Abstract
The aims of this study were to explore the environmental factors that determine the distribution of plant communities in temporary rock pools and provide a quantitative analysis of vegetation–environment relationships for five study sites on the island of Gavdos, southwest of Crete, Greece. Data from 99 rock pools were collected and analysed using Two-Way Indicator Species Analysis (TWINSPAN), Detrended Correspondence Analysis (DCA) and Canonical Correspondence Analysis (CCA) to identify the principal communities and environmental gradients that are linked to community distribution. A total of 46 species belonging to 21 families were recorded within the study area. The dominant families were Labiatae, Gramineae and Compositae while therophytes and chamaephytes were the most frequent life forms. The samples were classified into six community types using TWINSPAN, which were also corroborated by CCA analysis. The principal gradients for vegetation distribution, identified by CCA, were associated with water storage and water retention ability, as expressed by pool perimeter and water depth. Generalised Additive Models (GAMs) were employed to identify responses of four dominant rock pool species to water depth. The resulting species response curves showed niche differentiation in the cases of Callitriche pulchra and Tillaea vaillantii and revealed competition between Zannichellia pedunculata and Chara vulgaris. The use of classification in combination with ordination techniques resulted in a good discrimination between plant communities. Generalised Additive Models are a powerful tool in investigating species response curves to environmental gradients. The methodology adopted can be employed for improving baseline information on plant community ecology and distribution in Mediterranean ephemeral pools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the occurrence of small, temporarily water-filled rock pools is associated with bare rock exposure on a variety of substrates and climates (Williams, 1987; Pinder et al., 2000; Bayly, 2002; Krieger et al., 2003). Temporary rock pools occur in clusters showing an island-like configuration, with varying morphometry and inundation period ranging from a few days to weeks (Deil, 2005). Therefore, they serve as model systems in ecology (Srivastava et al., 2004; De Meester et al., 2005) to evaluate metapopulation dynamics (Spencer et al., 2002), dispersal processes (Vanschoenwinkel et al., 2007) and species competition (Laurilla, 2000).
The plant communities found in temporary rock pools are usually quite simple floristically with low species richness (Bayly, 1997), probably due to the limited carrying capacity and relatively small size of the pools (Blaustein & Schwartz, 2001). In arid and semi-arid environments, the short and unpredictable aquatic phase coupled with pronounced fluctuations of biotic and abiotic conditions (Scholnick, 1994) have led plant species occurring in pools to develop special adaptations. These include phenological plasticity, a tendency to nanism, autogamy or cleistogamy for annuals (Deil, 2005), while perennials have slow growth and rosette leaves (Keeley, 1999). This specialization often results in a high level of endemism/rarities in rock pool environments, which are considered diversity hot spots (Pinder et al., 2000). Collectively, they support considerably more species, and specifically more scarce species, than other freshwater water body types. Due to their unique edaphic and microclimatic conditions, they possess vegetation that is distinct from their surroundings.
In the Mediterranean, where ponds are common, due to climatic conditions, islands such as Corsica and Sardinia are still important havens for this habitat, whereas Sicily and Malta have witnessed significant losses (Grillas et al., 2004). In Greece, there are 34 Mediterranean Temporary Ponds (MTP) sites, 68% of which occur in islands (Dimitriou et al., 2006; Zacharias et al., 2007), with the island of Crete hosting the highest number of MTPs (20.5%) compared to any other region of the country (Zacharias et al., 2008). On the island of Gavdos, southwest of Crete (Fig. 1), the extensive presence of limestone in combination with semi-arid conditions has resulted in the occurrence of numerous temporary rock pools (Bergmeier, 2001).
The formation of these irregularly shaped rock pools is due to rainwater collection in natural depressions and hollows on crystalline limestone karst during the wet season. These hollows were originally formed by the action of CO2-acidified rainwater dissolving away the limestone substrate over many years (Kruckeberg, 2002). Rock pools are usually very transient and rapidly dry up, especially with the onset of the dry season. Nevertheless, they are important to humans and biota alike. In places like Gavdos, where the availability of freshwater resources is limited, people have striven to overcome water scarcity (Rackham & Moody, 1996). Therefore, rock pools have played an important role as water reservoirs for both people and livestock with some of the pools modified to retain and increase their water retention capacity. Moreover, in this dry environment, they play a unique ecological role by offering a resting place for migratory birds, a habitat for many freshwater plant rarities and supporting valuable catchment functions at the national or European level (Céréghino, 2008).
In addition, and due to their ephemeral nature, rock pools are subjected to drastic environmental changes (e.g. in irradiation, temperature, moisture and nutrient availability), and therefore, merit considerable research attention (Wessels & Budel, 1989; Ott et al., 1996, 1997). Hence, there is growing awareness in Europe of the importance of temporary ponds, which culminated in their inclusion as priority habitat in the EU Habitats Directive (Council of Europe, 1992). Although in the case of Greece, this has resulted in greater efforts for their protection (Dimitriou et al., 2006), community studies of freshwater rock pool systems are still limited. Bergmeier & Raus (1999) summarised the state of knowledge for the vegetation of vernal pools and seasonally wet riverbanks and lakeshores in Greece with data on ecology and distribution of the plant communities. Further contributions on ephemeral wetland vegetation in Greece come from: Bergmeier & Abrahamczyk (2008), De Bolos et al. (1996), Oberdorfer (1952), Krause et al. (1963) and Sarika-Hatzinikolaou et al. (2003). With few exceptions (e.g. Bergmeier, 2001), the factors that affect plant community structure and species richness have not been examined thoroughly. There is therefore an urgent need to increase research efforts in these environments to contribute to their understanding and protection. The objectives of this paper are to: (a) classify and describe the rock pool vegetation on the island of Gavdos using multivariate analysis techniques; (b) explore by means of direct gradient analysis the relationship between vegetation types and environment; (c) identify the ecological niches of the dominant rock pool macrophytes.
Materials and methods
Study area
Gavdos (or Gavdhos) lies 28 miles off the island of Crete and 150 miles off the shores of northern Africa (34°52′36″ N and 24°05′25″ E) (Fig. 1). It has an area of 30 km2, a maximum altitude of 362 m and geologically consists of upper Cretaceous limestones. Rainfall is limited to an annual average of c.400 mm that falls on about 90 days a year, mostly from October to March (Bergmeier, 2001). The vascular flora comprises c.460 taxa, 30 of which (6%) are of south Mediterranean/north African origin, which on the island are at their European northern distributional limit (Bergmeier et al., 1997). The vegetation in Gavdos is mainly dominated by pine, juniper and lentisk formations, in a unique cultural landscape of terraces, where wheat and barley are still grown (Rackham & Moody, 1996). Following the implementation of European Habitats directive, Gavdos has been designated a Natura 2000 site. Rock pools are widely spread over the island occurring on limestone, ranging from 50 to 300 m altitude and are located mostly close to abandoned settlements, with the exception of Kastri (Fig. 1).
Data collection
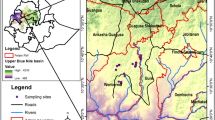
Fieldwork took place during April 2007 when a total of 99 rock pools (Table 1) were examined from five sites on the island, namely, Agios Panteleimonas, Agios Ioannis, Kastri, Korfos and Frageliana (Fig. 1). For every pool, species composition was recorded, while abundance was estimated using a scale from 1% to 100%. Plants’ nomenclature follows Turland et al. (1993) and life-form spectrum classification follows Raunkiaer (1934). Chorology was based on Jahn & Schönfelder (1995).
The environmental variables recorded were pool perimeter, surface, soil depth, water depth and Total depth. The exact location, pool area and perimeter were mapped using a differential GPS. Water depth was measured using a measuring pole, while soil depth was measured with a soil auger. For the measurement of the pool depth, the depth of the organic matter and sediment formed by the dominant species over the bedrock was also taken into account. At the time of sampling, pool conditions ranged from inundated to dry pools. Therefore, a qualitative index was used to describe this condition for every pool, as observed in the field. This index (PC) comprises four classes (Table 2): (1) rock pools full of water (PCa), (2) dried rock pools (PCb), (3) rock pools with moist soil (PCc) and (4) patchy rock pools (intermediate condition between 2 and 3) (PCd). The size of the pools ranged from 0.06 to 30.98 m2, water depth ranged from 0 to 44 cm and the total depth from 7 to 70 cm. Water presence and retention varied greatly, depending on pool morphology and the presence of soil.
Data analysis
Using PC-ORD version 4 (McCune & Mefford, 1999), the Two-Way Indicator Species Analysis (TWINSPAN; Hill, 1979) was used to classify vegetation data. Presence/absence data were analysed and the classification was stopped at the third level of division, so that the resulting groups would contain a sufficient number of samples to characterise the vegetation communities. Fourteen rock pools that contained no species were excluded from TWINSPAN and subsequent gradient analysis. Gradient analysis was carried out with CANOCO package 4.5 for Windows (ter Braak & Smilauer, 1998). Correspondence Analysis was used to detect any outliers in the species data, whereas Detrended Correspondence Analysis (DCA; Hill, 1979) was employed to evaluate the type of response model for selection. Based on the DCA results, a unimodal response model for the data was accepted (Jongman et al., 1987). Therefore, Canonical Correspondence Analysis (CCA; ter Braak & Smilauer, 1998) was used to assess the relative importance of each environmental variable in vegetation variation, by forward selection of explanatory variables. A randomised Monte Carlo permutation test was used (999 permutations) to select the most important variables that explained variations in the dataset (P ≤ 0.001) and to test the significance of the canonical axes (P ≤ 0.001) (ter Braak & Smilauer, 1998). Since the inclusion of a moderately to strongly intercorrelated group of variables in the ordination may yield unreliable results (ter Braak & Smilauer, 1998), the variables employed were tested first for correlation using the Pearson correlation coefficient. Surface was highly correlated with perimeter (r = 0.9) and therefore excluded from the analysis. The PC index was included in the analysis as a dummy variable. Although nine variables were initially evaluated, only water depth, perimeter, PCb and PCc (Table 2) were proven to be statistically significant and therefore were included in the final model.
Separate CCA analyses, performed for the samples found on dried rock pools and rock pools with moist soil, did not result in significant models and therefore are not reported. The samples for the other two conditions (pools full of water and patchy pools) were too few to allow separate analysis.
Species response curves
Generalised Additive Models (GAMs) were used to construct species response curves along a water depth gradient for the dominant rock pool species, namely, Chara vulgaris, Callitriche pulchra, Zannichellia pedunculata and Tillaea vaillantii. GAMs are semi-parametric extensions of GLMs that assume no a priori responses of a species to an environmental gradient (Guisan et al., 2002). GAMs with a Poisson distribution were employed using the CANOCO software. The starting model included the predictor smoothed with four degrees of freedom (Heikkinen et al. 2007). The Akaike information criterion (AIC) was used to select the best of the GAM models.
Results
Species composition
The total number of species recorded in the five sites was 46 belonging to 21 different families. The dominant families were Labiatae (15%), Gramineae (11%) and Compositae (11%), followed by Plantaginaceae (8%) and Rubiaceae (8%). Therophytes are by far the dominant life form (82% of total species) followed by Chamaephytes (7%). The dominant chorological elements were the Mediterranean (22.4%) and the East Mediterranean (8%). Species with particular ecological interest recorded by this study include Matricaria aurea and Callitriche pulchra, which are unique to the Greek flora. Most of the species (78%) showed a low frequency (less than 5%), i.e. they were found only on a small number of pools. Species frequency ranged from 1.2% (Anagallis arvensis, Matricaria aurea, Plantago lagopus) to 44.7% (Zannichellia pedunculata).
Vegetation classification
The results of the TWINSPAN analysis are summarised in Fig. 2. Although the total number of the groups was 8, due to the presence of the same characteristic species between the first two groups (1, 2) as well as the last two groups (7, 8), these were finally amalgamated to one community type. Therefore, six community types were identified and are discussed herein (Table 3):
1. Zannichellia pedunculata–Chara vulgaris community:
Differential species: Zannichellia pedunculata and Chara vulgaris
Ecology: This community was identified in the deepest pools full of water, with no species present other than Zannichellia pedunculata and Chara vulgaris.
Distribution in Greece: This community has been reported from Crete and Gavdos (Bergmeier, 2001; Dimitriou et al., 2006).
2. Zannichellia pedunculata–Callitriche pulchra community:
Differential species: Zannichellia pedunculata and Callitriche pulchra
Ecology: This community was also found in deepest inundated pools; however, it was marked by the absence of Chara vulgaris.
Distribution in Greece: This community has been reported from Crete and Gavdos (Bergmeier, 2001; Dimitriou et al., 2006).
3. Callitriche pulchra–Tillaea vaillantii community:
Differential species: Callitriche pulchra and Tillaea vaillantii
Ecology: This community occurs in pools with intermediate pool depth and intermediate water level.
Distribution in Greece: Gavdos (Bergmeier, 2001).
4. Tillaea vaillantii community:
Differential species: Tillaea vaillantii and Lythrum hyssopifolia
Ecology: This community occurs in relatively shallow pools with relatively low water level.
Distribution in Greece: Similar communities have been reported from North and Central Aegean, Gavdos (Bergmeier, 2001; Dimitriou et al., 2006).
5. Tillaea vaillantii–Polypogon maritimus community:
Differential species: Tillaea vaillantii and Polypogon maritimus
Ecology: This community occurs in relatively shallow pools as well but with lower water depth than the previous community type.
Distribution in Greece: Gavdos (Bergmeier, 2001).
6. Tillaea alata–Crepis pusilla community:
Differential species: Tillaea alata and Crepis pusilla
Ecology: This community was found on dry, shallow rock pools and included some typical perennial garrigue species.
Distribution in Greece: Similar community reported from S. Crete and Gavdos (Bergmeier, 2001; Dimitriou et al., 2006).
Ordination
Detrended Correspondence Analysis did not detect any outliers in the species data. The length of the first DCA axis amounted to 4.28 SD and therefore a unimodal response model for the data was assumed (Jongman et al., 1987). The eigenvalues of the first two CCA axes for the whole dataset are 0.76 and 0.33 (Table 4). Table 5 shows the canonical coefficients of the environmental factors taken into account. Axis 1 is strongly correlated with PCb (r = 0.91), while Axis 2 is strongly correlated with pool perimeter (r = 0.6) and water depth (r = −0.57). These first two axes of CCA account for c.15% of the total variance in the sample data (Table 4). CCA axes were statistically tested with a Monte Carlo permutation test (999 permutations) and were proven to be significant (P = 0.001).
Species ordination
The species with high negative scores on Axis 1 (Fig. 3) include Callitriche pulchra, Zannichellia pedunculata and Chara vulgaris. These species are highly positively related to water depth and rock pool perimeter. The species found in the top of Axis 2 (Fig. 3) with high positive score include Tillaea vaillantii, Plantago bellardi, Lagoecia cuminoides, Nigella arvensis, Trigonella monspeliaca, Centaurium spicatum and Psilurus incurvus. These species are positively correlated with pool condition C of the PC index and negatively correlated with condition Β of the PC index (Table 2). These species were found in rock pools with relatively wet soil.
CCA biplot for species–environmental variables. Species labels are slightly shifted to improve graph clarity. Perimete: Pool Perimeter, WaterD: Water Depth, PCb and PCc as in Table 2
In the lower end of Axis 2, species such as Valantia muralis, Trifolium suffocatum, Crepis cretica, Plantago weldenii, Plantago lagopus and Erodium cicutarium are located. These species are positively correlated with condition B of the index PC and negatively correlated with condition C of the PC index and water depth. They were found in dry rock pools.
Pools ordination
In order to compare the classification and ordination results, the TWINSPAN community types are superimposed onto the CCA samples ordination in Fig. 4. The derived community types are well separated, although there is some degree of overlap between them. More specifically, the CCA analysis of the rock pools showed three distinct groups (Fig. 4). The first group (left lower side of Fig. 4) comprises rock pools with large perimeter and high water depth. These deep rock pools contained very few species, were dominated by Zannichellia pedunculata, Chara vulgaris and Callitriche pulchra and belong to the Zannichellia pedunculata–Chara vulgaris and the Zannichellia pedunculata–Callitriche pulchra community types (Table 3). The group in the middle of the first axis of the graph comprises rock pools with relatively small perimeter, moist soil and few species. They belong to Callitriche pulchra–Tillaea vaillantii and Tillaea vaillantii community type. In the lower right of the graph, the third group comprises dry rock pools with relatively small perimeter and belong to the Tillaea alata–Crepis pusilla and Tillaea vaillantii–Polypogon maritimus community types. Diversity is high (Table 3) and many species present are typical of phryganic communities (e.g. Sarcopoterium spinosum, Satureja thymbra and Coridothymus capitatus). The almost ‘linear’ grouping of the samples is a result of high species similarity and small number of species in samples of the same community types (i.e. reduced variation).
CCA biplot for samples–environmental variables. Continuous environmental variables biplot values have been multiplied by two. PCb and PCc as in Table 2
Species response curves
Selected models explained much of the deviance in species data and faired better than GLMs with a linear form of dependence, with the exception of Tillaea vaillantii (Table 6). The response curves of the four species examined show differentiation of their realised niches (Fig. 5). Tillaea vaillantii shows a decreasing monotonic response curve along the water gradient.
The species response curve for Zannichellia pedunculata shows a double hump along the water gradient. The trough on the graph between the two peaks coincides with the peak of Chara vulgaris (Fig. 5). The decrease of Zannichellia pedunculata at higher depths coincides with a monotonic increase of Chara vulgaris for this depth. This suggests that the two species that were found in pools full of water are in direct competition. There is an overlap of the ecological amplitudes of Callitriche pulchra and Zannichellia pedunculata, although their optima differ. For Callitriche pulchra, abundance peaks at shallow inundated pools and decreases with increased depth. For intermediate depths, the species amplitude coincides with that of Zannichellia pedunculata.
Discussion
The rock pools of Gavdos investigated in this study are typical of ephemeral environments (see Deil, 2005; Krieger et al., 2003), where physical and chemical conditions fluctuate. As a result, they are rich in annual (therophytes) and poor in perennial species. However, there were no endemics recorded other than Matricaria aurea and Callitriche pulchra, which are unique for the Greek flora (Phitos et al., 1996). Compared to temporary pools in California, where the environmental conditions are similar, Mediterranean pools have low degree of endemism (Grillas et al., 2004; Deil, 2005).
These findings corroborate the previous findings by Bergmeier (2001) who also reported five of the six communities identified herein (Table 3), in Gavdos rock pools. We distinguish one more community type in the deepest inundated pools Zannichellia pedunculata–Chara vulgaris. Although we did not record Tillaea alata or Crepis pusilla, we named one of the communities identified Tillaea alata–Crepis pusilla due to its floristic similarity (e.g. the presence of Filago cretensis and Sedum littoreum) and habitat similarity (shallow pools with low water level) with the one identified by Bergmeier (2001). Despite the minor differences, both studies (ours and Bergmeier’s) agree on the importance of water level and pool depth in determining community composition in Gavdos’ rock pools.
The community types derived from TWINSPAN were corroborated by CCA. The CCA analysis for the whole dataset suggested that the two main compositional gradients represent water storage (Axis 1) and water retention ability (Axis 2), as expressed by pool perimeter and water depth. Water storage is directly responsible for spatial and temporal variations of vegetation in these environments (Grillas et al., 2004; Müller & Deil, 2005; Delipetrou, 2007). Inundated rock pools, were dominated by Zannichellia pedunculata, Chara vulgaris and Callitriche pulchra with few other species present. Water is a prime determinant of plant community structure affecting colonisation, germination and growth. In pools where there was visibly moist soil (PCc), or patchy (PCd), Tillea vaillantii dominates. The margins (perimeter length) of the pools heat up and dry up faster than the centre, and are the first to be colonised. Perimeter is also related to surface and therefore indirectly to water storage and retention. In general, the larger the surface of a pool, the more water is stored and the faster evaporation takes place. This is also dependent on pool depth. Therefore, in pools that are very deep and narrow, as many of those seen in Gavdos, water simply evaporates along a vertical gradient. Seasonality and the presence of water is linked to distinct ecophases, which result in many kinds of succession that may not be progressive, particularly when habitats are of primary nature and when there are continuous hydrological or geomorphological processes in place (Deil, 2005). In the case of the pools recorded along a steep stream in the site of Kastri (Fig. 1), the slope gradient facilitates water runoff during the rainy season at a faster rate than the pace at which successional processes take place. In addition, in the island’s karstic environment, stochastic events are also related to geomorphologic processes, such as limestone weathering. The nature of rock pool vegetation as a non-equilibrium system has been proposed by work on Ivorian inselbergs (Krieger et al., 2003).
Various studies have demonstrated species response curves other than Gaussian/unimodal, such as asymmetric and bimodal (Austin & Gaywood, 1994; Bio et al., 1998). Compared to GLMs, GAMs are more flexible permitting both linear and complex additive response shapes to be modelled. Coudun & Gegout (2006) suggested that a minimum value of 50 occurrences is necessary to derive acceptable ecological responses curves. Nevertheless, examples where less than 50 samples have been used have been reported (e.g. Horsak, 2006). In this case, the occurrences of the species used for modelling ranged from 20 to 47 and the derived GAM models perform well for most of the species, apart from Tillaea vaillantii (Table 6). In addition, the response curves along the water depth gradient are ecologically interpretable and revealed relationships between species as observed on the ground and also reported by Bergmeier (2001). These include similarities in ecological amplitudes in relation to water depth between Zannichellia pedunculata and Callitriche pulchra but also in higher depths direct competition between Zannichellia pedunculata and Chara vulgaris.
In rock pool environments, interannual variability of the floristic composition and abundance is high as a result of small-scale fluctuations and high turnover rate following rainfall events (Deil, 2005; Delipetrou, 2007). Therefore, and although this study was based on one sampling season, it is part of an ongoing monitoring programme, which will enable insight to be gained into large-scale patterns and temporal variability that will assist with conservation activities in the area. Monitoring in ephemeral pools is very important, due to their small extent and the potential threats they face. At a European level, there is a need to strengthen, develop and coordinate existing initiatives, to establish a sound scientific and practical basis for pond conservation (Zacharias et al., 2007; Céréghino, 2008). Rather than single pond protection, Grillas et al. (2004) advocate the protection of several pools with a range of environmental conditions. Gavdos is one such example, where the protection of the island’s pond sites may result in maximum benefits for biodiversity conservation.
References
Austin, M. P. & M. J. Gaywood, 1994. Current problems of environmental gradients and species response curves in relation to continuum theory. Journal of Vegetation Science 5: 473–482.
Bayly, I. A. E., 1997. Invertebrates of temporary waters in gnammas on granite outcrops in Western Australia. Journal of the Royal Society of Western Australia 80: 167–172.
Bayly, I. A. E., 2002. The life of temporary waters in Australian gnammas (rock-holes). Verhandlungen der Internationalen Vereinigung fur Theoretische und Angewandte Limnologie 28: 1–8.
Bergmeier, E., 2001. Seasonal pools in the vegetation of Gavdos (Greece)—in situ conservation required. Bocconea 13: 511–516.
Bergmeier, E. & S. Abrahamczyk, 2008. Current and historical diversity and new records of wetland plants in Crete. Willdenowia 38: 433–453.
Bergmeier, E. & Th. Raus, 1999. Verbreitung und Einnischung von Arten der Isolto-Nanojuncetea in Griechenland. Mitteilungen des Badischen Landesvereins für Naturkunde und Naturschutz 17: 463–479.
Bergmeier, E., R. Jahn & A. Jagel, 1997. Flora and Vegetation of Gavdos (Greece), the southernmost European island. I. Vascular flora and chorological relations. Candollea 52: 305–358.
Bio, A. M. F., R. Alkemade & A. Barendregt, 1998. Determining alternative models for vegetation response analysis: a non-parametric approach. Journal of Vegetation Science 9: 5–17.
Blaustein, L. & S. S. Schwartz, 2001. Why study ecology in temporary pools? Israel Journal of Zoology 47: 303–312.
Céréghino, R., 2008. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 1–6.
Coudun, C. & J. C. Gegout, 2006. The derivation of species response curves with Gaussian logistic regression is sensitive to sampling intensity and curve characteristics. Ecological Modelling 199: 164–175.
Council of Europe, 1992. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Communities L 206: 7–50.
De Bolos, O., R. M. Masalles, J. M. Ninot & J. Vigo, 1996. A survey on the vegetation of Cephalonia (Ionian islands). Phytocoenologia 26: 81–123.
De Meester, L., S. Declerck, R. Stoks, G. Louette, F. Van De Meutter, T. De Bie, E. Michels & L. Brendonck, 2005. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 715–725.
Deil, U., 2005. A review on habitats, plant traits and vegetation of ephemeral wetlands—a global perspective. Phytocoenologia 35: 533–705.
Delipetrou, P., 2007. Monitoring Plan for the Habitat Vernal Pools in Cyprus. University of Athens, Athens (in Greek).
Dimitriou, E., I. Karaouzas, N. Skoulikidis & I. Zacharias, 2006. Assessing the environmental status of Mediterranean temporary ponds in Greece. Annales de Limnologie-International Journal of Limnology 42: 33–41.
Grillas, P., P. Gauthier, N. Yavercovski & C. Perennou, 2004. Mediterranean Temporary Pools; Volume 1—Issues Relating to Conservation, Functioning and Management. Station Biologique de la Tour du Valat.
Guisan, A., T. Edwards & T. Hastie, 2002. Generalised linear and generalised additive models in studies of species distribution: setting the scene. Ecological Modelling 157: 89–100.
Heikkinen, R. K., M. Luoto, M. Kuussaari & T. Toivonen, 2007. Modelling the distribution of a threatened butterfly: impacts of scale and statistical technique. Landscape and Urban Planning 79: 347–357.
Hill, M. O., 1979. TWINSPAN—A FORTRAN Program for Arranging Multivariate Data in Ordered Two Way Table by Classification of the Individuals and the Attributes. Cornell University, Department of Ecology and Systematics, Ithaca, New York.
Horsak, M., 2006. Mollusc community patterns and species response curves along a mineral richness gradient: a case study in fens. Journal of Biogeography 33: 98–107.
Jahn, R. & P. Schönfelder, 1995. Exkursionflora für Kreta. Ulmer, Stuttgart.
Jongman, R. H. G., C. J. F. ter Braak & O. F. R. van Tongeren (eds), 1987. Data Analysis in Community and Landscape Ecology. Pudoc, Wageningen.
Keeley, J. E., 1999. Photosynthetic pathway diversity in a seasonal pool community. Functional Ecology 13: 106–118.
Krause, W., W. Ludwig & F. Seidel, 1963. Zur Kenntnis der Flora und Vegetation auf Serpentinstandorten des Balkans. 6. Vegetationsstudien in der Umgebung von Mantoudi (Euböa). Botanische Jahrbücher für Systematik 82: 337–403.
Krieger, A., S. Porembski & W. Barthlott, 2003. Temporal dynamics of an ephemeral plant community: species turnover in seasonal rock pools on Ivorian inselbergs. Plant Ecology 167: 283–292.
Kruckeberg, A. R., 2002. Geology and Plant Life: The Effects of Landforms and Rock Types on Plants. University of Washington Press, Washington.
Laurilla, A., 2000. Competitive ability and the co-existence of anuran larvae in freshwater rock-pools. Freshwater Biology 43: 161–174.
McCune, B. & M. J. Mefford, 1999. PC-ORD, Multivariate Analysis of Ecological Data. MJM Software, Glenden Beach.
Müller, J. V. & U. Deil, 2005. The ephemeral vegetation of seasonal and semi-permanent ponds in tropical West Africa. Phytocoenologia 35: 327–388.
Oberdorfer, E., 1952. Beitrag zur Kenntnis der nordagaischen Kustenvegetation. Vegetatio 3: 329–349.
Ott, S., U. Elders & H. M. Jahns, 1996. Vegetation of the rock-alvar of Gotland I. Microhabitats and succession. Nova Hedwigia 64: 433–470.
Ott, S., E. Osenberg & H. M. Jahns, 1997. Vegetation of the rock-alvar of Gotland II. Microclimate of lichens in a rock habitat. Nova Hedwigia 64: 87–101.
Phitos, D., A. Strid, S. Snogerup & W. Greuter (eds), 1996. The Red Data Book of Rare and Threatened Plants of Greece. WWF, Greece, Athens.
Pinder, A. M., S. A. Halse, R. J. Shiel & J. M. Mcrae, 2000. Granite outcrop pools in south-western Australia: foci of diversification and refugia for aquatic invertebrates. Journal of the Royal Society of Western Australia 83: 149–161.
Rackham, O. & J. Moody, 1996. The Making of the Cretan Landscape. Manchester University Press, Manchester.
Raunkiaer, C., 1934. The Life Forms of Plants and Statistical Plant Geography. Oxford University Press, Oxford.
Sarika-Hatzinikolaou, M., A. Yannitsaros & D. Babalonas, 2003. The macrophytic vegetation of seven aquatic ecosystems of Epirus (NW Greece). Phytocoenologia 35: 93–151.
Scholnick, D. A., 1994. Seasonal variation and diurnal fluctuations in ephemeral desert pools. Hydrobiologia 294: 111–116.
Spencer, M., S. S. Schwarz & L. Blaustein, 2002. Are there fine-scale spatial patterns in community similarity among temporary freshwater pools? Global Ecology and Biogeography 11: 71–78.
Srivastava, D. S., J. Kolasa, J. Bengtsson, A. Gonzalez, S. P. Lawler, T. E. Miller, P. Munguia, T. Romanuk, D. C. Schneider & M. K. Trzcinski, 2004. Are natural microcosms useful model systems for ecology? Trends in Ecology & Evolution 19: 379–384.
ter Braak, C. J. F. & P. Smilauer, 1998. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4). Microcomputer Power, Ithaca, NY, USA.
Turland, N. J., L. Chilton & J. R. Press, 1993. Flora of the Cretan Area. Annotated Checklist and Atlas. HMSO, London.
Vanschoenwinkel, B., C. De Vries, M. Seaman & I. Brendonck, 2007. The role of metacommunity processes in shaping invertebrate rock pool communities along a dispersal gradient. Oikos 116: 1255–1266.
Wessels, D. C. J. & B. Budel, 1989. A rock pool lichen community in Northern Transvaal, South Africa: composition and distribution patterns. The Lichenologist 21: 259–277.
Williams, D. D., 1987. The Ecology of Temporary Waters. Timber Press, Oregon.
Zacharias, I., E. Dimitriou, A. Dekker & E. Dorsman, 2007. Overview of temporary ponds in the Mediterranean region: threats, management and conservation issues. Journal of Environmental Biology 28: 1–9.
Zacharias, I., A. Parasidou, E. Bergmeier, G. Kehayias, E. Dimitriou & P. Dimopoulos, 2008. A “DPSIR” model for Mediterranean temporary ponds: European, national and local scale comparisons. Annales de Limnologie-International Journal of Limnology 44: 243–256.
Acknowledgements
This research was funded by the LIFE Nature Programme Actions for the conservation of Mediterranean Temporary Ponds in Crete (LIFE04NAT/GR/000105). We are grateful to Mrs. Christina Fournaraki, curator at the Herbarium of MAICh, for her help in species identification, and two anonymous reviewers whose comments resulted in an improved manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. M. Thomaz
Rights and permissions
About this article
Cite this article
Vogiatzakis, I.N., Kazakis, G. & Ghosn, D. Macrophyte community structure and species occurrence in relation to environmental determinants in the ephemeral aquatic habitats of Gavdos, Greece. Hydrobiologia 630, 127–138 (2009). https://doi.org/10.1007/s10750-009-9785-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9785-x