Abstract
We studied the attachment strength and aggregation behaviour of Dreissena polymorpha in the presence of large roach Rutilus rutilus (>180 mm total length) (efficient molluscivore), small roach (<110 mm) (unable to feed on zebra mussels) and perch Perca fluviatilis (not feeding on mussels). The intention was to check whether small (<10 mm) and large (>10 mm) mussels would respond specifically to fish capable of consuming them (i.e. large roach). After 1 day of exposure, we found no significant differences in mussel attachment strength. After 6 days in the presence of large roach, mussels were attached more strongly than in the other treatments. After a 1-day exposure to all kinds of fish, mussels were more aggregated than in the control treatment. After 6 days, the largest percentage of aggregated mussels was found in the presence of large roach, while the aggregation levels in the other treatments were lower and did not differ from one another. Perhaps, an initial response was a non-specific reaction to the presence of any fish, while a specific response to large roach appeared later. Thus, zebra mussels were able to recognize their potential predators. The observed behaviour of mussels may enhance their resistance to molluscivores in the field by limiting the access of predators to their potential prey (due to the increased aggregation of prey) and by increasing predator handling costs (due to the stronger attachment of prey).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of predators induces behavioural defensive responses of many aquatic organisms, including such distinct taxa as: protozoans (Wiąckowski et al., 2004), rotifers (Lass & Spaak, 2003), molluscs (Orr et al., 2007; Kobak & Kakareko, 2009), arthropods (Koperski, 1997; De Meester et al., 1999; Baumgärtner et al., 2002) and fish (Magurran, 1990; Gliwicz, 2005; Wisenden et al., 2008). These anti-predator defences can be induced by alarm pheromones released by wounded conspecifics (Pijanowska & Kowalczewski, 1997; Cheung et al., 2004; Wisenden et al., 2008) or directly by predator kairomones, which are chemical substances released by one species (here: a predator) and beneficial for another species, receiving the signal (here: a potential prey) (De Meester et al., 1999; Lass & Spaak, 2003). Common examples of anti-predator responses include swarming (Pijanowska & Kowalczewski, 1997), active selection of predator-free habitats (De Meester et al., 1999; Baumgärtner et al., 2002; Gliwicz, 2005) and/or reduction of activity (Koperski, 1997; Naddafi et al., 2007; Wisenden et al., 2008). These defences can be very efficient, allowing for survival of prey organisms under strong predator pressure (Gliwicz, 2005). Moreover, they can evolve very rapidly and substantially alter predator–prey dynamics (Yoshida et al., 2003). Thus, they cannot be neglected in attempts to understand ecological processes taking place in nature.
Defensive mechanisms of sessile organisms have not been studied as comprehensively as those of freshwater zooplankton (De Meester et al., 1999; Lass & Spaak, 2003) and planktivorous fish (Gliwicz, 2005). Blue mussels Mytilus sp. exposed to predators were found to increase their attachment strength, become more aggregated, and reduce their locomotion (Reimer & Tedengren, 1997; Côté & Jelnikar, 1999; Reimer & Harms-Ringdahl, 2001; Nicastro et al., 2007). Other epifaunal marine mussels: Hormomya mutabilis (Gould), Perna viridis (L.) and Brachidontes variabilis (Krauss) also modify their byssal thread production in the presence of predators (Ishida & Iwasaki, 2003; Cheung et al., 2004; Cheung et al., 2006).
Freshwater zebra mussel, Dreissena polymorpha (Pallas) is an expansive, invasive Ponto-Caspian bivalve, inhabiting various hard substrata in lakes, large lowland rivers and artificial reservoirs. It strongly affects invaded ecosystems by filtration, fouling and increasing substratum heterogeneity (Karatayev et al., 2002). Zebra mussel life cycle includes a planktonic larva that, after spending several days in the water column, attaches to substratum by byssal threads and metamorphoses into an adult. However, mussels retain the ability to abandon their byssi at any time and crawl over substratum to find a new site (Ackerman et al., 1994). Detachment of mussels occurs in the field when they are dislodged by water flow or after substratum disintegration, e.g. when macrophytes decay (Lewandowski, 2001). Such individuals must find a new, appropriate site and attach as soon as possible. Moreover, mussels quite often detach actively (Kobak et al., 2009) and relocate onto new substrata, such as newly deployed surfaces (Ackerman et al., 1994; Czarnecka, 2006), materials cleaned from epiphytic growth (Lauer & Spacie, 2004) or nearby conspecifics (Stańczykowska, 1964). Despite the short duration of such detachment events in a mussel life, they are critical periods, when an animal is particularly exposed to adverse environmental factors, including predation risk. That is why its behaviour (e.g. reattachment, locomotion or site selection) during such periods may be especially important for its survival.
Zebra mussels are commonly consumed by waterfowl, fish and crayfish (Molloy et al., 1997), which can considerably affect its population structure and abundance (Molloy et al., 1997; Bartsch et al., 2005; Werner et al., 2005). One of the most efficient predators of D. polymorpha is roach Rutilus rutilus (L.). Fish of this species start to feed on zebra mussels at the body length of ca. 17–18 cm (Molloy et al., 1997). Roach that have just started to feed on mussels take mainly small animals, ca. 8–10 mm shell length, while larger fish (body length > 210 mm), being typical molluscivores, prefer larger individuals (11–17 mm) (Prejs et al., 1990). Zebra mussels smaller than 8 mm are usually rejected by roach of all sizes and those larger than 17 mm are also less often consumed (Prejs et al., 1990). Various interactions between zebra mussels and their predators, including behavioural defences, can be expected. So far, a reduction of the filtration rate of D. polymorpha and changes in its feeding selectivity in the presence of molluscivorous roach Rutilus rutilus and a crayfish Pacifastacus leniusculus (Dana) have been documented (Naddafi et al., 2007), though it is uncertain whether the mussels in this study were affected directly by predators or by conspecifics consumed by them. Furthermore, in populations assumed to live under high predation pressure, small mussels (8–10 mm shell length) have heavier shells and the growth rate of medium mussels (12–14 mm) is reduced (Czarnołęski et al., 2006), either due to individual plasticity or evolutionary changes. Small and medium-sized zebra mussels (<17 mm shell length) were also found to respond to the presence of large roach (>15 cm) by increasing their attachment strength, forming larger aggregations and reducing upward movement (Kobak & Kakareko, 2009), while large mussels (>17 mm) did not respond to any roach cues (Kobak & Kakareko, 2009).
In the present study, we address further questions dealing with zebra mussel responses to predators: (1) the effect of predator size, to check their capability of discriminating between fish constituting a real danger and small individuals, feeding on other types of food, and (2) the effect of exposure time in the presence of predators. We carried out a laboratory experiment to check zebra mussel attachment and aggregation behaviour in the presence of fish with different feeding capabilities and habits: large roach Rutilus rutilus, a well adapted molluscivore, small roach, unable to consume zebra mussels (Molloy et al., 1997) and European perch Perca fluviatilis (L.), not reported to feed on them (Craig, 1987; Molloy et al., 1997; Brylińska, 2000). On the basis of our previous study (Kobak & Kakareko, 2009), we hypothesised that mussels would attach more strongly and form aggregations more frequently in the presence of roach, especially large fish. We expected a stronger reaction of mussels after a longer exposure time and in the presence of large roach, being a real danger for them (Prejs et al., 1990; Molloy et al., 1997).
Methods
Experimental animals
Mussels were collected by divers from the dam of the Włocławek Reservoir (the lower Vistula River, central Poland) from the depth of ca. 5 m. They were kept in a 500-l aquarium filled with aerated water (temperature 15–20.5°C). Ca. 20% of water volume was exchanged every 3–4 weeks to avoid the accumulation of wastes in the aquarium. The mussels were divided into small (mean shell length: 9.5 mm, range: 8.2–9.9 mm) and large (15.4 mm, 13.3–16.9 mm) size classes. Selection of the size classes was based on previous research, dealing with the feeding preferences of roach (Prejs et al., 1990) and the responses of mussels to predator cues (Kobak & Kakareko, 2009). We did not test mussels within 2 weeks of collection to allow them to acclimate to laboratory conditions or later than 4 months after collecting, to avoid the potential effects of prolonged captivity on their behaviour. The mussels were not fed during the study course. However, they are known to tolerate long periods of food deprivation without substantial loss of condition (Chase & McMahon, 1995). We only used animals attached to the substratum in the rearing aquarium. Each individual was only examined once.
We tested the following fish: (1) large roach (180–250 mm total length), capable of feeding on zebra mussels (Prejs et al., 1990); (2) small roach (80–110 mm), having pharyngeal teeth too weak to crush zebra mussel shells (Prejs et al., 1990) and feeding on other types of food: soft zoobenthos, small thin-shelled molluscs (Sphaeriidae), zooplankton and plants (Brylińska, 2000; Kakareko, 2002); (3) European perch (100–180 mm), which have never been reported to feed on zebra mussels (Craig, 1987; Molloy et al., 1997; Brylińska, 2000). In this study, perch was included to control for potential mussel responses to cues other than kairomones, such as changes in water chemistry (e.g. due to faeces).
All studied species occur in the reservoir, from which the mussels were collected (Kakareko, 2000). They were collected in eutrophic lakes of North-Eastern Poland by anglers and commercial fishermen using seine nets, as well as in the Włocławek Reservoir by electrofishing. The fish were transported to the laboratory in plastic bags with oxygenated water and placed in 100–500 l tanks (each species and size class separately) with filtered, aerated water at temperature of 12.8–17.9°C. Every 2 weeks, we exchanged ca. 25% of water volume to keep the tanks clean.
Before the experiments, the fish were adapted to laboratory conditions for at least 1 month. The fish were fed on frozen chironomids and blended beef heart. Mussels were excluded from their diet to avoid potential signals from crushed conspecifics.
Experimental setup
We conducted the experiments in 4 100-l tanks (treatments) containing randomly selected fish of a given kind (2 large roach, 5 small roach or 3–4 perch individuals). We assumed that the numbers of fish were sufficient to produce the amount of kairomones (provided that they were produced at all) capable of triggering mussel responses. At the same time, the numbers of fish were low enough to ensure suitable life conditions for them during the trials (20–50 l of water per fish, depending on their sizes). The fourth, control treatment was a tank devoid of any fish. Water in all the tanks was aerated, filtered and mixed using aquarium pumps (Fan-3 Plus Filter, Aquael, Warsaw, Poland). The pumps allowed for even mixing of a potential fish kairomone in water and its access to the tested mussels. We checked the water quality using a multimeter Multi340i (WTW GmbH, Weilheim, Germany). Temperature (mean: 15.8°C, range: 14.2–19.1°C), conductivity (558, 525–581 μS/cm), pH (8.2, 7.8–8.6) and oxygen concentration (8.0, 6.9–9.4 mg/l) were similar among treatments. The tanks were illuminated by natural light scattered by the closed blinds in the window of the laboratory room (ca. 30–300 lx at the water surface, depending on the weather; the natural photoperiod between November and May).
To study mussel attachment strength, we used boxes made from polyvinyl chloride (PVC) tiles (100 × 100 × 5 mm), a suitable substratum for zebra mussels (Walz, 1973; Ackerman et al., 1995; Kobak, 2004). The boxes consisted of the bottom and four walls joined by rubber bands and roofed with 1-mm nylon mesh (Kobak, 2006). The boxes prevented mussels from leaving the tiles and protected them from predators. Before each trial, we cleaned the tiles with sandpaper and put them in water without fish for 1 week before use. This period is sufficient for the development of a biofilm, making the substratum more suitable for mussels (Kavouras & Maki, 2003). At the beginning of each trial, we put the boxes with 10 small or large mussels into the experimental tanks. The fish could move all over the tanks, also around and above the boxes. Thus, any substances released by the potential predators could easily reach the mussels. Mussels were exposed in the experimental tanks for 1 or 6 days. At the end of a trial, we counted aggregated (clustered) individuals (i.e. attached in a direct physical contact with another mussel) and singletons (without any physical contact with conspecifics). Then we disassembled the boxes and measured the attachment strength of each mussel using a digital dynamometer (FG-5000A, Lutron Electronic Enterprise Co., Ltd, Taipei, Taiwan) connected with a forceps holding a mussel. We pulled the device gently perpendicular to the tile until the mussel was detached (Hubertz, 1994). The attachment strength of individuals that were too crowded to access with the forceps was not measured. The adhesion of those mussels that attached to conspecifics, rather than to the tiles, was also not tested, so that all analysed individuals were attached to the same substratum type (PVC). Altogether, only ca. 3% of mussels were excluded from the analysis due to the above reasons, which should not bias the results in any way. We used the mussels from the vertical tiles, as mussels attach similarly to vertical and horizontal surfaces (Kobak, 2006). In another study, conducted according to a similar procedure, mussel attachment strength increased significantly during the first 4 days and then stabilized at a constant level (Kobak, 2006). Thus, we measured attachment strength in its initial (day 1) and stable (day 6) phases.
The treatments (tanks with 2 large roach, 5 small roach, 3–4 perch and control) were replicated 10 times. In each tank, 4 boxes (with large or small mussels, exposed for 1 or 6 days) were exposed simultaneously in each replicate. Thus, altogether 16 different variants were tested. We cleaned the tanks and changed water and fish between the replicates. The 1-day trials started not earlier than 2 days after a water change to assure the sufficient concentration of a potential fish kairomone.
Statistical analysis
We analysed mussel attachment strength using a four-way ANOVA with 3 fixed factors: (1) fish presence (large roach, small roach, perch, control), (2) mussel size (small, large) and (3) exposure time (1 day, 6 days) and 1 random factor: replicate. We compared the percentages of mussels forming aggregations in various treatments with a three-way ANOVA (factors: fish presence, mussel size, exposure time). We used Tukey HSD test as a post-hoc procedure. To avoid violations of the ANOVA assumptions of normality and homoscedasticity (checked with Kolmogorov–Smirnov and Levene tests, respectively), we log-transformed the data for both analyses.
Results
Attachment strength
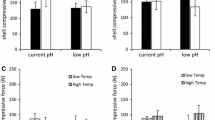
In the analysis of mussel attachment strength, we found a significant fish presence x exposure time interaction (ANOVA: F 3, 27 = 14.0, P < 0.001, Table 1A). It resulted from the fact that there was no significant effect of the presence of fish after a 1-day exposure, whereas after a 6-day exposure the attachment strength of the mussels kept in the presence of large roach was the highest (Fig. 1) and differed significantly from that of the other individuals (Tukey test, P < 0.001, Table 1B). Attachment also increased with mussel size and exposure time (Fig. 1), with a greater change exhibited by large mussels (a significant mussel size x exposure time interaction in ANOVA: F 1, 9 = 96.3, P < 0.001, Table 1). The interactions involving the mussel size and fish presence factors were insignificant (ANOVA: P > 0.05, Table 1A), showing that both size classes responded similarly to the presence of fish. The interactions among the random factor replicate and the fixed factors were also insignificant (ANOVA: P > 0.05), indicating that the mussel responses to the tested variables did not change during the duration of the entire experiment.
Aggregation behaviour
The mussels rarely attached to conspecific shells, but often formed clusters by attaching to the PVC substratum in a direct physical contact with the conspecifics. The percentage of animals forming aggregations ranged from 22% of small mussels after a 1-day exposure in the control treatment to 77% of large mussels after a 6-day exposure in the presence of large roach (Fig. 2). The mussel reactions to the presence of fish depended on the exposure time, as indicated by the significant interaction between these factors (ANOVA: F 3, 144 = 3.2, P = 0.025, Table 2A). After 1 day, the mussels were more aggregated in the presence of all kinds of fish than in the control treatment (Fig. 2A, C), as shown by the Tukey test (P < 0.001, Table 2B). In the control and large roach treatments, the percentages of clustered mussels increased significantly after a longer exposure (Tukey test, P < 0.001 and P < 0.01, respectively), while the aggregation levels of the mussels exposed to small roach and perch did not change significantly between 1 and 6 days (Tukey test, P > 0.05, Table 2B). As a result, after 6 days the mussels exposed to large roach were the most aggregated (Fig. 2B, D) and differed significantly with this respect from those from the other treatments (Tukey test, P < 0.05, Table 2B), except that with small roach. The aggregation levels in the small roach and perch treatments after 6 days no longer differed significantly from that observed in the control treatment (Tukey test, P > 0.05, Table 2B).
Discussion
Attachment strength
The mussels after a longer exposure to large roach were more strongly attached to the substratum than the individuals in the other treatments (Fig. 1). A similar response of zebra mussels was observed in our previous study (Kobak & Kakareko, 2009). Anti-predator defences can be also triggered by alarm substances released by wounded conspecifics (Pijanowska & Kowalczewski, 1997; Cheung et al., 2004; Wisenden et al., 2008). This phenomenon has been also observed in zebra mussels (Toomey et al., 2002), but, as in our study the predators were not fed on molluscs, this was almost certainly due to the effects of fish. Roach occur in the native range of zebra mussels (Brylińska, 2000), so a long co-evolution of interactions between both species was possible. Dreissenids and other byssate bivalves (e.g. Mytilus sp.), can constitute most of the diet of large roach (Prejs et al., 1990; Westerbom et al., 2006). Thus, the effect of large roach on bivalve populations can be considerable and mussels could benefit from their anti-predator behaviour in the presence of this fish.
The mussel adhesion strength in the presence of non-molluscivorous perch did not differ from that observed in the control treatment (Fig. 1). Thus, the observed modifications of attachment resulted from detecting a potential predator (large roach), and not from the changes in water quality caused by any kind of fish. The attachment strength increased with time and the differences among treatments became evident after a longer exposure (Table 1B). Probably, a detached mussel tries to attach itself as soon as possible, regardless of the presence or absence of other cues, including predators. The prolonged presence of predators, indicating their permanent high local density and hence a high level of predation risk, could affect the subsequent behaviour of mussels and result in their stronger attachment after a longer time. Anti-predator defences were observed to differ depending on exposure time. Reduced activity was an initial response of Mytilus sp. to predators, later followed by a period of higher activity, leading to greater attachment strength and searching for a refuge. Similarly to our results, responses of Mytilus sp. after a longer exposure to predators were stronger (Reimer & Tedengren, 1997; Reimer & Harms-Ringdahl, 2001). At even longer time scales (weeks or months), yet different responses to predators take place, such as changes of shell thickness and shape and/or growth rate (Reimer & Harms-Ringdahl, 2001; Krist, 2002; Weber, 2003; Czarnołęski et al., 2006).
Adhesion to hard substratum is essential for survival of byssate bivalves and protects them against adverse environmental factors, such as waves (Bell & Gosline, 1997) and toxins (Rajagopal et al., 2005). Our study has shown that it may also be a form of an anti-predator defence, as we observed the higher attachment strength of mussels in the presence of efficient molluscivores. Breaking strong byssal threads needs considerable energy investments and increases the risk of injury (Smallegange & Van Der Meer, 2003). Therefore, predators may abandon potential prey which is too strongly attached (Nagelkerke & Sibbing, 1996). The efficiency of this type of behaviour has been demonstrated by Green et al. (2008), who observed that detached zebra mussels were more vulnerable to the predation by crayfish Orconectes rusticus than attached individuals. Strong attachment also increased the survival of Mytilus sp. in the presence of predatory starfish and crabs (Reimer & Tedengren, 1997; Reimer & Harms-Ringdahl, 2001).
Aggregation behaviour
After 1 day, the mussels responded to all kinds of fish by forming clusters more often than in the control treatment (Fig. 2, Table 2B). However, the mussels tested in the presence of perch and small roach did not increase significantly their aggregation level after a longer exposure (Table 2B). In contrast to them, the control mussels kept clumping and after 6 days their aggregation level no longer differed significantly from that observed in the presence of perch and small roach (Fig. 2, Table 2B). The gradual increase of the aggregation level of zebra mussels with time was also observed by Stańczykowska (1964) on various types of substrata. The rate of this process was probably increased by the presence of any kind of fish, while the final percentage of clustered individuals was not affected. The mussels exposed to large roach for 6 days continued aggregating and finally significantly exceeded the clumping level observed in the other treatments (Fig. 2, Table 2B). Thus, the aggregative response of mussels to large roach seems to be distinct from their reaction to the other fish.
Zebra mussels are gregarious animals, living in dense clusters or druses and exhibiting a natural tendency to aggregate, independent of the substratum quality (Stańczykowska, 1964; Chase & Bailey, 1999; Czarnołęski et al., 2003). Our study shows that the presence of predators can further increase this tendency. Aggregation forming is a common anti-predator defence used by various organisms, such as cladocerans (Pijanowska & Kowalczewski, 1997), bivalves (Reimer & Tedengren, 1997; Côté & Jelnikar, 1999) and fish (Magurran, 1990). It is effective because capturing an individual from the centre of an aggregation is more difficult due to the reduced accessibility and smaller exposed surface of such prey (Cheung et al., 2004). A group of mussels attached to one another is also more difficult to handle than a single individual.
Two mechanisms of forming conspecific aggregations of mussels are possible: (1) increased random movement during which mussels stop after encountering a conspecific and (2) movement mediated by chemical attraction to conspecifics. In the former case, it could be expected that aggregated mussels moved for a longer time, and therefore, started to attach to the substratum later than singletons. In consequence, the attachment strength of clustered mussels, having less time for attachment, should be lower (or at least not higher) than that of single individuals. However, our results did not confirm this expectation, as the predator-exposed mussels, which were more aggregated than the control individuals (Fig. 2), were also more strongly attached to the substratum (Fig. 1). Furthermore, in an earlier study, we did not observe any increase of the horizontal locomotion of zebra mussels in the presence of roach (Kobak & Kakareko, 2009). Actually, mussels were even found to reduce their movement after detecting crushed conspecifics (Toomey et al., 2002) or strong light (Kobak & Nowacki, 2007, Kobak et al., 2009). These results suggest that mussel aggregation behaviour was mediated by the chemical attraction to conspecifics, rather than by the increased random movement, though the exact mechanism of this phenomenon is yet to be explained in the future. Côté & Jelnikar (1999) and Nicastro et al. (2007) also postulated the chemical attraction among individuals as an aggregation mechanism in Mytilus sp. On the other hand, Uryu et al. (1996) explained the clumping of another bivalve Limnoperna fortunei (Dunker) by simple random movement. It is also possible that the mussels exposed to roach increased the rate of byssal thread production. In this case, they would have been attached more strongly independent of the actual attachment time.
The effect of predator size and mussel size
Zebra mussels responded with stronger attachment only to the presence of large, molluscivorous roach. The cues responsible for differentiation between a dangerous predator and a harmless specimen from the same species are unknown. We can assume that in all treatments the concentration of a potential kairomone was sufficient to trigger mussel responses, due to the considerable density of fish in a relatively small tank and quite a long time of their presence in the tank. Perhaps, mussels could detect some substances released to water by sexually mature roach. They mature at the age of 3 years, when their body length is ca. 110–120 mm (Brylińska, 2000). Thus, the large roach in our study were probably mature, while the small fish were close to this threshold size. Obviously, further studies are needed to confirm this hypothesis. Nevertheless, zebra mussels seem to be able to recognize a predator constituting a real danger for them. Other taxa, such as blue mussels, cladocerans and gammarids were also observed to discriminate among various types of predators and vary their defences accordingly (Baumgärtner et al., 2002; Weber, 2003; Freeman, 2007).
The studied mussels, independent of their size, responded similarly to the presence of large roach by attaching more strongly to the substratum (Fig. 1) and more often selecting sites in the direct proximity of conspecifics (Fig. 2). Previous research has shown that yet larger zebra mussels (>17 mm shell size) do not exhibit any defensive responses to roach (Kobak & Kakareko, 2009). According to Prejs et al. (1990), the mussels tested in our study (<17 mm) were within the range endangered by roach predation and consequently they responded to the presence of fish. Similarly to other studies, smaller mussels were less strongly attached than larger individuals (Ackerman et al., 1995; Kobak, 2006) and attachment strength increased with time in all treatments (Clarke & McMahon, 1996; Kobak, 2006).
Conclusions
Direct or indirect symptoms of the increased predation risk stimulate zebra mussels to select sites within bivalve colonies (the present study), as well as sheltered, dark, near-bottom locations (Thorp et al., 1994; Lewandowski, 2001; Kobak & Nowacki, 2007; Kobak & Kakareko, 2009). Such behaviour protects mussels from predation, but at the same time leads to occupying places, which can be suboptimal in terms of food availability and water quality due to the strong intraspecific competition (Tuchman et al. 2004) and accumulation of waste in dense mussel colonies (Burks et al., 2002). Furthermore, mussels exposed to predators allocate more energy into attachment to the substratum. That is why the observed defences are inducible and appear only after detecting a predator, instead of being exhibited continuously. A similar trade-off was observed in other prey taxa, which reduce their feeding activity (damselflies; Koperski, 1997), spend more time in places with low food availability (zooplankton and planktivorous fish; De Meester et al., 1999; Gliwicz, 2005) and/or become less resistant to starvation due to attaining smaller size (zooplankton; Gliwicz, 1990; Weber, 2003) in order to decrease the predation risk.
It should be noted that the roach density (and thus the concentration of a potential fish kairomone) in the relatively small, closed tanks used in our study probably exceeded that found in the field, though, due to the occurrence of roach in large shoals (Pavlov et al., 1986), local concentrations of their kairomones can be quite high. Other organisms, e.g. planktonic cladocerans, can respond differently to various concentrations of predator kairomones, with stronger reactions observed at higher predator densities (e.g. von Elert & Pohnert, 2000; Baumgärtner et al., 2002; Weetman & Atkinson, 2002). A threshold level of kairomone sufficient to trigger the mussel responses observed in our study needs to be estimated in further research. Nevertheless, it seems unlikely that mussels do not exhibit similar predator-induced behavioural changes in the field. Their responses certainly involve complex adaptations of sensory systems (to detect predators and conspecifics), behaviour (to move towards a conspecific) and physiology (to modify the byssus production rate). Thus, it is improbable that these traits are only manifested in artificial laboratory conditions and have (or had in the past) no effect on the animal fitness in the field.
Our study does not show directly whether the observed behavioural changes increase chances of mussel survival in the presence of large roach. Moreover, it is not known if mussel reactions depend on the predator diet (neutral or including conspecific prey specimens), which was observed in other bivalves (Smith & Jennings, 2000; Cheung et al., 2006; Griffiths & Richardson, 2006), fish (Pettersson et al., 2000) and cladocerans (Pijanowska & Kowalczewski, 1997). These questions should be addressed by the future studies.
References
Ackerman, J. D., B. Sim, S. J. Nichols & R. Claudi, 1994. A review of the early life history of zebra mussels (Dreissena polymorpha) comparisons with marine bivalves. Canadian Journal of Zoology 72: 1169–1179.
Ackerman, J. D., C. M. Cottrell, C. R. Ethier, D. G. Allen & J. K. Spelt, 1995. A wall jet to measure the attachment strength of zebra mussel. Canadian Journal of Fisheries and Aquatic Sciences 52: 126–135.
Bartsch, M. R., L. A. Bartsch & S. Gutreuter, 2005. Strong effects of predation by fishes on an invasive macroinvertebrate in a large floodplain river. Journal of the North American Benthological Society 24: 168–177.
Baumgärtner, D., A.-D. Jungbluth, U. Koch & E. von Elert, 2002. Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli. Archiv für Hydrobiologie 155: 353–367.
Bell, E. C. & J. M. Gosline, 1997. Strategies for life in flow: tenacity, morphometry, and probability of dislodgment of two Mytilus species. Marine Ecology – Progress Series 159: 197–208.
Brylińska, M., 2000. Ryby słodkowodne Polski. (Freshwater fishes of Poland). PWN, Warsaw: 521 pp (in Polish).
Burks, R. L., N. C. Tuchman, C. A. Call & J. E. Marsden, 2002. Colonial aggregates: effects of spatial position on zebra mussel responses to vertical gradients in interstitial water quality. Journal of the North American Benthological Society 21: 64–75.
Chase, R. & R. F. McMahon, 1995. Starvation tolerance of zebra mussels, Dreissena polymorpha. Proceedings of the Fifth International Zebra Mussel and Other Aquatic Nuisance Organisms Conference, Toronto, Canada, February 1995: 31–38.
Chase, M. E. & R. C. Bailey, 1999. The ecology of the zebra mussel (Dreissena polymorpha) in the Lower Great Lakes of North America: I. Population dynamics and growth. Journal of Great Lakes Research 25: 107–121.
Cheung, S. G., P. Y. Tong, K. M. Yip & P. K. S. Shin, 2004. Chemical cues from predators and damaged conspecifics affect byssus production in the green-lipped mussel Perna viridis. Marine and Freshwater Behaviour and Physiology 37: 127–135.
Cheung, S. G., K. C. Luk & P. K. S. Shin, 2006. Predator-labeling effect on byssus production in marine mussels Perna viridis (L.) and Brachidontes variabilis (Krauss). Journal of Chemical Ecology 32: 1501–1512.
Clarke, M. & R. F. McMahon, 1996. Effects of temperature on byssal thread production by the freshwater mussel, Dreissena polymorpha (Pallas). American Malacological Bulletin 13: 105–110.
Côté, I. M. & E. Jelnikar, 1999. Predator-induced clumping behaviour in mussels (Mytilus edulis Linnaeus). Journal of Experimental Marine Biology and Ecology 235: 201–211.
Craig, J. F., 1987. The biology of perch and related fish. Timber Press Portland, Oregon: 333 pp.
Czarnecka, M., 2006. Ekologiczny status epifauny zasiedlającej sztuczne podłoża podwodne. (Ecological status of epifauna on artificial underwater substrata). Ph. D. Thesis, N. Copernicus University, Toruń: 178 pp (in Polish).
Czarnołęski, M., J. Kozłowski, A. Stańczykowska & K. Lewandowski, 2003. Optimal resource allocation explains growth curve diversity in zebra mussels. Evolutionary Ecology Research 5: 571–587.
Czarnołęski, M., J. Kozłowski, P. Kubajak, K. Lewandowski, T. Müller, A. Stańczykowska & K. Surówka, 2006. Cross-habitat differences in crush resistance and growth pattern of zebra mussels (Dreissena polymorpha): effects of calcium availability and predator pressure. Archiv für Hydrobiologie 165: 191–208.
De Meester, L., P. Dawidowicz, E. Van Gool & C. J. Loose, 1999. Ecology and evolution of predator-induced behavior of zooplankton: depth selection behavior and diel vertical migration. In Tollrian, R. & C. D. Harvel (eds), The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton: 160–176.
Freeman, A. S., 2007. Specificity of induced defenses in Mytilus edulis and asymmetrical predator deterrence. Marine Ecology – Progress Series 334: 145–153.
Gliwicz, Z. M., 1990. Food thresholds and body size in cladocerans. Nature 343: 638–640.
Gliwicz, Z. M., 2005. Food web interactions: why are they reluctant to be manipulated? Plenary Lecture. Verhandlungen Internationale Vereinigung für theoretische und angewandte Limnologie 29: 73–88.
Green, N. S., B. A. Hazlett & S. Prueff-Jones, 2008. Attachment and shell integrity affects the vulnerability of zebra mussels (Dreissena polymorpha) to predation. Journal of Freshwater Ecology 23: 91–99.
Griffiths, C. L. & C. A. Richardson, 2006. Chemically induced predator avoidance behaviour in burrowing bivalve Macoma balthica. Journal of Experimental Marine Biology and Ecology 331: 91–98.
Hubertz, E., 1994. Procedure for measuring the force required to remove zebra mussels from substrate. Zebra Mussel Technical Notes Collection, U.S. Army Engineer Research & Development Center, Vicksburg, MS. ZMR-1-19: 1–4.
Ishida, S. & K. Iwasaki, 2003. Reduced byssal thread production and movement by the intertidal mussel Hormomya mutabilis in response to effluent from predators. Journal of Ethology 21: 117–122.
Kakareko, T., 2000. The ichthyofauna of the lower Vistula River. The state of art and the program of research. Acta Universitatis Nicolai Copernici, Limnological Papers 21: 85–100.
Kakareko, T., 2002. The importance of benthic fauna in the diet of small common bream Abramis brama (L.), roach Rutilus rutilus (L.), pikeperch Sander Lucioperca (L.) and ruffe Gymnocephalus cernuus (L.) in the Włocławek Reservoir. Archives of Polish Fisheries 10: 221–231.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2002. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Leppäkoski, E., S. Gollasch & S. Olenin (eds), Invasive Aquatic Species of Europe: Distribution, Impacts and Management. Kluwer Academic Publishers, Boston: 433–446.
Kavouras, J. H. & J. S. Maki, 2003. The effects of natural biofilms on the reattachment of young adult zebra mussels to artificial substrata. Biofouling 19: 247–256.
Kobak, J., 2004. Recruitment and small-scale spatial distribution of Dreissena polymorpha (Bivalvia) on artificial materials. Archiv für Hydrobiologie 160: 25–44.
Kobak, J., 2006. Factors influencing the attachment strength of Dreissena polymorpha (Bivalvia). Biofouling 22: 153–162.
Kobak, J. & T. Kakareko, 2009. Attachment strength, aggregation and movement of the zebra mussel (Dreissena polymorpha, Bivalvia) in the presence of potential predators. Fundamental and Applied Limnology (Archiv für Hydrobiologie) 174: 193–204.
Kobak, J. & P. Nowacki, 2007. Light-related behaviour of zebra mussel (Dreissena polymorpha, Bivalvia). Fundamental and Applied Limnology/Archiv für Hydrobiologie 169: 341–352.
Kobak, J., M. Poznańska & T. Kakareko, 2009. Effect of attachment status and aggregation on behaviour of the zebra mussel, Dreissena polymorpha, Bivalvia. Journal of Molluscan Studies 75: 109–117.
Koperski, P., 1997. Changes in feeding behaviour of the larvae of the damselfly Enallagma cyathigerum in response to stimuli from predators. Ecological Entomology 22: 167–175.
Krist, A. C., 2002. Crayfish induce a defensive shell shape in a freshwater snail. Invertebrate Biology 121: 235–242.
Lass, S. & P. Spaak, 2003. Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491: 221–239.
Lauer, T. E. & A. Spacie, 2004. Space as a limiting resource in freshwater systems: Competition between zebra mussels (Dreissena polymorpha) and freshwater sponges (Porifera). Hydrobiologia 517: 137–145.
Lewandowski, K., 2001. Development of populations of Dreissena polymorpha (Pall.) in lakes. Folia Malacologica 9: 171–213.
Magurran, A. E., 1990. The adaptive significance of schooling as an antipredator defence in fish. Annales Zoologici Fennici 27: 51–66.
Molloy, D. P., A. Y. Karatayev, L. E. Burlakova, D. P. Kurandina & F. Laruelle, 1997. Natural enemies of zebra mussels: predators, parasites, and ecological competitors. Reviews in Fisheries Science 5: 27–97.
Naddafi, R., P. Eklöv & K. Pettersson, 2007. Non-lethal predator effects on the feeding rate and prey selection of the exotic zebra mussel Dreissena polymorpha. Oikos 116: 1289–1298.
Nagelkerke, L. A. J. & F. A. Sibbing, 1996. Efficiency of feeding on zebra mussel (Dreissena polymorpha) by common bream (Abramis brama), white bream (Blicca bjoerkna), and roach (Rutilus rutilus): the effects of morphology and behavior. Canadian Journal of Fisheries and Aquatic Sciences 53: 2847–2861.
Nicastro, K. R., G. I. Zardi & C. D. McQuaid, 2007. Behavioural response of invasive Mytilus galloprovincialis and indigenous Perna perna mussels exposed to risk of predation. Marine Ecology-Progress Series. 336: 169–175.
Orr, M. V., M. El-Bekai, M. Lui, K. Watson & K. Lukowiak, 2007. Predator detection in Lymnaea stagnalis. Journal of Experimental Biology 210: 4150–4158.
Pavlov, D. S., A. G. Gusar, A. Pyanov & A. N. Gorin, 1986. The results of hydroacoustic observations on roach in Lake Glubokoe in winter. Hydrobiologia 141: 125–132.
Pettersson, L. B., P. A. Nilsson & C. Brönmark, 2000. Predator recognition and defence strategies in crucian carp, Carassius carassius. Oikos 88: 200–212.
Pijanowska, J. & A. Kowalczewski, 1997. Predators can induce swarming behaviour and locomotory responses in Daphnia. Freshwater Biology 37: 649–656.
Prejs, A., K. Lewandowski & A. Stańczykowska, 1990. Size-selective predation by roach (Rutilus rutilus) on zebra mussel (Dreissena polymorpha): field studies. Oecologia 83: 378–384.
Rajagopal, S., G. Van der Velde, M. Gaag & H. A. van der Jenner, 2005. Byssal detachment underestimates tolerance of mussels to toxic compounds. Marine Pollution Bulletin 50: 20–29.
Reimer, O. & S. Harms-Ringdahl, 2001. Predator-inducible changes in blue mussels from the predator-free Baltic Sea. Marine Biology 139: 959–965.
Reimer, O. & M. Tedengren, 1997. Predator-induced changes in byssal attachment, aggregation and migration in the blue mussel, Mytilus edulis. Marine and Freshwater Behaviour and Physiology 30: 251–266.
Smallegange, I. M. & J. Van Der Meer, 2003. Why do shore crabs not prefer the most profitable mussels? Journal of Animal Ecology 72: 599–607.
Smith, L. D. & J. A. Jennings, 2000. Induced defensive responses by the bivalve Mytilus edulis to predators with different attack modes. Marine Biology 136: 461–469.
Stańczykowska, A., 1964. On the relationship between abundance, aggregations and “condition” of Dreissena polymorpha Pall. in 36 Mazurian Lakes. Ekologia Polska A 34: 653–690.
Thorp, J. H., J. E. Alexander, K. S. Greenwood, A. F. Casper, R. K. Kessler, A. R. Black, W. Fang, A. F. Westin, R. B. Summers, T. W. Sellers & B. Lewis, 1994. Predicting success of riverine populations of zebra mussels (Dreissena polymorpha) – early colonization and microhabitat distribution in the Ohio River. Proceedings of The Fourth International Zebra Mussel Conference, Madison, Wisconsin, March 1994: 456–476.
Toomey, M. B., D. McCabe & J. E. Marsden, 2002. Factors affecting the movement of adult zebra mussels (Dreissena polymorpha). Journal of the North American Benthological Society 21: 468–475.
Tuchman, N. C., R. L. Burks, C. A. Call & J. Smarrelli, 2004. Flow rate and vertical position influence ingestion rates of colonial zebra mussels (Dreissena polymorpha). Freshwater Biology 49: 191–198.
Uryu, Y., K. Iwasaki & M. Hinoue, 1996. Laboratory experiments on behaviour and movement of a freshwater mussel, Limnoperna fortunei (Dunker). Journal of Molluscan Studies 62: 327–341.
von Elert, E. & G. Pohnert, 2000. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos 88: 119–128.
Walz, N., 1973. Studies on the biology of Dreissena polymorpha in Lake Constance. Archiv für Hydrobiologie (Supplement) 42: 452–482.
Weber, A., 2003. More than one ‘fish kairomone’? Perch and stickleback kairomones affect Daphnia life history traits differently. Hydrobiologia 498: 143–150.
Weetman, D. & D. Atkinson, 2002. Antipredator reaction norms for life history traits in Daphnia pulex: dependence on temperature and food. Oikos 98: 299–307.
Werner, S., M. Mörtl, H. G. Bauer & K. O. Rothhaupt, 2005. Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at Lake Constance, Germany. Freshwater Biology 50: 1412–1426.
Westerbom, M., A. Lappalainen & O. Mustonen, 2006. Invariant size selection of blue mussels by roach despite variable prey size distributions. Marine Ecology – Progress Series 328: 161–170.
Wiąckowski, K., J. Fyda & A. Ciećko, 2004. The behaviour of an omnivorous protozoan affects the extent of induced morphological defence in a protozoan prey. Freshwater Biology 49: 801–809.
Wisenden, B. D., J. Karst, J. Miller, S. Miller & L. Fuselier, 2008. Anti-predator behaviour in response to conspecific chemical alarm cues in an esociform fish, Umbra limi (Kirtland 1840). Environmental Biology of Fishes 82: 85–92.
Yoshida, T., L. E. Jones, S. P. Ellner, G. F. Fussmann & N. G. Hairston Jr, 2003. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424: 303–306.
Acknowledgements
We wish to thank to Andrzej Denis, Szymon Denis, Hubert Denis and Józef Liczkowski for collecting mussels. We are also extremely grateful to Arkadiusz Mierzejewski and Krzysztof Puwalski for their help in capturing fish. Our study was supported by the Grant of the Polish Ministry of Science and Higher Education No. N N304 1530 33.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: L. B. Kats
Rights and permissions
About this article
Cite this article
Kobak, J., Kakareko, T. & Poznańska, M. Changes in attachment strength and aggregation of zebra mussel, Dreissena polymorpha in the presence of potential fish predators of various species and size. Hydrobiologia 644, 195–206 (2010). https://doi.org/10.1007/s10750-010-0113-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0113-2