, an unfiltered 2-l subsample was stored in an opaque Nalgene container for total nutrient analysis, while a 1-l sample of unsieved water was filtered onto a Whatman GF/F filter to concentrate phytoplankton. The filtrate was stored in a 500-ml Nalgene for total dissolved nutrient analyses, and the filter placed in a petri dish wrapped with tinfoil. Another 1-l of unsieved water was filtered through an ashed Whatman GF/F filter for analysis of particulate C, N, P. All samples were stored on ice and transported back from each remote site to Canmore AB via helicopter. Thereafter, samples were transported 500 km to the University of Alberta to conduct nutrient bioassays and chemical analysis. The remaining unfiltered water was sieved through a stainless steel sieve (64-μm mesh size) to remove crustacean zooplankton and rotifers, and stored in an acid-washed 2-l amber Nalgene container until conducting the nitrogen-amendment bioassay. The bioassays were started within 3 days of water collection.
Analysis of water chemistry was performed at the University of Alberta Biogeochemical Analytical Facility (URL link: http://www.biology.ualberta.ca/facilities/limnology). Total nitrogen (TN), total dissolved nitrogen (TDN), ammonia (NH4), and nitrate/nitrite (NO3 + NO2) were measured colorimetrically via NaOH–persulfate oxidation and cadmium reduction using a Lachat Quikchem 8500 FIA automated ion analyzer with detection limits of 7, 7, 2, and 1 μg l−1, respectively. Particulate nitrogen (PN) was determined using an Exeter 440 CHN analyzer. Dissolved organic carbon (DOC) was measured using a Shimadzu 5000A TOC Analyzer with a detection limit of 0.1 mg l−1. Total phosphorus (unfiltered) and total dissolved phosphorus (0.45-μm filtered) were determined via automated ascorbic acid reduction using a Lachat QuickChem 8500 multichannel flow analyzer with a detection limit of 1 μg l−1. DIN:TP ratios were calculated based on weight (i.e., μg DIN:μg TP) to enable direct comparison with values reported previously by Morris & Lewis (1988) and Sickman et al. (2003).
To test for nitrogen limitation in each water body, we performed a single-factor (control, nitrogen-amended) experiment in triplicate for a total of six culture vessels. Sieved water from each water body was mixed vigorously and transferred into 250 ml clear, vented, polystyrene culture vessels (Corning, USA). Three culture vessels were enriched with N to a final concentration of 1 mg l−1 by adding 1 ml of an ammonium nitrate solution, while 1 ml of double deionized water was added to the controls. We conducted the experiment in a growth chamber at 12°C using Phillips® 44 watts fluorescent bulbs to produce 4,100 lx over a 12-h light:dark cycle. During the 12-day experiment, the culture vessels were shaken every 3 days, and randomly repositioned.
On day 12, each culture vessel was shaken rigorously for 30 s and 150 ml of its contents concentrated onto Whatman GF/F filters to determine final total chlorophyll concentration. An Agilent 1100 Series high performance liquid chromatography (HPLC) unit equipped with a Varian Microsorb-100Å C18 column was used to perform a standard procedure for quantifying chlorophyll concentrations (Vinebrooke et al., 2002). Chlorophylls were identified using in-line diode array and fluorescence detectors having tested detection limits of 0.2 and 0.1 μg l−1, respectively. Total chlorophyll concentrations were quantified via calibration equations and an electronic spectral library constructed using standards of chlorophylls a, b, and c purchased from DHI Water and Environment (Agern Alle 5, DK-2970 Hoersholm, Denmark).
We tested statistically for both significant predictive relationships between measured chemical variables and total chlorophyll using regression analysis, and differences in total chlorophyll between controls and nitrogen-amended phytoplankton communities using analysis of variance (ANOVA). Two-way ANOVAs were also conducted to determine if phytoplankton response to nitrogen amendment depended on water body type (lake vs. pond), or period of sampling (early vs. late summer). Regression analysis and ANOVA were performed using SPSS Version 11 for Macintosh®. Data were log10-transformed prior to analysis to improve normality and homogeneity of variance.
Results
Limnological conditions varied substantially among the surveyed alpine sites (Appendix in Electronic Supplementary Material). TDN varied from 15 to 935 μg l−1, while dissolved inorganic nitrogen (NH4 + NO X ) ranged from only 1 to 373 μg l−1, indicating that a large portion of TDN was organically bound, especially in the shallow water bodies. In comparison, TP varied from 1 to 72 μg l−1 across the surveyed sites. As a result, DIN:TP ratios ranged from <1 to 109. DOC concentrations were relatively low (<2 mg l−1), except in certain visibly stained ponds (e.g., Snowflake ponds). Similarly, total chlorophyll levels were relatively low (<10 μg l−1) across most sites, reflecting their oligotrophic status. Significant chemical predictors of total chlorophyll concentrations were DIN (r = 0.52, P < 0.001), TP (r = 0.51, P < 0.001), and DOC (r = 0.45, P = 0.001).
The surveyed alpine ponds were more chemically concentrated than the lakes (Fig. 2). TN levels were over two-fold times greater in ponds than in lakes, owing primarily to higher concentrations of TDN. DIN levels were only slightly elevated in ponds relative to lakes, while TP concentrations were three-fold times greater in ponds. Consequently, the mean DIN:TP ratio of 7.4 was significantly (F 12,34 = 6.23 P = 0.01) lower by 50% in the ponds compared to the lakes. Ponds also contained significantly (F 13,35 = 9.11, P = 0.004) greater concentrations of total chlorophyll than did lakes, averaging 0.4 μg l−1 ± 1.29 SE and 0.1 μg l−1 ± 0.16 SE, respectively.
Inferences of nutrient limitation based on DIN:TP ratios revealed that most surveyed water bodies were likely limited by phosphorus and not nitrogen (i.e., DIN:TP >4), especially during early summer (Fig. 3). However, DIN:TP ratios were lower for many sites sampled during late summer, resulting in several relatively shallow ecosystems (Elk Lake, Sparrowhawk Pond 1, Upper Sue Lake, Snowflake Ponds 2 and 3) appearing to be co-limited or limited by nitrogen. Only certain ponds (Snowflake Ponds 1 and 4, Cathedral Pond) showed sufficiently low DIN:TP ratios to be considered nitrogen-limited (i.e., DIN:TP <1).
Dissolved inorganic nitrogen to total phosphorus ratios (DIN:TP) for surveyed lakes and ponds from the early (solid bars, July 10–20), and late (open bars, Aug 7–Sep 13) sampling periods. Values above the solid line (DIN:TP >4) are inferred to be phosphorus-limited. Values below the dashed line (DIN:TP <1) are categorized as nitrogen-limited. Intermediate values falling between the solid and dashed lines are inferred to be co-limited by nitrogen and phosphorus
Bioassays showed that nitrogen amendment did not significantly (F 1,268 = 2.1, P = 0.15) increase final total phytoplankton chlorophyll concentration across all of the water bodies sampled during either early (Fig. 4) or late summer 2007 (Fig. 5). Although phytoplankton collected from ponds showed several positive responses to nutrient amendment, the effect of nitrogen on total chlorophyll did not differ significantly (nitrogen–water body interaction; F 1,268 = 0.02, P = 0.96) between pond and lake communities. Harvested final total chlorophyll concentrations from the bioassays were significantly (water body effect; F 1,268 = 2.9, P = 0.05) higher for ponds than lakes.
A comparison of our chemically inferred and bioassay-based lines of evidence of nitrogen limitation revealed a level of agreement equaling 74.5% (Appendix in Electronic Supplementary Material). Chemical inferences of nitrogen limitation (i.e., DIN:TP <1) was coupled with significant positive algal responses to added nitrogen in four cases, namely Capricorn Lake, Cathedral Pond, Snowflake Ponds 1 and 4 (Appendix in Electronic Supplementary Material). Otherwise, chemically inferred nitrogen limitation was not supported by a positive chlorophyll response only in the case of David Lake (Appendix in Electronic Supplementary Material). High DIN:TP ratios (i.e., >10) indicating phosphorus limitation were supported by nonsignificant effects of nitrogen enrichment on total chlorophyll, except in few cases. Here, DIN:TP ratios greater than 10 underestimated the positive effect of nitrogen amendment on the total chlorophyll content of phytoplankton collected in July (e.g., B95, Middle Devon, Sparrowhawk Pond 1, Sue Lake) (Fig. 4). Similarly, high DIN:TP ratios for certain sites sampled during late summer 2007 ran counter to increased levels of chlorophyll in the experimentally nitrogen-enriched phytoplankton (e.g., Elk Lake, McConnell Lake, Nymph Pond, Opabin Lake, Snowflake Lake) (Fig. 5).
Discussion
Our limnological survey and bioassays of phytoplankton from the eastern front ranges of the Canadian Rockies generated two lines of evidence suggesting that most will not respond to rising levels of anthropogenic nitrogen deposition. The chemical inferences of nutrient limitation in these ecosystems agreed with our experimental evidence in most cases. The surveyed lakes and many ponds contained high DIN:TP ratios and phytoplankton that did not usually show significant positive responses to nitrogen enrichment, suggesting that phosphorus limitation of aquatic ecosystems is common place in this region. Low DIN:TP values coupled with positive increases in chlorophyll following experimental enrichment with nitrogen revealed that certain ponds were nitrogen-limited, and thus, shallow aquatic alpine ecosystems can function as useful indicators of increases in nitrogen deposition. Therefore, synoptic surveys of water chemistry from this area appear in most cases to provide information that is consistent with bioassay-derived evidence regarding nitrogen limitation and the sensitivity of the lakes and ponds to elevated nitrogen deposition.
Several potential explanations exist for the few discrepancies that we detected between the survey-based and experimental evidence of nutrient limitation. Specifically, nitrogen enrichment on eight occasions enhanced the total chlorophyll content of phytoplankton collected from sites that were chemically inferred to be phosphorus-limited (i.e., DIN:TP >10). Depletion of dissolved gaseous nitrogen (N2) in the culture vessels may have impeded N2-fixation (Schindler, 1998), thereby generating false positive responses to nitrogen enrichment during the bioassays. Further, we chose a relatively long incubation period of 12 days to better insure detection of changes in chlorophyll given initially sparse phytoplankton concentrations; however, this may have worsened the lack of N2 and contributed to limitation of N2-fixation. In comparison, potential carbon limitation owing to the poor availability of gaseous carbon dioxide in the culture vessels was unlikely given the water used in the bioassays were collected from slightly alkaline (pH 8–9), carbonate-rich lakes and ponds. Another possible explanation involves the lack of grazing pressure in nutrient-enrichment bioassays (Schindler, 1998), which may have enabled certain highly edible phytoplankton that are otherwise suppressed by predation in the lakes and ponds to respond positively to nitrogen enrichment. We are confident that our reported DIN:TP ratios provided reliable indication of nutrient limitation and were not skewed by sampling or analytical error since our values were consistent with earlier chemical reports (McMaster & Schindler, 1995) for many of these sites.
Only four ponds and two shallow lakes showed evidence that consistently demonstrated nitrogen- or co-limitation (Appendix in Electronic Supplementary Material). These sites had low DIN:TP values (i.e., <4) and contained phytoplankton that increased in total chlorophyll following enrichment with nitrogen. Their responsiveness to nitrogen may be attributed to a variety of factors. Wind-driven events can facilitate rapid nitrogen assimilation by benthic biofilms, thereby outcompeting phytoplankton by drawing down DIN levels in the water column (Axler & Reuter, 1996). Further, the relatively elevated TP levels found in these high-elevation ecosystems suggest that internal loading of phosphorus is sufficient to negate the possibility of phosphorus limitation. Wind-driven resuspension of sediments and pronounced release of phosphorus into the water column occur more frequently in shallower systems where the smaller volume of water does not allow for dilution as it would in larger lakes (Schallenberg & Burns, 2004). Interestingly, evidence of nitrogen limitation in our study originated most often with samples collected during late summer, suggesting that elevated nitrogen deposition that occurs in the form of summer rainfall would have more pronounced immediate impacts on these shallow ecosystems than nitrogen inputs from melting snow packs in June.
Although DIN:TP and chlorophyll responses to nitrogen enrichment both indicated that certain shallow ecosystems were either co-limited or limited by nitrogen, these indicators were not supported by the Redfield index of the nutritional status of the phytoplankton, namely, particulate nitrogen:particulate phosphorus (PN:PP). In comparison, DIN:TP is considered to better reflect the nutrient supply to the phytoplankton because it incorporates both the external availability of nitrogen and internal cellular storage of phosphorus (Morris & Lewis, 1988). The six phytoplankton communities that responded positively to nitrogen enrichment exhibited a mean PN:PP of 14.9, which suggested a deficiency in phosphorus. Similar disagreement between DIN:TP and PN:PP estimates of nutrient limitation in high-elevation have been previously reported, and attributed to the potentially confounding effect of micronutrient limitation involving trace metals (Sickman, 2001). Another potential confounding factor involves DOC affecting the bioavailability of the dissolved nitrogen fraction of TN. In particular, large differences between TDN and DIN levels within the nitrogen-limited ponds indicated that a large proportion (82%) of the dissolved nitrogen was organically bound, and therefore, not likely directly available for uptake by the phytoplankton (Antia et al., 1991). Similarly, remote shallow lakes in the Andes, Antarctica, and Himalayas also appear to be nitrogen-limited, containing low TN concentrations consisting mainly of organic nitrogen (Rogora et al., 2008).
An important question arising from our findings is whether the observed general lack of nitrogen limitation in water bodies along the eastern front ranges of the Canadian Rockies is attributable to recent anthropogenic events or long-term natural conditions. For example, shifts toward increased phosphorus limitation of phytoplankton in high-elevation lakes have been attributed to high levels of anthropogenic nitrogen deposition (Goldman et al., 1993; Sickman, 2001; Bergström et al., 2008). However, moderately elevated levels of nitrogen deposition are considered relatively recent phenomena in the less developed Canadian Rockies (Schindler et al., 2006), and paleolimnological evidence suggests that nitrogen levels in several of the non-glaciated alpine lakes have remained stable over the past century (R.D. Vinebrooke, unpublished data; Bunting et al., 2010). Therefore, widespread phosphorus limitation of these and other large alpine lake ecosystems in the region likely reflects their natural trophic status. In comparison, evidence of nitrogen limitation being pond-specific reflects the stronger land–water linkage between nitrogen-limited terrestrial and shallow freshwater ecosystems (Graham et al., 2006).
References
Antia, N. J., P. J. Harrison & L. Oliveira, 1991. The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia 30: 1–89.
Axler, R. P. & R. E. Reuter, 1996. Nitrate uptake by phytoplankton and periphyton: whole-lake enrichments and mesocosm-15N experiments in an oligotrophic lake. Limnology and Oceanography 41: 659–671.
Bergström, A. K. & M. Jansson, 2006. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Change Biology 12: 635–643.
Bergström, A. K., A. Jonsson & M. Jansson, 2008. Phytoplankton responses to nitrogen and phosphorus enrichment in unproductive Swedish lakes along a gradient of atmospheric nitrogen deposition. Aquatic Biology 4: 55–64.
Bunting, L., P. R. Leavitt, R. P. Weidman & R. D. Vinebrooke, 2010. Regulation of nitrogen biogeochemistry of mountain lakes by subsidies of terrestrial dissolved organic matter and the implications for climate studies. Limnology and Oceanography 55: 333–345.
Das, B., R. D. Vinebrooke, A. Sanchez-Azofelfa, B. Rivard & A. P. Wolfe, 2005. Inferring sedimentary chlorophyll concentrations with reflectance spectroscopy: a novel approach to reconstructing historical changes in the trophic status of mountain lakes. Canadian Journal of Fisheries and Aquatic Sciences 62: 1067–1078.
Elser, J. H., M. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Fenn, M. E., J. S. Baron, E. B. Allen, H. M. Rueth, K. R. Nydick, L. Geiser, W. D. Bowman, J. O. Sickman, T. Meixner, D. W. Johnson & T. Neitlich, 2003. Ecological effects of nitrogen deposition in the western United States. Bioscience 53: 404–420.
Goldman, C. R., A. D. Jassby & S. H. Hackley, 1993. Decadal, interannual, and seasonal variability in enrichment bioassays at Lake Tahoe, California-Nevada, USA. Limnology and Oceanography 50: 1489–1496.
Graham, M. D., R. D. Vinebrooke & M. A. Turner, 2006. Coupling of boreal forests and lakes: effects of conifer pollen on littoral communities. Limnology and Oceanography 51: 1524–1529.
Lafrancois, B. B., K. R. Nydick & B. Caruso, 2003. Influence of nitrogen on phytoplankton biomass and community composition in fifteen Snowy Range lakes (Wyoming, USA). Arctic Antarctic and Alpine Research 35: 499–508.
Levine, M. A. & S. C. Whalen, 2001. Nutrient limitation of phytoplankton production in Alaskan arctic foothill lakes. Hydrobiologia 455: 189–201.
McMaster, N. L. & D. W. Schindler, 2005. Planktonic and epipelic algal communities and their relationship to physical and chemical variables in alpine ponds in Banff National Park, Canada. Arctic, Antarctic and Alpine Research 37: 337–347.
Morris, D. P. & W. M. Lewis, 1988. Phytoplankton nutrient limitation in Colorado Mountain Lakes. Freshwater Biology 20: 315–327.
Nydick, K. N., B. M. Lafrancois, J. S. Baron & B. M. Johnson, 2004. Nitrogen regulation of algal biomass, productivity, and composition in shallow mountain lakes, Snowy Range, Wyoming, USA. Canadian Journal of Fisheries and Aquatic Sciences 61: 210–223.
Rogora, M., J. Massaferro, A. Marchetto, G. Tartari & R. Mosello, 2008. The water chemistry of some shallow lakes in Northern Patagonia and their nitrogen status in comparison with remote lakes in different regions of the globe. Journal of Limnology 67: 75–86.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Biodiversity—global biodiversity scenarios for the year 2100. Science 287: 1770–1774.
Saros, J. E., S. J. Interlandi, A. P. Wolfe & D. R. Engstrom, 2003. Recent changes in the diatom community structure of lakes in the Beartooth Mountain Range, USA. Arctic Antarctic and Alpine Research 35: 18–23.
Schallenberg, M. & C. W. Burns, 2004. Effects of sediment resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal enrichment. Freshwater Biology 49: 143–159.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 195: 260–262.
Schindler, D. W., 1998. Replication versus realism: the need for ecosystem-scale experiments. Ecosystems 1: 323–334.
Schindler, D. W. & J. P. Smol, 2006. Cumulative effects of climate warming and other human activities on freshwaters of Arctic and subarctic North America. Ambio 35: 160–168.
Schindler, D. W., P. J. Dillon & H. Schreier, 2006. A review of anthropogenic sources of nitrogen and their effects on Canadian aquatic ecosystems. Biogeochemistry 79: 25–44.
Sickman, J. O., 2001. Comparative analyses of nitrogen biogeochemistry in high-elevation ecosystems. Doctoral dissertation, University of California, Santa Barbara.
Sickman, J. O., J. M. Melack & D. W. Clow, 2003. Evidence of nutrient enrichment of high-elevation lakes in the Sierra Nevada, California. Limnology and Oceanography 48: 1885–1892.
Vinebrooke, R. D., S. S. Dixit, M. D. Graham, J. M. Gunn, Y. W. Chen & N. Belzile, 2002. Whole-lake algal responses to a century of acidic industrial deposition on the Canadian Shield. Canadian Journal of Fisheries and Aquatic Sciences 59: 483–493.
Wetzel, R. G. & G. Likens, 2000. Limnological Analyses. Springer-Verlag, New York.
Acknowledgments
This study was supported by grants from the Alberta Ingenuity Centre for Water Research, and the Natural Sciences and Engineering Research Council of Canada to RDV. We also thank Parks Canada for their permission to conduct our research in the national mountain parks of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Hilde Eggermont, Martin Kernan & Koen Martens / Global change impacts on mountain lakes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murphy, C.A., Thompson, P.L. & Vinebrooke, R.D. Assessing the sensitivity of alpine lakes and ponds to nitrogen deposition in the Canadian Rocky Mountains. Hydrobiologia 648, 83–90 (2010). https://doi.org/10.1007/s10750-010-0146-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0146-6