Abstract
Reference conditions and boundary values between Water Framework Directive status classes were estimated for phytoplankton biomass from empirical relationships relating: (1) nitrogen inputs from land to total nitrogen (TN) concentrations and (2) TN concentrations to chlorophyll a (chl a) concentrations. Different periods during the last >100 years were used to characterise hypothesised ecological status, and a hind-casted time series was used to define boundary values for nitrogen inputs. Nitrogen levels in 35 coastal water bodies around Denmark were significantly related to inputs from land to various degrees (factor of 50) reflecting gradients from open coastal to freshwater-influenced estuaries. Significant differences in the relationship between chl a and TN across sites were found, suggesting that previous response models have been too simple and uncertain. Reference and boundary values for chl a, estimated with a relative uncertainty of 5–20%, varied substantially between sites, and the boundary value between good and moderate status was 6–81% higher than the reference condition with an average of 28%. Differences in bioavailability of nutrient sources and grazing pressure are important factors controlling site-specific phytoplankton biomass, and models for predicting phytoplankton responses to nutrient reductions must account for these. The boundary setting must be adaptive to incorporate improved quantitative knowledge and effects of shifting baselines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The estuaries and coastal areas in Denmark are generally shallow (<3 m) with short residence times and for the most part heavily loaded with nutrients (Conley et al., 2000). These water bodies receive nutrients from various, mostly small, streams discharging at different locations and have been defined as coastal waters sensu the Water Framework Directive (WFD), since they do not fulfil the classical definition of an estuary as a (1) drowned river valley, (2) fjord-type estuary, (3) bar-built estuary or (4) a tectonic produced feature (Pritchard, 1967). The coastal sites generally fall within the physical categories of broad, bay, inlet, cove, sound or strait (Conley et al., 2000). Tidal influence is small, whereas wind may change water levels significantly and break down stratification. The frequent mixing of the entire water column and high primary productivity (Conley et al., 2000; Carstensen et al., 2003) implies a substantial benthic grazing pressure on phytoplankton, mainly by blue mussels, Mytilus edulis L. (Møhlenberg, 1995).
Coastal eutrophication in Denmark became extremely pronounced in the mid-1980s with widespread hypoxia (Conley & Josefson, 2001) and loss of seagrasses (Boström et al., 2003), and in 1987 a national action plan on the aquatic environment was enacted aiming to reduce nutrient inputs by 50% for nitrogen and 80% for phosphorus. A harmonised monitoring program, the Danish National Aquatic Monitoring and Assessment Program (DNAMAP), was established to evaluate the effectiveness of the reduction measures and ecological consequences (Kronvang et al., 1993). The extensive data set obtained through DNAMAP has provided a fundamental basis for establishing reference conditions and boundary values between ecological status classes.
Phytoplankton is one of the biological quality elements to be used for assessing the ecological status of coastal waters according to the European WFD (Directive, 2000). While several sub-elements of phytoplankton (biomass, composition and bloom frequency) are described in the WFD only the concentration of chlorophyll a (chl a) (as a proxy of biomass) and abundance of very few area-specific indicator species, e.g. Phaeocystis sp. in the north-east Atlantic region, have been analysed at part of the WFD intercalibration process at present (Carletti & Heiskanen, in press).
Pivotal to the assessment is the definition of reference conditions (i.e. conditions representing no anthropogenic disturbance) and acceptable deviations from the reference conditions for the given quality element. Danish waters have been affected by anthropogenic activities for a long time. In particular, the intensified use of agricultural fertilizers since the 1950s has made nutrient loading from land and eutrophication a major pressure on all Danish marine areas. The Danish national marine monitoring was initiated in the 1970s, and therefore phytoplankton data representing reference conditions are not available and reference conditions for this quality element can only be established through expert judgement or different types of modelling. A preliminary approach to defining Danish reference conditions for chl a at WFD intercalibration sites was based historical data on Secchi depth combined with Secchi depth-chl a relationships derived from the national monitoring program (Henriksen, 2009). A major limitation of this method is the availability of historical Secchi depth measurements that represent only few areas of the open waters and none of the coastal areas. Alternatively reference conditions for phytoplankton can be calculated through hind-casted inputs of nutrients to coastal areas combined with serial empirical modelling of nutrient inputs versus nutrient concentrations and nutrient concentrations versus chl a concentration relationships, the concept employed in the present study.
Summer primary production in Danish coastal waters is nitrogen limited due to the exchange with phosphate-rich open waters. Therefore, the summer phytoplankton biomass is considered related to the nitrogen levels. Conley et al. (2007) hind-casted nutrient inputs from Denmark to the Danish straits based on estimates of the nitrogen surplus from Danish agriculture and estimated changes in point sources. These estimates of N inputs to Danish waters were subsequently combined with expert judgement of different ecological status classes during different periods in time. Based on general knowledge on the marine environment, the ecological status of different time periods since 1900 can roughly be characterised as follows with corresponding N inputs given in Table 1. The period up to 1950 is considered having a high ecological status. In the 1950s and early 1960s, the ecological status was considered to be good. In the late 1960s and 1970s, the situation started worsening and the ecological status was considered to be moderate. In the 1980s, the conditions were poor and during several years in the mid-1980s with onset of severe events of wide spread hypoxia and anoxia, the status may have been considered bad.
The objective of this study is to estimate reference conditions and boundary values for phytoplankton biomass by translating (1) total nitrogen (TN) inputs (Table 1) to TN concentrations and (2) TN concentrations to chl a concentrations. Monitoring data and statistical regression models provide the basis for analysis.
Materials and methods
Monitoring data and sites
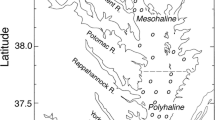
Data from all regular monitoring stations belonging to DNAMAP were extracted from the national monitoring database (http://mads.dmu.dk) and divided into 39 monitored water bodies (sites) for this study (Fig. 1). These sites displayed large differences with respect to salinity, nutrient concentrations and phytoplankton biomass, assessed by means of chl a. The large estuarine complex Limfjorden in North-western Denmark was divided into five separate water bodies, because of the large differences in both physical and biological characteristics of the different basins within Limfjorden. One site category covered the open waters of the Danish straits and was provided for comparison with the water bodies under WFD.
The geographic distribution of the Danish study sites. Open water stations (site #25) are scattered through the Danish straits and not indicated on the map. Dotted lines encircle the Limfjorden complex that has been separated into five distinct sites. Site names corresponding to the site number can be found in Tables 2 and 3
Yearly means for chl a and TN
For the WFD intercalibration in the Baltic Sea, summer chl a (May–September) was selected as indicator of phytoplankton biomass, so far the only operational indicator for the WFD biological quality element phytoplankton in the Baltic Sea. Yearly summer chl a means were computed for each site by means of the following general linear model containing three categorical factors,
which describe variations in the log-transformed chl a observations as a function of station j (variations between multiple stations within sites, j = 1–6 levels for estuarine and coastal sites and j = 17 for open waters), year i (variations between years monitored at the site, i = 3–34 levels for the different sites) and month k of monitoring (variations between months, k = 5 levels for the five summer months). Marginal means for year i , representing the mean over all stations and months, were computed from the parameter estimates of Eq. 1 after back-transformation using the exponential function to derive geometric means. A total of 849 means were computed representing the 39 sites.
Summer chl a means were compared to winter–spring (January–June) means of TN, computed in a similar manner from the model
as the marginal means for the factor year i . The factors year i and station j describe the same as for Eq. 1, whereas month k describes variations between winter and spring months (k = 6 levels). A total of 857 means were computed representing the 39 sites. For both chl a and TN, there were large differences between sites in the number of means computed, ranging from 3 up to 31 years. Due to large variations in the number of observations used for calculating the yearly means, TN means with a relative standard error >15% and chl a means with a relative error >25% were discarded from the further analyses. This reduced the number of yearly means to 621 and 626 for chl a and TN, respectively, and three sites were insufficiently monitoring to provide precise mean estimates leaving 36 sites for the analysis below (Fig. 1).
Estimating cause–effect relationships
The relationship between nitrogen input and TN was modelled by individual weighted regressions as well as a weighted regression model with site-specific slopes and a common intercept:
where TN was represented by marginal means from Eq. 2. The assumption underlying this regression is that TN concentrations in all coastal waters will attain a similar level, if local nitrogen sources are completely removed. Since all the 36 sites discharge to and mix with the open waters of the Danish straits and have relatively short residence times (Rasmussen & Josefson, 2002), it is reasonable to assume a common intercept for all sites. Two advantages of this model are that the common intercept estimate will have a relatively good precision and that regression lines cannot cross, i.e. there will be proportionality in the site-specific responses.
The second step in linking nutrient inputs to phytoplankton biomass was to identify relationships between TN levels (January–June) and chl a (May–September) by weighted linear regression. Since the pioneering work of Vollenweider (1968) documented the effects of normalised phosphorus inputs on chl a in lakes, the concept of a universal relationship between nutrient stress/status and response has underpinned environmental management. The lack of long time series had also led scientists to resort to broad-brushed relationships between nutrient and chlorophyll concentrations (e.g. Guildford & Hecky, 2000; Hoyer et al., 2002; Nielsen et al., 2002; Smith, 2006) assuming that time could be substituted with space. However, the uncertainty of using these relationships for predicting management responses has so far not been considered, and the validity of disregarding spatial differences in the relationships has never been proven.
Interannual variations in TN levels can be linked to nitrogen inputs from land (this study and Carstensen et al., 2006), and a large fraction of TN is actually bioavailable for primary production, particularly when TN is mainly of terrestrial, anthropogenic origin. TN can be considered composed of a bioavailable and a refractory part, and in this study, it was assumed that there is a generic functional relationship between phytoplankton biomass and bioavailable nitrogen. However, the fraction of bioavailable nitrogen could potentially depend on site-specific features such as influence of terrestrial nitrogen, retention time and exchange with Baltic Sea water that has a relatively high refractory part of TN (Ærtebjerg et al., 2003, Spokes et al., 2006).
Let us assume that we can describe the generic relationship between phytoplankton biomass, proxied by chl a (summer means), and bioavailable TN by means of a power function:
where p(bioavailable) is the bioavailable proportion of TN (winter–spring mean). Using a log-transform on this equation yields
which is a linear relationship between log(TN) and log(chl a) with an intercept, \( \log \left( a \right) + b \cdot \log \left( {p\left( {\text{bioavailable}} \right)} \right), \) that might be constant if the proportion of bioavailable TN is constant throughout the data set or alternatively, it could depend on the specific site of the data. The constancy of the bioavailable proportion over sites was investigated by comparing two models:
i.e. a log–log model with a constant intercept (Eq. 6) versus a site-specific intercept (Eq. 7).
All regression models were formulated within the framework of general linear models, using the inverse variance of the marginal means as weights (Best Linear Unbiased Estimate, BLUE) and analysed using SAS/PROC GLM.
Results
Nutrient status versus nutrient inputs
The first step in the cause–effect chain from nutrient inputs to phytoplankton biomass was to establish relationships between N inputs and TN concentrations. Consistent time series for the site-specific inputs were not available and for local inputs to coastal sites may only have a small contribution to nutrient enrichment of the water body relative to the regional inputs due to intensive transports of water masses. Therefore, the time series of N inputs (Danish inputs updated from Conley et al., 2007) was used for establishing relationships between regional inputs and site-specific concentrations.
Site-specific weighted regressions between N inputs and TN concentrations demonstrated significant effects of land-based inputs on concentrations in the sea for 29 out of the 36 sites, and consistent tendency for all the significant regressions to intercept at a common TN concentration (Fig. 2). The only exception was the hypertrophic Mariager Fjord, which is the only fjord-type estuary in Denmark characterised by a deep trough (almost permanently anoxic bottom water) in the inner part of the estuary (>25 m) and long sill in the outer part with an average depth of 1.1 m (Fallesen et al., 2000). Those sites that did not have a significant relationship were sites characterised by large exchanges with Baltic Sea water (Bornholm, Fakse Bugt, Hjelm Bugt, and Præstø Fjord) and sites with relatively few mean values (2–6 yearly means of both chl a and TN for Dybsø Fjord, Karrebæksminde Bugt and Korsør Nor). Most of the significant relationships had intercepts in the range from 10 to 30 μmol l−1 (Fig. 3).
Regression lines between TN mean concentrations (January–June) and regional nitrogen input from land obtained from 36 estuarine and coastal sites in Denmark. The regression line for open-water stations in the Danish straits is highlighted with a thick grey line. The site Mariager Fjord is also emphasised since it gave a deviating regression from the overall pattern. Solid lines represent significant linear relationships (P < 0.05) and dashed lines show relationships that were not significant
Estimated intercept values for the 36 site-specific regressions with 95% confidence intervals for the estimates. Estimates have been sorted by increasing intercept estimate with open symbols for non-significant regressions. Dotted line shows the common intercept from the model in Eq. 3
Mariager Fjord, where the permanently anoxic deep waters have considerably longer residence times, was excluded from the analysis of Eq. 3 and a common intercept was estimated at 16.6 (±1.16) μmol l−1 that fell nicely within most of the 95% confidence intervals of the site-specific intercepts (Fig. 3). The open-water TN means ranged from 17 to 24 μmol l−1, but these are also affected by inputs from land (Carstensen et al., 2006). This may suggest that N inputs from Denmark contribute about 1–7 μmol l−1 in addition to the TN levels in the Danish straits determined by exchanges between the Skagerrak and the Baltic Sea. Thus, the model implies that if all land-based inputs were reduced to zero, all sites would approach a common TN level of 16.6 μmol l−1, corresponding to the TN concentration in the open waters.
The estimated site-specific slopes from Eq. 3 varied from 0.05 to 2.25 μmol l−1 ktonnes−1, i.e. by a factor of 50. These estimates clearly displayed spatial patterns with sites strongly influenced by freshwater discharges, such as Odense Fjord and Randers Fjord, having high slopes and coastal sites dominated by water exchanges with the open waters having low slopes (Fig. 4). All sites characterised as open coastal areas had slopes <0.15 μmol l−1 ktonnes−1, whereas estuaries and semi-enclosed embayments had slopes above 0.2 μmol l−1 ktonnes−1. Thus, a reduction of ~20% of the present input level, corresponding to ~10 ktonnes N for the total Danish input to the Danish straits, would reduce TN concentrations by 0.5 μmol l−1 in the open coastal sites and up to 22.5 μmol l−1 in the inner part of Odense Fjord.
Estimated site-specific slopes in regression between N input and concentrations with common intercept (Eq. 3) with 95% confidence intervals for the estimate. Estimates have been sorted by increasing slope estimate with open symbols for slopes not significantly different from zero
The proposed threshold values for N inputs (Table 1) were converted into corresponding values for TN concentration in the different sites by means of Eq. 3 with uncertainties deriving from the regression only, i.e. assuming thresholds values for N inputs to be fixed (Table 2). The spatial pattern of the estimated threshold values followed the results from the regression model in Eq. 3 with wide spans for WFD status classes for estuaries strongly influenced by land (high slopes in Fig. 4) and narrow spans for coastal sites. Estimated reference values ranged from 17.2 μmol l−1 at Bornholm to 48.1 μmol l−1 in the inner part of Odense Fjord. Similarly, estimated boundaries between good and moderate status ranged from 19 to 135 μmol l−1. It should also be stressed that the uncertainty of the reference conditions (Table 2) was relatively small for all sites because the common intercept was relatively well determined. The largest uncertainties were found for the boundary between poor and bad, because the N input data set contained a few years for that range only.
Phytoplankton response to nutrient status
Significant relationships between chl a and TN were obtained for both the model with a constant intercept (Eq. 6; F 1,602 = 1280; P < 0.0001) and the model with site-specific intercepts (Eq. 7; F 1,562 = 116.6; P < 0.0001), but the overall slopes of the two models differed (Fig. 5). The simpler of the two models, Eq. 6, had a slope of 0.92 (±0.26) suggesting almost proportionality (slope of 1) in the response of chl a to TN. In addition to the significant overall relationship with log(TN), the second model, Eq. 7, showed significant different site-specific intercepts (F 35,562 = 65.9; P < 0.0001), however, with a significantly lower slope of 0.53 (±0.049) than for Eq. 6. The lower slope means that increasing TN concentrations will give less than a proportional yield.
Summer chl a versus winter–spring TN mean concentrations for 36 different sites, indicated by different symbols. The grey line shows the simple regression line without site-specific features, whereas the black solid line shows the estimated site-specific relationship averaged over all sites, and dotted lines show relationship with the lowest factor (Dybsø Fjord) and the highest factor (Skive Fjord). Means from Mariager Fjord were not included
The site-specific intercepts varied from −1.72 in Dybsø Fjord to 0.10 in Skive Fjord (Fig. 5), which back-transformed by the exponential function give site-specific factors for the power relation in Eq. 4 ranging from 0.18 to 1.11 (Fig. 6). The ranking of sites by the site-specific factors corresponded to the expectation with sites strongly influenced by terrigenous inputs had the highest factors, but there were some exceptions. In order to further investigate the interpretation of the site-specific factor, the bioavailable fraction of TN was calculated during the winter months (January–February) when most of the bioavailable fraction was either in the form of dissolved inorganic nitrogen (DIN) or bound in phytoplankton biomass. Therefore, the bioavailable nitrogen was calculated as the sum of DIN and chl a converted to nitrogen by means of Redfield ratios and a carbon:chl a ratio of 50.
Estimated site-specific factors from Eq. 4 ranked by magnitude. Error bars show the 95% confidence intervals of the estimates
Most sites showed a good agreement between the estimated factors and the winter bio-available nitrogen fraction (Fig. 7). The intercept of the regression was not significantly different from zero (t = 0.42; P = 0.6769) resulting in a proportional relationship between the bioavailable nitrogen fraction in winter and the estimated site-specific yield factor from the chl a–TN regression. This is in agreement with the conceptual theory described in Eq. 4.
There were, however, a number of sites that deviated from the overall pattern. Randers Fjord and Odense Fjord inner part are strongly influenced, particularly in winter time, by riverine inputs. These two sites deviate because (1) TN in January–June may not be a good nutrient status indicator for summer chl a due to low retention times and (2) phytoplankton primary production in summer may additionally be limited by high flushing rates and light limitation. Skive Fjord is very productive with high biomasses throughout summer, and summer algae blooms are fuelled by nutrients from the sediments following events of hypoxia (Carstensen et al., 2007). In this sense, Skive Fjord behaves partly like Mariager Fjord with an intensified internal recycling of nutrients and no benthic grazing in the deeper parts where the monitoring station is located. Finally, Dybsø Fjord has a lower chl a yield from TN than most other sites, because nutrient inputs to Dybsø Fjord are small, and primary production is dominated by benthic vegetation.
Reference conditions and boundary values were calculated from the chl a–TN regression (Table 3) using the corresponding values for TN as input (Table 2). Standard errors for the chl a values (Table 3) were found by Monte Carlo simulation (10,000 simulations) including uncertainty of the estimated chl a–TN model as well as uncertainty of the TN reference condition and boundary values. Reference conditions ranged from 0.97 μg l−1 in Dybsø Fjord to 6.27 μg l−1 in Skive Fjord, whereas the boundary between good and moderate ranged from 1.20 μg l−1 in Karrebæksminde Bugt to 9.93 μg l−1 in Skive Fjord. The boundary between good and moderate was on average 28% above the reference conditions but it varied from 6% for Bornholm to 81% for the inner part of Odense Fjord. These differences were a combination of the response in TN to nitrogen inputs and the factor used in the chl a–TN relationship. The relative uncertainties of the values in Table 3 were typically about 5–20%, however, considerably higher for sites with fewer monitoring data such as Dybsø Fjord and Korsør Nor (~30%).
Discussion
The European WFD has the noble objective to stop further deterioration of aquatic ecosystems and that European waters should have at least good ecological status by 2015. Since the WFD was adopted in 2000 (Directive, 2000), water managers have been striving to transform the good political intentions into operational managerial frameworks, and scientists have been engaged in improving our scientific understanding of ecosystem functioning. The quantitative knowledge, so far, is mostly based on empirical studies lumping data from monitored sites together to obtain sufficient power for estimating relationships, under the general assumption that space can substitute time and universal relationships between stress and response exist. Although this line of thinking is now over 40 years old (going back to Vollenweider, 1968), these underlying assumptions have never been validated or for that matter actually investigated. Most studies have been concerned with modifying TN measurements to exclude the contribution from phytoplankton (Borum, 1996; Nielsen et al., 2002) or extending the linear log–log relationship between chl a and TN to higher order parametric relationships (e.g. quadratic relationship in Smith, 2006) or non-parametric relationships (e.g. LOWESS relationship in Phillips et al., 2008). Although some studies have acknowledged and discussed potential site-specific effects (e.g. Phillips et al., 2008), this study actually documents significant differences in the chl a–TN relationship between sites. In the earlier studies, site-specific effects could not be investigated due to data limitations, but extensive data set is now largely available to test for such differences and empirical relationships applied to ecosystem management should at least include site-specific features or document that these are negligible.
Site-specific features are important for Danish coastal sites. The summer phytoplankton biomass results mainly from the balance between production and grazing processes (benthic and pelagic). Primary production in the summer period comprised by new production due to direct nutrient inputs from land and atmosphere and regenerated production based on remineralisation of nutrients in the water column and releases from the sediments. The bioavailability of nitrogen varies with land use in the watershed with the highest values from urban areas and arable land and lower values from natural and forested lands (Seitzinger et al., 2002). Urban areas and arable land comprise >76% of the land in Denmark, whereas the remaining part accounts for forests, natural lands, wetlands and lakes (http://www.dst.dk). Land use differences across watersheds are mainly shift from urban to arable land, and thus, the site-specific differences in nitrogen bioavailability of N inputs from land are small. On the other hand, the open waters of the Danish straits have strong gradients of salinity from mixing of saline Skagerrak water with brackish Baltic Sea water, but also gradients of nitrogen bioavailability because a relatively large fraction of the Baltic Sea dissolved organic nitrogen is refractory and inorganic nutrients are low (Ærtebjerg et al., 2003). This will lead to differences in the site-specific bioavailability in Eq. 4. Nutrient input from the sediments is enhanced through hypoxia (Mortimer, 1941; Smith & Hollibaugh, 1989), which may lead to excessive algae growth (Carstensen et al., 2007). The investigated sites are variously affected by hypoxia with Mariager Fjord (permanently hypoxic) and Skive Fjord (hypoxic every summer) as the most affected sites. Thus, there are significant differences in the nitrogen bioavailability across sites.
Grazing pressure on phytoplankton may also exert significant differences between sites. Blue mussels are widespread in Danish estuaries and play a major regulatory role for the plankton, including copepod eggs (Conley et al., 2000). The investigated sites range from permanently over intermittently stratified to well mixed. Bivalves can effectively control phytoplankton biomass as exemplified in Ringkøbing Fjord on the west coast of Denmark, where a small change in salinity allowed the clam Mya arenaria (L.) to settle and reduce chl a from around 50 to about 10 μg l−1 (Petersen et al., 2008). Large variations in the benthic fauna biomass and composition (Josefson & Hansen, 2004) signify large differences across sites with respect to benthic grazing pressure. The effect of pelagic grazing, mostly by protozoa (Conley et al., 2000), may also vary substantially between sites, but the biomass of microzooplankton will also be controlled by filter feeders. Thus, differences in the phytoplankton biomass relative to TN levels must be expected due to variations in grazing pressure across sites.
The overall uncertainty was reduced by including significant site-specific features. In this study, the residual standard error was reduced from 0.35 using Eq. 6 to 0.22 using Eq. 7, which corresponds to a reduction of the relative uncertainty from 42 to 22%, i.e. almost a halving of the uncertainty. Reducing the uncertainty of boundary values is crucial to the WFD implementation and methods to achieve this should be emphasised. Carstensen (2007) documented a need for extensive monitoring efforts to obtain sufficient precision in the status classification using fixed boundary values. Substantial uncertainty in the boundary setting further adds to the overall uncertainty of the WFD implementation with a risk of jeopardising the WFD objectives. Thus, including site-specific features will provide more realistic boundary values for managing specific water bodies as well as provide more certainty in the boundary estimates.
The WFD boundary setting for biological elements cannot be entirely objective, but it has to be based on the best available knowledge. In this study, we have presented a method for deriving boundary values for chl a based on empirical relationships between TN inputs and concentrations, and TN and chl a concentrations. The choice of TN input levels for defining reference conditions and boundary values remains subjective but are, however, linked to periods in the past that will qualify these values more than expert judgement. The WFD proposes several approaches for deriving reference and boundary values and given the still relatively large uncertainty for these values in this study, other approaches should be pursued to either corroborate or falsify the results of the present study. Henriksen (2009) estimated reference conditions for summer chl a (also May–September) by means of historical Secchi depths from beginning of the twentieth century, corresponding to the same period defined in this study, and obtained generally lower values than in the present study (up to 50% lower). However, his method involved a number of subjective parameter settings in the statistical analysis, and reference condition was derived from historical Secchi depths measured in open waters, assuming these also to represent status of the WFD intercalibration coastal sites.
The identified empirical relationships present an advance compared to other studies in its statistical rigour, but improved precision and knowledge must continue to be developed. It is implicitly assumed that the estimated relationships are static, i.e. that they do not change with time. This assumption is unlikely to be valid, since many other factors (e.g. global warming) affect the chl a–TN relationship. Duarte et al. (2009) have shown that ecosystems going through the phases of eutrophication and oligotrophication do not necessarily return to the point of departure, and shifting baselines will affect the likelihood of complying with the boundary for good ecological status. Therefore, the WFD should not consider managing nutrient inputs only but must consider the multitude of stressors affecting marine ecosystems, and the boundary setting procedure is an adaptive process that should incorporate new knowledge as it becomes available and keep focus on establishing good ecological status in a changing world rather than focusing on allowable deviations from unattainable historical reference conditions.
Conclusions
Water Framework Directive boundary setting will inevitably include elements of subjectivity, such as expert judgement, but it must also be accompanied by quantitative relationships when present, to qualify chosen values. Such relationships can be based on empirical evidence of links between nutrient input and concentrations as well as links between nutrient status and ecological responses using data from a broad range of ecosystems. However, differences between ecosystems must be taken into account to establish the most correct and precise relationships. The presented boundary setting approach advances our quantitative knowledge for establishing such relationships.
References
Ærtebjerg, G., J. H. Andersen & O. S. Hansen, 2003. Nutrients and Eutrophication in Danish Marine Waters: A Challenge for Science and Management. National Environmental Research Institute, Roskilde, Denmark: 126 pp.
Borum, J., 1996. Shallow waters and land/sea boundaries. In Jørgensen, B. B. & K. Richardson (eds), Eutrophication in Coastal Marine Ecosystems. Coastal and Marine Studies, Vol. 52. American Geophysical Union, Washington, DC: 179–203.
Boström, C., S. P. Baden & D. Krause-Jensen, 2003. The seagrasses of Scandinavia and the Baltic Sea. In Green, E. P. & T. F. Short (eds), World atlas of Seagrasses. California University Press, Berkeley: 27–37.
Carletti, A. & A.-S. Heiskanen, in press. Water Framework Directive Intercalibration Technical Report. Part 3: Coastal and Transitional Waters. European Commission Joint Research Centre, Institute for Environment and Sustainability.
Carstensen, J., 2007. Statistical principles for ecological status classification of Water Framework Directive monitoring data. Marine Pollution Bulletin 55: 3–15.
Carstensen, J., D. J. Conley & B. Müller-Karulis, 2003. Spatial and temporal resolution of carbon fluxes in a shallow coastal ecosystem, the Kattegat. Marine Ecology Progress Series 252: 35–50.
Carstensen, J., D. J. Conley, J. H. Andersen & G. Ærtebjerg, 2006. Coastal eutrophication and trend reversal: a Danish case study. Limnology and Oceanography 51: 398–408.
Carstensen, J., P. Henriksen & A.-S. Heiskanen, 2007. Summer algal blooms in shallow estuaries: definition, mechanisms and link to eutrophication. Limnology and Oceanography 52: 370–384.
Conley, D. J. & A. Josefson, 2001. Hypoxia, nutrient management and restoration in Danish waters. In Rabalais, N. N. & R. E. Turner (eds), Coastal Hypoxia: Consequences for Living Resources and Ecosystems. Coastal and Estuarine Studies, Vol. 58. American Geophysical Union, Washington, DC: 425–434.
Conley, D. J., H. Kass, F. Møhlenberg, B. Rasmussen & J. Windolf, 2000. Characteristics of Danish estuaries. Estuaries 23: 820–837.
Conley, D. J., J. Carstensen, G. Ærtebjerg, P. B. Christensen, T. Dalsgaard, J. L. S. Hansen & A. B. Josefson, 2007. Long-term changes and impacts of hypoxia in Danish coastal waters. Ecological Applications 17(5): S165–S184.
Directive, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Official Journal of the European Communities L 327: 1–72.
Duarte, C. M., D. J. Conley, J. Carstensen & M. Sánchez-Camacho, 2009. Return to Neverland: shifting baselines affect eutrophication restoration targets. Estuaries and Coasts 32: 29–36.
Fallesen, G., F. Andersen & B. Larsen, 2000. Life, death and revival of the hypertrophic Mariager Fjord, Denmark. Journal of Marine Systems 25: 313–321.
Guildford, S. J. & R. E. Hecky, 2000. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnology and Oceanography 45: 1213–1223.
Henriksen, P., 2009. Reference conditions for phytoplankton at Danish Water Framework Directive intercalibration sites. Hydrobiologia 629: 255–262.
Hoyer, M. V., T. K. Frazer, S. K. Notestein & D. E. Canfield Jr., 2002. Nutrient, chlorophyll, and water clarity relationships in Florida’s nearshore coastal waters with comparisons to freshwater lakes. Canadian Journal of Fishery and Aquatic Science 59: 1024–1031.
Josefson, A. B. & J. L. S. Hansen, 2004. Species richness of benthic macrofauna in Danish estuaries and coastal areas. Global Ecology and Biogeography 13: 273–288.
Kronvang, B., G. Ærtebjerg, R. Grant, P. Kristensen, M. Hovmand & J. Kirkegaard, 1993. Nationwide monitoring of nutrients and their ecological effects: state of the Danish Aquatic Environment. Ambio 22: 176–187.
Møhlenberg, F., 1995. Regulating mechanisms of phytoplankton growth and biomass in a shallow estuary. Ophelia 42: 239–256.
Mortimer, C. H., 1941. The exchange of dissolved substances between mud and water in lakes. Journal of Ecology 29: 280–329.
Nielsen, S. L., K. Sand-Jensen, J. Borum & O. Geertz-Hansen, 2002. Phytoplankton, nutrients, and transparency in Danish coastal waters. Estuaries 25: 916–929.
Petersen, J. K., J. W. Hansen, M. B. Laursen, P. Clausen, J. Carstensen & D. J. Conley, 2008. Regime shift in a coastal marine ecosystem. Ecological Applications 18: 497–510.
Phillips, G., O.-P. Pietiläinen, L. Carvalho, A. Solimini, A. Lyche-Solheim & A. C. Cardoso, 2008. Chlorophyll–nutrient relationships of different lake types using a large European dataset. Aquatic Ecology 42: 213–226.
Pritchard, D. W., 1967. What is an estuary: physical viewpoint. In Lauff, G. H. (ed.), Estuaries. American Association for the Advancement of Science, Publication No. 83, Washington, DC: 3–5.
Rasmussen, B. & A. B. Josefson, 2002. Consistent estimates for the residence time of micro-tidal estuaries. Estuarine, Coastal and Shelf Science 54: 65–73.
Seitzinger, S. P., S. W. Sanders & R. Styles, 2002. Bioavailability of DON from natural and anthropogenic sources to estuarine plankton. Limnology and Oceanography 47: 353–366.
Smith, V. H., 2006. Responses of estuarine and coastal marine phytoplankton to nitrogen and phosphorus enrichment. Limnology and Oceanography 51: 377–384.
Smith, S. V. & J. T. Hollibaugh, 1989. Carbon-controlled nitrogen cycling in a marine ‘macrocosm’: an ecosystem-scale model for managing cultural eutrophication. Marine Ecology Progress Series 52: 103–109.
Spokes, L. & 29 others, 2006. MEAD: an interdisciplinary study of the marine effects of atmospheric deposition in the Kattegat. Environmental Pollution 140: 453–462.
Vollenweider, R. A., 1968. Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters with Particular Reference to Nitrogen and Phosphorus as Factors of Eutrophication. Technical Report DA S/SCI/68.27.250, Organisation for Economic Cooperation and Development, Paris, France.
Acknowledgements
This study used data collected within the National Danish Aquatic Monitoring and Assessment Programme, and financial support was given by the Danish Environmental Protection Agency. Henning Karup and Jens Brøgger Jensen are acknowledged for constructive discussions of the approach and comments to the manuscript. This research is a contribution to the Thresholds Integrated Project, funded by Framework Program 6 of the European Commission (contract # 003933-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: P. Nõges, W. van de Bund, A. C. Cardoso, A. Solimini & A.-S. Heiskanen
Assessment of the Ecological Status of European Surface Waters
Rights and permissions
About this article
Cite this article
Carstensen, J., Henriksen, P. Phytoplankton biomass response to nitrogen inputs: a method for WFD boundary setting applied to Danish coastal waters. Hydrobiologia 633, 137–149 (2009). https://doi.org/10.1007/s10750-009-9867-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9867-9