Abstract
Phenotypic plasticity of the two salt marsh grasses Spartina alterniflora and Phragmites australis in salt marshes is crucial to their invasive ability, but the importance of phenotypic plasticity, nitrogen levels, and intraspecific competition to the success of the two species is unclear at present. Spartina alterniflora Loisel. is an extensively invasive species that has increased dramatically in distribution and abundance on the Chinese and European coasts, and has had considerable ecological impacts in the regions where it has established. Meanwhile, Phragmites australis Cav., a native salt marsh species on the east coast of China, has replaced the native S. alterniflora in many marshes along the Atlantic Coast of the US. This study determined the effects of nitrogen availability and culm density on the morphology, growth, and biomass allocation traits of Spartina alterniflora and Phragmites australis. A large number of morphological, growth, and biomass parameters were measured, and various derived values (culm: root ratio, specific leaf area, etc.) were calculated, along with an index of phenotypic plasticity. Nitrogen addition significantly affected growth performance and biomass allocation traits of Spartina alterniflora, and culm density significantly affected morphological characteristics in a negative way, especially for Spartina alterniflora. However, there were no significant interactions between nitrogen levels and culm density on the morphological parameters, growth performances parameters, and biomass allocation parameters of the two species. Spartina alterniflora appears to respond more strongly to nitrogen than to culm density and this pattern of phenotypic plasticity appears to offer an expedition for successful invasion and displacement of Phramites australias in China. The implication of this study is that, in response to the environmental changes that are increasing nitrogen levels, the range of Spartina alterniflora is expected to continue to expand on the east coast of China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental conditions occupied by plants are highly variable, both spatially and temporally. As a consequence of such environmental heterogeneity, a remarkable phenotypic variation can be observed among individuals of the same genotype developing in different habitats (Avramov et al., 2007). Phenotypic plasticity is the ability of a genotype to produce distinct phenotypes when exposed to different environments during its ontogeny and it has been regarded as an important ecological agent in expanding the habitat range of a species (Zhu et al., 2007). It is essential for the survival of most plant species that they show phenotypic plasticity via variations of morphological and ecophysiological traits in heterogeneous and variable environments, especially for invasive plants.
Rapid changes in morphology and growth characteristics often occur in plants in response to variation in resource availability (Maherali & Delucia, 2001; Wang & Feng, 2004). Because there is an intimate relationship between these changes and resource acquisition, the changes often facilitate the tolerance of stress and invasiveness (Tyler et al., 2007). Some researchers suggested that one of the most significant ways in which plants respond to changing environments is to change root morphology (Schlichting, 1986; Ryser & Lambers, 1995; Fransen et al., 1998), leaf shape and area (Perez et al., 1994), and biomass allocation pattern (Idestam-Almquist & Kautsky, 1995).
Coastal ecosystems are highly variable in various ways because they are affected by the processes of marine, terrestrial, and sometimes riverine ecosystems (Wang et al., 2006) and numerous studies have found that environmental factors play important roles in structuring and functioning of coastal salt marshes (Hemminga & Buth, 1991; Grace & Pugesek, 1997). Nitrogen is the primary factor limiting plant production in many coastal ecosystems. However, as a result of human development, eutrophication of coastal zones caused by nutrient input from watersheds has been one of the most pressing environmental concerns worldwide (Cloern, 2001). One consequence of excessive nutrient loading is the facilitation of species invasions (Dukes & Mooney, 1999). The way in which plant species adapt to changed nutrient conditions may be determined by analyzing their phenotypic response to different nitrogen levels (Elberse & Damme, 2003). In addition, alterations in culm density may crucially affect phenotypic plasticity (Chen, 2000; Cipollini & Bergelson, 2001). In plant populations, sizes of individuals are generally far from uniform. The inequality in plant size, or size hierarchy, has been well documented in even-aged monocultures (Nagashima & Terashima, 1995). Most of the skewed distributions reported are considered to be consequences of competition, because skewness of distribution increases with increase in initial plant density.
Another important factor in structuring salt marshes is plant invasion. Phenotypic plasticity is often cited as an important mechanism of plant invasion (Funk, 2008). However, few studies have evaluated the plasticity of a diverse set of traits among invasive and native species, particularly in different nitrogen resource and culm density habitats, and none have examined the functional significance of these traits.
Spartina alterniflora and Phragmites australis (hereafter referred to as Spartina and Phragmites) are rhizomatous perennial graminoid grasses that grow in the coastal marshes in dense monocultures. As invasive species, both Spartina and Phragmites present serious threats to the ecosystems they invade. Spartina, native to the East and Gulf coasts of North America, has become a highly invasive weed throughout coastal marshes of the Pacific (Poulin et al., 2002; Vasquez et al., 2006; Wang et al., 2006). In China, Spartina was first introduced in 1979 to stabilize shorelines, and now flourishes in coastal intertidal zones from Guangxi northward to Tianjin. Phragmites, a dominant salt marsh species in the east coast of China, also has replaced the native species, such as Spartina, in many marshes along the Atlantic coast of the US (Marks et al., 1994; Windham & Lathrop, 1999). The dramatic invasions of both Spartina and Phragmites have caused many ecological and economic problems in China and North America. However, it is interesting that in North America Phragmites is moving from high to low marshes to invade Spartia-dominated ecosystems, while in China Spartina is threatening high Phragmites salt marshes. One of the possible explanations for the reciprocal invasions is that the environmental conditions in North America favor Phragmites, whereas those in China allow Spartina to express its competitiveness (Wang et al., 2006). Recently, it has been reported that increasing nutrient supply in coastal zones has positively promoted the invasion of both exotic species (Tyler et al., 2007), and analyzing the phenotypic plasticity of the two species in response to the increased nitrogen supply and culm density will enable us to examine the validity of this explanation for the range expansions of both species.
Here, the alien Spartina and native Phramites were studied by adjusting nitrogen level and culm density under greenhouse condition, while maintaining mono-cultures of the two species. The primary purpose was to evaluate statistically the impact of nitrogen level and culm density on the morphological characteristics, growth performances, and biomass allocation traits of two species and thus to identify optimal environmental conditions for plant growth. In particular, we wished to determine how phenotypic plasticity of the two species responded to different nitrogen level and culm density, and thus to explain and predict their invasive abilities in different coastal ecosystems.
Materials and methods
Plant and substrate materials
The individuals of two species and saline soil were collected at the end of March 2006 from the same area of Yancheng Natural Reserve (32°34′–34°28′N, 119°48′–121°150′E), Jiangsu Province, China. Table 1 shows the environmental background values of the two species on the coast of Jiangsu. For each species, individuals consisting of a single tiller with attached root material were separated, moved to the greenhouse and planted into plastic tanks (75 cm × 50 cm × 40 cm, length × width × height), which were filled with saline soil up to a depth of 20 cm. The plants were grown in the greenhouse for 4 weeks and watered daily to provide a large pool of healthy individuals for use in the following experiments.
Experimental design
Experiments were set up in the greenhouse of Nanjing University, China (32°10′37″N, 118°41′57″E) on 24 April 2006. They aimed to test the effects of nitrogen level (low, medium or high) and intra-specific competition on the morphology, growth and biomass allocation of Spartina and Phragmites growing at different densities. Thirty-six units of Spartina (34.4 ± 1.1 cm tall) and 36 Phragmites (44.6 ± 2.3 cm tall) were planted into 10 l plastic pots (28 cm in diameter, 25 cm in height) with 20 cm deep saline sand (12.5 kg dry weight) using 1, 2, and 3 stems per pot. This represented 4 repetitions × 2 species × 3 nitrogen levels × 3 densities. After planting, carbamide solution (with 46% available nitrogen) was added once every 2 weeks to the tanks from the top of the saline soil to maintain the soil nitrogen contents close to 0 mg/kg (N0 treatment), 60 mg/kg (N1) and 120 mg/kg (N2), to represent low, medium and high nitrogen treatments, respectively. The N loading expressed as 60 and 120 mg/kg of soil dry weight and calculated as available nitrogen (nitrogen weight/saline sand dry weight per tank). Each treatment has four replicate in this study. During the experiment, the salinity of substrates was maintained at 8–10‰ (to match field conditions) and all tanks were immersed to a depth of 3–5 cm above the sand. Salinity and water levels were monitored weekly, and adjusted to initial conditions by adding freshwater and crude salt.
Parameters measured
After treated with nitrogen for 15 weeks, all the plants were harvested and morphological and biomass allocation parameters were measured.
Parameters measured to reflect morphological characteristics were max-height of culm (MHC), stem diameter (SD), mean height of total culms (MHTC), total leaf areas (TLA), and specific leaf area (SLA, total leaf areas/total leaf weight). Those reflecting biomass allocation were above-biomass ratio (ABR, the above-ground biomass/the total biomass), below-biomass ratio (BBR, the belowground biomass/the total biomass), culm biomass ratio (CBR, culm biomass/the total biomass), leaf biomass ratio (LMR, leaf biomass/the total biomass), root biomass ratio (RMR, root biomass/the total biomass), rhizome biomass ratio (RhMR, rhizome biomass/the total biomass), root biomass/crown mass (R/C) and leaf area to root mass ratio (LARMR, total leaf areas/root biomass). Parameters measuring growth were RGR, NAR and LARm. For biomass allocation parameters, the plants from each replicate were divided into leaves and stems, and rhizomes and roots, which were, respectively, dried to constant weight at 80°C for 72 h and weighed (±0.1 mg). The weights of culm and leaves were combined to give the total aboveground biomass and those of roots and rhizomes to give the total belowground biomass of each plant.
The relative growth rate (RGR), net assimilation rate (NAR) and mean leaf area ratio (LARm) were quantified using the methods of Poorter (1999):
where \( \overline{{W_{1} }} \), \( \overline{{L_{1} }} \) are measures of the dry weight of initial total biomass (g) and total leaf area (cm2) (n = 10) of a plant, \( \overline{{W_{2} }} \), \( L_{2} \)are measures of the dry weight of harvest total biomass (g) and total leaf area (cm2), and \( \Updelta t \) is the time with nitrogen treatment. Phenotypic plasticity index (PPI) was calculated according to the formulas given by Valladares et al. (2000). PPI = 1 − \( {x \mathord{\left/ {\vphantom {x X}} \right. \kern-\nulldelimiterspace} X} \) where x and X are the minimum and the maximum mean values among the three density/N level treatments.
Data analysis
Two-way ANOVA was used to test the statistical difference of the effects on nitrogen treatments, culm density and their interactions on parameter variation. Tukey HSD test were conducted to examine the differences among the treatments. One-way ANOVA was used to test the statistical difference of the related parameters of the two species under the same nitrogen treatment/culm density. If necessary, biomass data were transformed (ln). Morphological data were transformed using either square root (max-height of culm, mean height of culms) or log10 (above-biomass ratio, below-biomass ratio and specific leaf area). All statistical analyses were conducted with SPSS 13.0 for Windows (SPSS Inc., USA). The figures are made with SigmaPlot 9.0.
Results
Effects of nitrogen levels and intraspecific competition on two species
Effects of nitrogen addition on two species
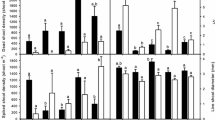
Nitrogen levels had a highly significant impact (P < 0.0001) on the morphological characteristic mean height of total culms of Spartina, with the individuals at medium nitrogen level producing the highest mean height of total culms (Table 2; Fig. 1a–d), while no significant effects of nitrogen on culm height were observed in Phragmites (Table 2; Fig. 2a–d). Nitrogen also showed highly significant effects (P < 0.001) on two growth parameters, relative growth rate and mean leaf area of Spartina but only on mean leaf area ratio of Phragmites (Table 2; Figs. 3a–c, 4a–c). In contrast, nitrogen had a similar, albeit slightly inconsistent, effects on biomass accumulation in both species with significant (P < 0.05) effects in one or both species for all parameters other than culm biomass ratio (Table 2; Figs. 5a–f, 6a–f). Under the same nitrogen level, Spartina generally had greater morphological parameter values and higher growth rate than Phragmites (Figs. 1, 2, 3, 4).
Effects of nitrogen levels and culm density on morphological traits of individual S. alterniflora.
a–f denotes, respectively: nitrogen levels (a–d) a MHC, b MHTC, c SD, d SLA; ( S,
S,  SS, ■ SSS); culm density (e–h) e MHC, f MHTC, g SD, h SLA (
SS, ■ SSS); culm density (e–h) e MHC, f MHTC, g SD, h SLA ( N0,
N0,  N1, ■ N2). N0, N1, N2: it means three nitrogen levels; N0 (0 mg kg−1), N1 (60 mg kg−1), N2 (120 mg kg−1). S, SS, SSS: it represents different density series of S. alterniflora. Each letter corresponds to one individual plant of that species. Treatments with different code letters are significantly different at the significance level of 5% (Tukey HSD test): uppercase letters denote differences between nitrogen levels or culm density; lowercase letters denote differences between different culm density under the same nitrogen level or different nitrogen level under the same culm density
N1, ■ N2). N0, N1, N2: it means three nitrogen levels; N0 (0 mg kg−1), N1 (60 mg kg−1), N2 (120 mg kg−1). S, SS, SSS: it represents different density series of S. alterniflora. Each letter corresponds to one individual plant of that species. Treatments with different code letters are significantly different at the significance level of 5% (Tukey HSD test): uppercase letters denote differences between nitrogen levels or culm density; lowercase letters denote differences between different culm density under the same nitrogen level or different nitrogen level under the same culm density
Effects of nitrogen levels and culm density on morphological traits of individual P. australis. a–f denotes, respectively: nitrogen levels (a–d) a MHC, b MHTC, c SD, d SLA; ( S,
S,  SS, ■ SSS); culm density (e–h) e MHC, f MHTC, g SD, h SLA(
SS, ■ SSS); culm density (e–h) e MHC, f MHTC, g SD, h SLA( N0,
N0,  N1, ■ N2). N0, N1, N2: it means three nitrogen levels; N0 (0 mg kg−1), N1 (60 mg kg−1), N2 (120 mg kg−1). P, PP, PPP: it represents different density of P. australis. Each letter corresponds to one individual plant of that species. Letters denote see Fig. 1
N1, ■ N2). N0, N1, N2: it means three nitrogen levels; N0 (0 mg kg−1), N1 (60 mg kg−1), N2 (120 mg kg−1). P, PP, PPP: it represents different density of P. australis. Each letter corresponds to one individual plant of that species. Letters denote see Fig. 1
Growth traits changes of individual plant of S. alterniflora. Nitrogen levels (a–c) a RGR, b NAR, c LARm; culm density (d–f) d RGR, e NAR, f LARm. Notes see Fig. 1
Growth traits changes of individual plant of P. australis. Nitrogen levels (a–c) a RGR, b NAR, c LARm; culm density (d–f) d RGR, e NAR, f LARm. Notes see Fig. 1
Biomass allocation traits changes of individual plant of S. alterniflora. Nitrogen levels (a–f) a CBR, b LBR, c RBR, d RhBR, e R/C, f LARMR; culm density (g–l) g CBR, h LBR, i RBR, j RhBR, k R/C, l LARMR. Notes see Fig. 1
Biomass allocation traits changes of individual plant of P. australis. Nitrogen levels (a–f) a CBR, b LBR, c RBR, d RhBR, e R/C, f LARMR; culm density (g–l) g CBR, h LBR, i RBR, j RhBR, k R/C, l LARMR. Notes see Fig. 1
Effects of culm density on two species
High culm density had significant inhibitory effect on all morphological traits of Spartina (Table 2; Fig. 1e–h) but affected only specific leaf area (P < 0.05) of Phragmites (Table 2; Fig. 2e–h). Increased culm density significantly (P < 0.001) reduced the relative growth rate of Spartina (Table 2; Fig. 3d–f) but no significant effects of density on Phragmites growth were found (Table 2; Fig. 4d–f). Significant effects of density (P > 0.05) on biomass allocation were observed only in the rhizome biomass ratio of Phragmites despite six biomass accumulation parameters being measured for each species (Table 2; Figs. 5g–l, 6g–l). Under the same culm density conditions, Spartina generally had greater morphological parameter values and higher growth rate than Phragmites (Figs. 1, 2, 3, 4).
Effects of interactions of nitrogen addition and culm density on two species
Two-way ANOVA analysis showed that there were no significant interactions between nitrogen levels and culm density on the morphological parameters, growth performances parameters, and biomass allocation parameters of either species (Table 2).
Phenotypic plasticity of morphologic character, growth performance and biomass allocation traits in response to different nitrogen level and plant density
Phenotypic plasticity of two species in response to nitrogen levels under different culm density
Mean phenotypic plasticity indices (MPPI) of morphological and biomass allocation parameters of the two species in response to nitrogen level generally decreased gradually with the increase of the culm density except for the MPPI of biomass allocation parameters of Phragmites, which showed a trend of first decrease and then increase. For MPPI of growth parameters, Spartina had its lowest response to nitrogen at medium culm density and highest one at high culm density, whereas the response of Phragmites had a subdued response with a maximum under medium and high culm density (Table 3). Under the same culm density Spartina generally showed greater response to nitrogen than did Phragmites.
Phenotypic plasticity of two species in response to intraspecific competition under different nitrogen levels
With increasing of nitrogen level, mean phenotypic plasticity indices (MPPI) of morphological traits of Spartina and Phragmites had a completely contrary trend in response to culm density, decreasing gradually for Spartina while increasing for Phragmites. For MPPI of growth parameters, Spartina had lowest response to culm density at medium nitrogen level and highest one at high nitrogen level, whereas the response of Phragmites was maximal under low nitrogen level. In addition, for the MPPI of the biomass allocation parameters, there was a decreased response for Spartina at the high nitrogen level, whereas for Phragmites the lowest response was at the intermediate nitrogen level. Under the same nitrogen level Spartina generally showed greater response to culm density than did Phragmites (Table 4).
Discussion
In the present study, Spartina responded more favorably to nitrogen availability and was less affected by culm density, which suggests that Spartina may acclimate better to high nitrogen environments and intraspecific competition than Phragmites. Nitrogen level had significant effects on several morphological (mean height of total culms), growth (the relative growth rate and mean leaf area ratio) and biomass allocation traits (leaf biomass ratio, rhizome biomass ratio, and leaf area to root mass ratio) of Spartina, while culm density only affected its morphological traits and the relative growth rate. Growth rate is thought to be an important trait associated with the invasiveness of plants. Grotkopp et al. (2002) found that invasive plants had higher relative growth rate than their noninvasive congeners and the relative growth rate was positively associated with invasiveness of invasive species. The higher relative growth rate of Spartina compared with Phragmites found in our study may contribute to its invasiveness. Net assimilation rate and mean leaf area ratio are regarded as two determinants of relative growth rate (Zheng et al., 2009). The higher net assimilation rate and mean leaf ratio of invaders may increase their relative growth rate, facilitating invasions of alien plants (Daehler, 2003; Zheng et al., 2009). Our results were consistent with these studies, with Spartina having higher net assimilation rate and mean leaf ratio of than Phragmites under the same nitrogen and culm density conditions, which may help the invader to form dense monoculture and outgrow native plant species, In addition, biomass allocation may also affect the success of invasive plants (Williams & Black, 1994; Wilsey & Polley, 2006). Our study provided support for this observation with the more invasive Spartina allocating more biomass to leaves and less to roots than Phragmites did (see also Williams & Black, 1994; Wilsey & Polley, 2006). Meanwhile, we also found that the nitrogen promoted an increase in the leaf biomass ratio and the leaf area to root mass ratio of Spartina, which suggests that more biomass was invested into the assimilative organ as nitrogen increased. This is likely to lead to greater carbon accumulation in Sparina and thus increase its relative growth rate and improve the competitive ability of this species.
It has been long suggested that high phenotypic plasticity is a characteristic of invasive species (Callaway et al., 2003; Funk, 2008). We found that Spartina exhibited higher plasticity for more morphological, growth, and biomass allocation traits than Phragmites in response to nitrogen and culm density. Difference in phenotypic plasticity between native and invasive species will influence how these species will respond to changing environmental conditions (Funk, 2008). Superior ability to capitalize on abundant resources is likely to enable a species to invade habitats with high resource availability (Vitousek, 1986; Jean & Alice, 2006). In our study, the high plasticity that we observed in Spartina, in terms of positive responses to nitrogen availability, may override the negative effects of plant density that most plants experience, and thus, in situations of high nitrogen availability Spartina may experience little intraspecific competition.
In the USA, nitrogen loading has promoted the invasion of Spartina in Willapa Bay and the invasion of its hybrid (S. alterniflora × S. foliosa) in San Francisco Bay, and the high response of Spartina to nitrogen has greatly contributed to its invasiveness, especially for the trait of aboveground to belowground biomass ratio in response to different nitrogen levels, which could strengthen not only aboveground but also belowground competition dominance relative to native species (Tyler et al., 2007). In our study no significant effect of nitrogen on biomass allocation below and aboveground was observed but the strong growth response, in terms of mean height of total culms and leaf biomass ratio, to nitrogen in Spartina compared with Phragmites will certainly enhance its aboveground competition dominance and thus its successful invasion, which is consistent with previous studies (Zhao et al., 2008). In China, eutrophication of coastal zones has been one of the most pressing environmental problems accompanying rapid economic growth and human development. The level of dissolved inorganic nitrogen in both seawater and sediments has significantly increased in the past two decades along the Chinese coast (Shen et al., 2001; Wang, 2006; Li et al., 2007). This recent increase in nitrogen availability may further facilitate invasions by Spartina and endanger the remaining Phragmites stands.
In our study, within the area where Phragmites occurs as a native species, Phragmites had lower morphological and growth trait values and lower phenotypic plasticity than invasive Spartina, whereas in its invasive range, Phragmites has been reported to have higher biomass and relative growth rate than Spartina (Farnsworth & Meyerson, 2003). There are several hypothesis to explain the mechanism of successful invasion for invasive plants, such as the natural enemies, evolution of invasiveness, empty niche, disturbance, and novel weapons hypotheses (Hierro et al., 2005). Because no adequate studies on Phragmites have been undertaken within its invasive range, it is difficult to develop explanations of why it outperforms Spartina in such areas. Further studies of Phragmites, both in its native and introduced ranges, are be required to explore the mechanism of its successful invasion of North America while it is declining in China.
Conclusions
Spartina and Phragmites are extensively invasive species around the world. The two species have strong ability to adapt to various habitats and to tolerate harsh environmental conditions, such as high salinity, high sulfur, changing nitrogen levels, and intraspecific competition. Our case study supports the idea that invasive species display high trait plasticityNitrogen level had significant effects on many morphological (mean height of total culms), growth (the relative growth rate and mean leaf area ratio) and biomass allocation traits (leaf biomass ratio, rhizome biomass ratio, and leaf area to root mass ratio) of Spartina, while culm density only affected its morphological traits and the relative growth rate. On average Spartina displayed higher trait plasticity compared with Phragmites in response to altered nitrogen availability and culm density. Understanding how the growing conditions alter the trait plasticity of invasive species has important implications for the management of plant invasions. However, this study examined the effects of two environmental factors on trait plasticity of the two species in China, thus further study should focus on comparative studies of two species in their native and invasive range to explore the mechanism of their successful invasions.
References
Avramov, S., D. Pemac & B. Tucic, 2007. Phenotypic plasticity in response to an irradiance gradient in Iris pumila: adaptive value and evolutionary constraints. Plant Ecology 190: 275–290.
Callaway, R. M., S. C. Pennings & C. L. Richards, 2003. Phenotypic plasticity and interactions among plants. Ecology 84: 1115–1128.
Chen, X. Y., 2000. Effects of plant density and age on the mating system of Kandelia candel Druce (Rhizophoraceae), a viviparous mangrove species. Hydrobiologia 432: 189–193.
Cipollini, D. F. & J. Bergelson, 2001. Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibiters in Brassica napus. Journal of Chemical Ecology 27: 593–610.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Daehler, C. C., 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics 34: 183–211.
Dukes, J. S. & H. A. Mooney, 1999. Does global change increase the success of biological invaders? Trends Ecology and Evolution 14: 135–139.
Elberse, I. A. M. & J. M. Damme, 2003. Plasticity of growth characteristics in wild barley (Hordeum spontaneum) in response to nutrient limitation. Journal of Ecology 91: 371–382.
Farnsworth, E. J. & L. A. Meyerson, 2003. Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands 23: 750–762.
Fransen, B. H., D. Kroon & F. Berendse, 1998. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 115: 351–358.
Funk, J. L., 2008. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology 96: 1162–1173.
Grace, J. B. & B. H. Pugesek, 1997. A structural equation model of plant species richness and its application to a coastal wetland. American Naturalist 149: 436–460.
Grotkopp, E., M. Rejmánek & T. L. Rost, 2002. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. American Naturalist 159: 396–419.
Hemminga, M. A. & G. J. C. Buth, 1991. Decomposition in saltmarsh ecosystems of the Sw Netherlands–the effects of biotic and abiotic factors. Vegetatio 92: 73–83.
Hierro, J. L., L. M. John & R. M. Callaway, 2005. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. Journal of Ecology 93: 5–15.
Idestam-Almquist, J. & L. Kautsky, 1995. Plastic responses in morphology of Potamogeton pectinatus L. to sediment condition at two sites in the northern Baltic proper. Aquatic Botany 52: 205–216.
Jean, H. B. & A. W. Alice, 2006. A comparison of plastic responses to competition by invasive and non-invasive congeners in the Commelinaceae. Biological Invasions 8: 797–807.
Li, M. T., K. Q. Xu, M. Watanabe & Z. Y. Chen, 2007. Long-term variations in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem. Estuarine Coastal and Shelf Science 71: 3–12.
Maherali, H. & E. H. DeLucia, 2001. Influence of climate-driven shifts in biomass allocation on water transport and storage in ponderosa pine. Oecologia 129: 481–489.
Marks, M., B. Lapin & J. Randall, 1994. Phragmites australis (P. communis): threats, management, and monitoring. Natural Areas Journal 14: 285–294.
Nagashima, H. & I. Terashima, 1995. Effects of plant density on frequency distributions of plant height in Chenopodium album stands: analysis based on continuous monitoring of height-growth of individual plants. Annals of Botany (London) 75: 173–180.
Perez, M., C. M. Duarte, J. Romero, K. Sand-Jensen & T. Alcoverro, 1994. Growth plasticity in Cymodocen nodosa stands: the importance of nutrient supply. Aquatic Botany 47: 249–264.
Poorter, L., 1999. Growth responese of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology 13: 396–410.
Poulin, B., G. Lefebvre & A. Mauchamp, 2002. Habitat requirements of passerines and phragmites australis bed management in Southern France. Biological Conservation 107: 315–325.
Ryser, P. & H. Lambers, 1995. Root and leaf attribute accounting for the performance of fast- and slow-growing grasses at different nutrient supply. Plant and Soil 170: 251–265.
Schlichting, C. D., 1986. The evolution of phenotypic plasticity in plant. Annual Review of Ecology and Systematics 17: 667–693.
Shen, Z. L., Q. Liu, S. M. Zhang, H. Miao & P. Zhang, 2001. The dominant controlling factors of high content inorganic N in the Changjing River and its mouth. Oceanologia et Limnologia Sinica 32: 465–473.
Tyler, A. C., J. G. Lambrinos & E. D. Grosholz, 2007. Nitrogen inputs promote the spread of an invasive marsh grass. Ecological Applications 17: 1886–1898.
Valladares, F., S. J. Wright, E. Lasso, K. Kitajima & R. W. Pearcy, 2000. Plastic phenotypic response to light of 16 congeneric shrubs from a panamanian rainforest. Ecology 81: 1925–1936.
Vasquez, E. A., E. P. Glenn, G. R. Guntenspergen & J. J. Brown, 2006. Salt tolerance and osmotic adjustment of Spartina alterniflora (Poaceae) and the invasive M haplotype of Phragmites australis (Poaceae) along a salinity gradient. American Journal of Botany 93: 1784–1790.
Vitousek, P. M., 1986. Biological invasions and ecosystem properties: can species make a difference? Ecological Studies 58: 163–176.
Wang, B. D., 2006. Cultural eutrophication in the Changjing (Yangtze River) plume: History and perspective. Estuarine, Coastal and Shelf Science 69: 471–477.
Wang, J. F. & Y. L. Feng, 2004. The effect of light intensity on biomass allocation, leaf morphology and relative growth rate of two invasive plants. Acta Phytoecologica Sinica 28: 781–786 (in Chinese with English abstract).
Wang, Q., C. H. Wang, B. Zhao, Z. J. Ma, Y. Q. Luo, J. K. Chen & B. Li, 2006. Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biological Invasions 8: 1547–1560.
Williams, D. G. & R. A. Black, 1994. Drought response of a native and introduced Hawaiian grass. Oecologia 97: 512–519.
Wilsey, B. & H. W. Polley, 2006. Aboveground productivity and root-shoot allocation differ between native and introduced grass species. Oecologia 150: 300–309.
Windham, L. & R. G. Lathrop, 1999. Effects of Phragmites australis invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22: 927–935.
Zhao, C. J., Z. F. Deng, C. F. Zhou, B. H. Guan & S. Q. An, 2008. Effects of nitrogen availability and competition on leaf characteristics of Spartina alterniflora and Phragmite australis. Journal of Plant Ecology 32: 392–401. (in Chinese with English abstract).
Zheng, Y. L., Y. L. Feng, W. X. Liu & Z. Y. Liao, 2009. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecology 203: 263–271.
Zhu, Z. H., J. X. Liu & X. A. Wang, 2007. Review of phenotypic plasticity and hierarchical selection in clonal plants. Jonrnal of Plant Ecology (Chinese Version) 31: 588–598. (in Chinese with English abstract).
Acknowledgement
This study is financially supported by the Special Research Program for Public- welfare Forestry (200804005), the Natural Scientific Foundation of Jiangsu Province, China (BK2009154), the National Natural Science Foundation of China (No. 30770358 and 30700061), the Fund of Post-doctor in Jangshu Province (0901017C) and Natural Science Foundation of Jiangsu Province (BK 2007152). The authors thank the colleagues and students from Nanjing University for helping with field and lab experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong jun Zhao and Hua Qing are the co-first authors.
Handling editor: S. A. Halse
Rights and permissions
About this article
Cite this article
Zhao, Y.j., Qing, H., Zhao, C.j. et al. Phenotypic plasticity of Spartina alterniflora and Phragmites australis in response to nitrogen addition and intraspecific competition. Hydrobiologia 637, 143–155 (2010). https://doi.org/10.1007/s10750-009-9992-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9992-5