Abstract
This study examined how sediment-sorbed PCBs and several large storms affected sediment nutrient dynamics based on potential nitrification rates and benthic flux measurements. PCBs were hypothesized to negatively affect potential nitrification rates due to the sensitivity of nitrifying bacteria. Sediment disturbance caused by the succession of storms, which can enhance nutrient inputs and phytoplankton production, was hypothesized to enhance both potential nitrification rates and benthic flux measurements as a result of higher nutrient and organic matter concentrations. Potential nitrification rates, benthic fluxes (NO3 − + NO2 −, NH4 +, and DIP), sediment PCB content, water content, organic content, salinity, bottom water dissolved oxygen, and sediment chlorophyll were measured at 13 different sites in Escambia Bay during the summer of 2005. Potential nitrification rates were highest at deep, organic-rich sites. Total PCB content did not have a direct effect on potential nitrification rates. An analysis of recent changes in benthic processes in relation to extreme meteorological events was performed by comparing the 2005 results with data from 2000, 2003, and 2004. Storm effects on sediment biogeochemistry were mixed with sediment nitrogen dynamics enhanced at some sites but not others. In addition, SOC and NH4 + fluxes increased in deeper channel sites after Hurricanes Ivan and Dennis, which could be attributed to the deposition of phytoplankton blooms. Sediment nutrient dynamics in Escambia Bay appear to be resilient to these extreme meteorological events since there were no significant effects on sediment processes in the Bay as a whole.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen is an important factor in the regulation of primary production in estuaries because nitrogen can be the primary limiting nutrient (Blackburn, 1988). Although nitrogen is a significant factor for the health of estuaries, excess nitrogen can lead to eutrophication (National Research Council, 2000). Sources such as fertilizers, sewage discharges, urban development, and aquaculture have greatly increased nitrogen concentrations in estuaries (Vitousek et al., 1997). Increased nitrogen inputs contribute to algal blooms which, may lead to acute and chronic effects on a wide variety of organisms, reduced oxygen availability in the water, bottom water hypoxia and anoxia, benthic species mortality, and elimination of nitrification (Diaz & Rosenberg, 1995; Shumway et al., 1995; Burkholder, 1998; Landsberg, 2002; Keppler et al., 2005).
Sedimentation of phytoplankton blooms transfers organic matter from the water column to sediments, which enhances mineralization of NH4 +. This NH4 + can either diffuse out of sediments or be nitrified. Nitrification is a two step microbially mediated process central to the nitrogen cycle. The first step is the aerobic oxidation of NH4 + to NO2 − (ammonia oxidation) and the second step is the oxidation of NO2 − to NO3 − (nitrite oxidation). Nitrification is often a key process supplying NO3 − for nitrogen removal processes such as denitrification, the reduction of NO3 − to dinitrogen gas (Seitzinger, 1988; Risgaard-Petersen, 2003), or anaerobic ammonium oxidation to dinitrogen gas (Risgaard-Petersen et al., 2005).
In addition to excess nitrogen, toxic contaminants from point and nonpoint sources also pollute estuaries. The impact of contaminants such as heavy metals, oil, and organochlorines depends on concentration, toxicity, and persistence in the environment (Kennish, 1992). Aroclor® 1254 is a common PCB mixture with an average chlorine content of 54% (USAF, 1989). In the environment, the behavior of PCB mixtures is directly correlated to the degree of chlorination. Although banned in 1977, PCBs such as Aroclor® 1254 continue to be released into the environment from hazardous waste sites, illegal or improper disposal of industrial wastes and consumer products, leaks from old electrical transformers containing PCBs, and burning of some wastes in incinerators. PCBs are stable compounds that can persist in sediments long after point source discharges are eliminated (Wilson & Forester, 1978). PCBs have been detected globally, from the most urbanized areas that contain high levels of PCB contamination to regions north of the Arctic Circle. The atmosphere serves as the primary route for global transport of PCBs, particularly for those congeners with 1–4 chlorine atoms. Due to lipophilicity, especially of highly chlorinated congeners, PCBs tend to accumulate in the fat of fish, birds, mammals, and humans (ATSDR, 1995). Although PCB toxicity and bioaccumulation to estuarine life is well documented (Olsson et al., 1992, 1994), few studies have examined the effects of PCBs on nitrogen biogeochemistry in saltwater environments. Sayler et al. (1982) reported that the ammonia oxidation was inhibited by PCBs in one reservoir, although this inhibition could not be reproduced with cultured nitrifiers. Nitrification potentials in grassland soils next to a municipal waste incinerator were inhibited at high PCB concentrations (Dušek, 1995). In addition to PCBs, other factors affect nitrification rates such as temperature (Hansen, 1980; MacFarlene & Herbert, 1984; Seitzinger et al., 1984), dissolved oxygen (Kemp et al., 1990), salinity (Rysgaard et al., 1999), organic loading (Caffrey et al., 1993), xenobiotics (Miller et al., 1996; Campos et al., 2001), macrofaunal activity (Henriksen et al., 1983; Herbert, 1999), and oxygen release by macrophyte roots (Kemp et al., 1982; Jaynes & Carpenter, 1986; Caffrey & Kemp, 1990).
Meteorological events can also affect water and sediment quality and, combined with anthropogenic impacts, can further stress the system. Episodic weather events such as tropical storms and hurricanes can cause sediment bound pollutants to reenter the water column and directly impact water and sediment quality. Natural disturbances such as these have been shown to greatly affect estuarine dynamics by depressing salinity, increasing nutrient concentrations, increasing algal blooms, increasing concentrations of organic matter, and causing bottom water hypoxia (DO < 2 mg l−1)/anoxia (DO = 0 mg l−1) in estuaries (Fogel et al., 1999; Mallin et al., 1999; Paerl et al., 2001; Hagy et al., 2006). Global climate change models and trend analyses of historic data suggest that the intensity and frequency of storm events will continue to increase with global warming (Meehl et al., 2007).
The objective of this study was to determine how human and meteorological impacts affect sediment nutrient dynamics based on potential nitrification rates and benthic flux measurements. Sediment-sorbed PCBs, an anthropogenic impact, were hypothesized to negatively affect potential nitrification rates due to the sensitivity of nitrifying bacteria. The meteorological impacts, which can enhance nutrient inputs and phytoplankton production following storm events, were hypothesized to enhance both potential nitrification rates and benthic flux measurements as a result of higher nutrient and organic matter concentrations.
Study area
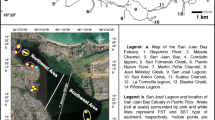
Escambia Bay, located in the western arm of the Pensacola Bay System in Northwest Florida, is an urban estuary (Fig. 1). Freshwater flow from the Escambia River averages about 180 m3 s−1 and represents 80% of the freshwater input to the bay. Tides are diurnal with a tide range of 0.35 m. Stratification during the summer months often leads to extended periods of hypoxia (Hagy & Murrell, 2007). Escambia Bay has a history of degraded sediment and water quality due to point and nonpoint source discharges (Thorpe et al., 1997). Because of poor tidal flushing, highly organic sediments, and a history of anthropogenic pollution, upper Escambia Bay has been considered the most anthropogenically stressed part of the Pensacola Bay System (Livingston, 1999). Polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), metals, pesticides, and other chemical contaminants pollute Escambia Bay (USEPA, 2004). Out of 281 estuarine sites sampled within the Gulf of Mexico, Escambia Bay had the third highest total sediment PCB content and the Escambia River had the fifth highest (Maruya et al., 1997). Although many of the contaminant inputs have been reduced, sediments in parts of the bay remain contaminated. One notable anthropogenic impact on Escambia Bay was a spill of Aroclor® 1254 in April 1969 due to an accidental leakage of heat exchange fluid from an industry adjacent to the Escambia River (Duke et al. 1970). Aroclor® 1254 had detrimental effects on Escambia Bay’s shrimp (Penaeus duorarum), oysters (Crassostrea virginica), and spot (Leoistomus xanthurus) (Nimmo et al., 1971, 1975). Shortly after the spill, PCB sediment concentrations in Escambia Bay were extremely high (486,000 μg kg−1; Nimmo et al., 1975, Wilson & Forester, 1978).
Hurricanes, tropical storms, and other extreme rainfall events periodically influence water quality and sediment transport in the region. Between 2004 and 2005, Escambia Bay was directly impacted by several events: Hurricane Ivan in September 2004, Hurricane Dennis in July 2005, Tropical storm Arlene in June 2005, and record breaking precipitation [April 2005, NOAA public communication (a)]. This followed 3 years of extreme drought between 1999 and 2001.
Materials and methods
Water column and sediment samples were collected at 13 different sites in the Escambia River and Escambia Bay, Florida (Fig. 1). Sampling sites had a range of water depth, sediment type, dissolved oxygen levels, water column chlorophyll a concentrations, and salinities. Three sites were in the river (R). Sampling locations in Escambia Bay were separated into channel (Ch) sites, which were down the main stem of the bay and shoal (S) sites on the eastern and western sides of the bay. Most shoal sites had water depths less than 2.5 m.
All sites, except R3, were sampled during the summer/fall of 2005. R3 was sampled during the spring of 2006 and R1 was resampled at this time giving 13 different sites but 14 sampling events. Physicochemical measurements were obtained with either a handheld YSI® 85 or a Hydrolab® Quanta 4 from 0.5 m below the surface and 0.5 m from bottom. The bottom measurements are reported in Table 1.
At each site, we collected six small cores (4.5 cm diameter), three large cores (8 cm diameter), and three liters of bottom water, which were used as replacement water for the benthic flux experiments. Potential nitrification, sediment chlorophyll a, water content, organic content (TOC), extractable ammonium, and extractable phosphate were analyzed in triplicate using sediment from small cores. The large cores were used for benthic flux measurements. All cores were collected either using divers for channel stations or push cores for the shoal and river stations (Table 2). Following the benthic flux incubations, samples for PCB content and grain size measurements were collected.
Sediment characteristics
Water content for 0–0.5, 0.5–1, 1–2, and 2–4 cm layers was determined for each site by sectioning the small core tubes and drying sediments in aluminum pans at 80°C for 24 h. Sediments were combusted at 500°C for 1 h and the weight was recorded (ashed weight) and used to calculate organic content.
In order to determine sediment chlorophyll a concentrations, sediment (0.5 g) from the top 0.5 cm of the small core tube was frozen at −17°C until analysis. The samples were homogenized and 0.5-g samples were extracted in 10 ml of 90% acetone and sonicated in an icebath for 5 min. Chlorophyll was extracted in the freezer (24 h), solutions centrifuged for 10 min, and concentrations were determined using a Turner Designs TD-700 Fluorometer (Welschmeyer, 1994). Water column chlorophyll a concentrations were also determined by filtering water samples (ca. 50 ml) through GF/F filters (nominal pore size 0.7 μm) and extracting the filters using the same procedure as the sediment samples.
Sediment grain size analysis followed Folk (1974) with slight modifications. A total of 20 cc of sediment was mixed with hydrogen peroxide and distilled water to digest organic matter. The samples were sieved through a 63-μm mesh sieve to separate the silt and clay from the sand. The sand was transferred into a pre-weighed beaker. The silt/clay mixture was diluted to one liter in 10% Calgon® solution. Two withdrawals were taken (the time between the withdrawals was determined from a temperature/fall distance table) and put into pre-weighed beakers. All fractions were placed into a drying oven for 24 h and reweighed. Percent sand, percent clay, and percent silt were calculated from these data.
Potential nitrification
Potential nitrification was measured in the top 0.5 cm of each small core of sediment. Approximately 1 g of sediment was incubated with GF/F filtered bottom water and ammonium chloride at 520 μM final concentration (Henriksen et al., 1981). Samples were incubated at room temperature (ca. 22°C) in the dark with continuous agitation. Initial (T = 0 h) and final (T = 24 h) samples were collected and filtered through a GF/F filter into sample vials and frozen immediately. Samples were analyzed for nitrate plus nitrite (NO3 − + NO2 −) and nitrite (NO2 −) (see nutrient analyses). Rates were expressed as μmol NO3 − + NO2 − per gram of wet sediment per day. NO2 − production was determined as a percentage of NO3 − + NO2 − production.
Benthic flux measurements
For benthic flux measurements, the batch method of incubation was used and the vertical structure of the core was maintained. Our method of collection was comparable to the benthic flux studies from 2000, 2003, and 2004 (Table 2). Most of the overlying water was removed from the sediment cores and replaced with bottom water collected at the site (Caffrey et al., 1996). Tops with magnetically driven stir bars were placed on the sediment cores to simulate turbulent mixing of the natural benthic environment, but below the resuspension threshold. The three large sediment cores plus a control core of bottom water only were covered with aluminum foil and placed in a water bath to maintain ambient bottom water temperatures. The height of the overlying water and the height of the sediment were recorded in each of the large core tubes. Across all the sites, the average height of the sediment and the average height of the overlying water were 26.7 and 17.2 cm, respectively. Overlying water samples were collected at 1, 3, 5, and 7 h for nutrient and dissolved oxygen analyses. Nutrient samples were filtered through a GF/F filter and frozen until analysis of NO3 − + NO2 −, DIP, and NH4 +. Samples for dissolved oxygen (DO) were collected into 7-ml vials and dissolved oxygen measurements were made using the Winkler technique modified for 7-ml samples (Parsons et al., 1984).
Nutrient analyses
For extractable NH4 + and phosphorous analyses, 10–20 g of sediment were taken from 0–0.5, 0.5–1, 1–2, and 2–4 cm layers and transferred into four different 50-ml centrifuge tubes. Each tube received 10 ml of 1 M NaCl if they contained less than 20 g of sediment and 20 ml of 1 M NaCl if they contained more than 20 g of sediment. The tubes were shaken and then centrifuged for 15 min. The supernatant was then filtered through a GF/F filter into sample vials that were frozen immediately and subsequently analyzed for NH4 + and DIP.
Extractable NH4 + and DIP were analyzed either on the Lachat Quickchem Series 8500 Flow Injection Autoanalyzer using the Quikchem® method 31-107-06-1-B (Liao, 2003) for NH4 + and method 31-115-01-1-I (Ammerman, 2003) for DIP or manually using the Holmes et al. (1999) method for NH4 + and Parsons et al. (1984) method for DIP. Both the Holmes and Parsons methods allow for highly reproducible results even at extremely low levels of ammonium and phosphate. NO3 − + NO2 − and NO2 − were analyzed on the Lachat Quickchem Series 8500 Flow Injection Autoanalyzer (Lachat) according to Quikchem® method 31-107-04-1-A (Diamond, 2003).
For PCB samples, 200 ml of sediment were collected and frozen until analysis. Analyses were conducted by Columbia Analytical Services, Inc. (CAS) in Houston, Texas for 209 PCB congeners using EPA method 1668A (USEPA, 1999).
Analysis of recent changes in Escambia Bay
In order to determine if there were differences between pre- and post-storm benthic flux data, we directly compared the sites from 2000, 2003, and 2004 to the same sites from 2005 (Fig. 2). Benthic nutrient flux measurements were first made in Pensacola Bay in the summer of 2000 (Didonato et al., 2006; Table 2). Four of the Didonato sites were revisited for this study (Mulat Bayou—S1, Trout Bayou—S3, Gull Point Shallow—S2, and Gull Point Deep—Ch2). Benthic nutrient and oxygen flux measurements were also made in the summers of 2003 and 2004 at sites throughout Pensacola Bay (Murrell et al., 2009). Six of those sites were revisited for this study (Table 2).
Statistical analyses
Differences between sites in water content, organic content, PCB content, extractable NH4 +, and extractable DIP were examined using univariate analysis of variance (ANOVA), with the Tamhane’s T2 or the Least Significant Difference (LSD) method for multiple comparisons. Pearson correlation analyses were used to examine the relationships among all measurements. When data were not normally distributed, log transformations or ranks were used. Potential nitrification rates, extractable NH4 +, extractable DIP, water content, and organic content data were obtained from 2004 and compared to the current data from 2005 to determine any significant differences between years using univariate ANOVA. Differences in individual PCB congeners between sites were determined by principal component analysis. Principal component analysis was also used to examine differences in sites based on sediment and bottom water characteristics. The composite variables created by this analysis were also used in Pearson correlation analysis with potential nitrification and benthic flux measurements.
Results
Physicochemical quality
The sites ranged in depth from 0.5 to 5.7 m (Table 1) and encompassed a wide salinity (0–28.7 PSU). Salinity was positively correlated with depth (r = 0.71; P = 0.01; Table 3). The samples were collected during the summer when surface water temperatures averaged 29°C, except for one sampling trip in March when temperatures averaged 21°C. The channel sites were strongly stratified with surface water salinity usually 10 PSU less than the bottom (data not shown). Two shoal sites (S5 and S6) were moderately stratified with surface water salinity between 6 and 9 PSU less than the bottom. The remaining shoal sites were weakly stratified, while the river sites were unstratified (data not shown). Three of the channel sites and one shoal site (S3) were hypoxic, while DO concentrations at the shoal sites averaged 5.3 mg l−1 (Table 1).
Sediment characteristics
The Escambia Bay sites varied in grain size from coarse sands (80% sands) to fine silts and muds (Table 1). All of the shoal sites were 80% sand or higher. These sandy sites had low water content (<30%) and low organic matter content (<1.2%), with the exception of S1. Water content ranged from 18% to 84% (Table 1) and was negatively correlated with DO (r = −0.71; P = 0.05; Table 3) and positively correlated with depth (r = 0.77; P < 0.01; Table 3) and organic content (r = 0.97; P = 0.01; Table 3). Organic content ranged from 0.3% to 13.3% (Table 1). The channel sites with fine grain sediments (at least 80% silt) generally had significantly higher organic content (greater than 7.8%) than shoal sites (Table 1).
Sediment chlorophyll ranged from 1.12 to 19.65 μg g−1 wet sediment (Fig. 3). Sediment chlorophyll concentrations were significantly higher at S5 than any other site. In general, the shallow sites had significantly higher sediment chlorophyll concentrations than the river and deep sites. Sediment chlorophyll was positively correlated with dissolved oxygen (r = 0.80; P = 0.02) and negatively correlated with bottom water NO3 − + NO2 − concentrations (r = −0.86; P < 0.001; Table 3).
Extractable NH4 + ranged from 3 to 88 nmol N cm−3 (Fig. 4). Ch2 and R2 had significantly higher NH4 + concentrations than any other site (greater than 105 μM), although these two sites also had the highest variability. Ch1, S2, S5, and S6 had the next significantly highest NH4 + concentrations, demonstrating that the channel sites were not significantly higher than the river or shoal sites as they were in water and organic content. Extractable PO4 3− ranged from near 0 to 2.3 nmol P cm−3 (Fig. 4). Shoal sites had the widest range of extractable PO4 3−. There were no significant trends among the shoal, river, and channel sites.
Total PCB content was positively correlated with organic content (r = 0.71; P = 0.02; Table 3) and water content (r = 0.73; P = 0.02; Table 3). Four sites (Ch1, Ch3, Ch4, and R3) had PCB concentrations above 21.55 μg kg−1, which exceeds the National Oceanic and Atmospheric Administration’s (NOAA) Threshold Effects Level (TEL) [NOAA public communication (b)]. R3, a freshwater site located downstream from the source of the PCB spill, had the highest total PCB content and a very different PCB composition compared to the other sites (Fig. 5). No relationship between PCB content and potential nitrification rates was evident (Table 3). In fact, three sites in Escambia Bay with high total PCB content also had high potential nitrification rates (Ch3, Ch4, and R3; Figs. 5, 6). Ch1 was the only site in Escambia Bay with high total PCB content (second highest of all the sites), but low potential nitrification rates. Ch1 had PCB congeners similar to Ch3 and Ch4 (Fig. 5), but significantly lower potential nitrification rates. While the distribution of PCB congeners was somewhat different between R3 and the other sites, potential nitrification rates were high at this site as well.
Principal components analysis of PCB composition for all sites showing PCA 1 versus PCA 2. Total PCB content (μg kg−1) is indicated by the size of the dots. Threshold Effects Level is (TEL = 21.55 μg kg−1) indicated by mid-sized dot. Four sites exceeded TEL, while none exceeded the Probable Effects Level (PEL) (188.79 μg kg−1)
Boxplot of potential nitrification rates (μmol g−1 day−1) for all sites. Top boxplot shows sites river and channel sites, while bottom boxplot shows shoal sites. The 25th percentile is the lower boundary of box and upper boundary of box is 75th percentile (n = 6); heavy line in box is mean, and light line is median
The principal components analysis of the water column and sediment parameters could explain 78% of the variance in the dataset with three factors. The first factor, which explained 40.5% of the variance, included bottom water NO3 − + NO2 −, PCB concentration, and sediment chlorophyll. The second factor included depth and salinity and explained 23% of the variance, while the third factor included water content, organic content, extractable NH4 +, and extractable PO4 3− and explained 15% of the variance (Table 4).
Potential nitrification
Average potential nitrification rates from the sampling sites ranged from near zero to 0.64 μmol g−1 wet sediment day−1 (Fig. 6). Two deeper, fine grained, saline sites (Ch3 and Ch4) had the highest potential nitrification rates, while the fresh, sandy, shallow sites (R1 2005 and R3) had equally high values. Shoal sites had potential nitrification rates less than 0.4 μmol g−1 day−1. Potential nitrification rates were positively correlated with depth (r = 0.63; P = 0.03), water content (r = 0.70; P = 0.01), and organic content (r = 0.71; P = 0.01; see Table 3). Potential nitrification was also significantly correlated with factor 3 (water content, organic content, extractable NH4 +, and extractable PO4 3−) from the principal components analysis (r = 0.61; P = 0.02).
Benthic flux measurements
NH4 + flux estimates ranged from −0.96 to 2.51 mmol m−2 day−1 (Table 5). The initial concentrations of NH4 + in the overlying water ranged from 0.20 to 12.3 μM. NH4 + fluxes were significantly different from zero at sites S2, S5, S6, R1, R2, Ch1, and Ch4. The highest NH4 + effluxes were observed at Ch3 and Ch4, two of the deeper sites. Only one site, Ch1, had significant NH4 + influx to the sediment. NH4 + fluxes were negatively correlated with the initial concentrations of DO in the overlying water (r = −0.61; P = 0.04; Table 5) and positively correlated with factor 2 (depth and salinity) from the principal components analysis (r = 0.65; P = 0.02).
NO3 − + NO2 − flux estimates ranged from −3.13 to 0.64 mmol m−2 day−1 (Table 5). The initial concentrations of NO3 − + NO2 − in the overlying water ranged from 0.36 to 16.7 μM. The NO3 − + NO2 − fluxes were negatively correlated with the initial concentrations of NO3 − + NO2 − in the overlying water (r = −0.76; P < 0.01; Table 3). At overlying water NO3 − concentrations greater than 3.6 μM, fluxes were into the sediment, while at concentrations less than 3.6 μM, fluxes were out of the sediment. NO3 − + NO2 − fluxes were significantly different than zero at Ch1, Ch4, S1, S2, S6, R1, and R2. Most of these sites had NO3 − + NO2 − flux into the sediments, but S1 and S6 had NO3 − + NO2 − efflux out of the sediments. The NO3 − + NO2 − fluxes were positively correlated with the initial concentration of oxygen in the overlying water (r = 0.67; P = 0.03; Table 3).
Fluxes of DIP in Escambia Bay ranged from −0.35 to 0.16 mmol m−2 day−1 (Table 5). The initial concentrations of DIP in the overlying water ranged from 0.05 to 0.94 μM. DIP fluxes were significantly different than zero at S4, Ch2, Ch3, and R1. Ch3 and S4 had DIP efflux, while R1 (2005) and Ch2 had DIP influx. There was a negative relationship between DIP flux and temperature (r = −0.78; P = 0.02; Table 3).
Sediment oxygen consumption (SOC) varied from 7.41 to 34.8 mmol m−2 day−1 and the initial concentrations of O2 in the overlying water ranged from 2.18 to 7.42 mg l−1. The sediment consumed oxygen at every site except for the Ch3 site where we were unable to maintain hypoxic conditions due to oxygen diffusing into the core tubes. The highest consumption of O2 by the sediment occurred at S9 and the lowest consumption of O2 into the sediment occurred at S1, where SOC was not significantly different than zero. Shallow sites, except for S3, had SOC less than 22 mmol m−2 day−1 (Table 5).
Discussion
Factors controlling potential nitrification
Potential nitrification rates in Escambia Bay were significantly influenced by water content, organic content, extractable NH4 +, and extractable PO4 3− (factor 3 from the principal components analysis). The highest, but most variable, rates were seen at R1 (2005), R3, Ch3, and Ch4, the four sites with the highest organic matter content (Fig. 6). It is interesting that higher potential nitrification rates occurred at deep organic-rich sediments and lower rates occurred at shallow organic-poor sediments. Deep sites with high organic matter may be expected to have low rates of nitrification because of corresponding low oxygen conditions. The absence of oxygen under hypoxic and anoxic conditions can virtually eliminate nitrification (especially in summer months) by limiting oxygen penetration in sediments, although nitrifiers can recover activity after exposure to low oxygen (Henriksen et al., 1981; Kemp et al., 1990). Because ammonium oxidizers have a higher affinity for oxygen than nitrite oxidizers, nitrite oxidation can be restricted under low oxygen conditions leading to an accumulation of NO2 − (Henriksen & Kemp, 1988). The lower portion of Escambia Bay (encompassing sites C3 and C7) often experiences extended periods of summer hypoxia due to stratification (Hagy & Murrell, 2007). While Ch3 and Ch4 had low oxygen concentrations during our sampling (0.08 and 0.9 mg l−1), their potential nitrification rates were among the highest. Nitrite oxidation at these two sites was limited, representing 48% and 66% of total potential nitrification, respectively (data not shown). Nitrifiers, especially the ammonium oxidizers, may not be as sensitive to oxygen levels as was once thought. This idea is consistent with some studies where nitrifiers were shown to be abundant at low oxygen levels (Goreau et al., 1980; Voytek & Ward, 1995). Lohse et al. (1993) also found higher nitrification (both in intact cores and with potentials) rates when the oxygen penetration depth was small compared to when the oxygen penetration depth was large. In Escambia Bay, the nitrifiers appear to be active even under low oxygen conditions. NH4 + supply (extractable NH4 + and organic content) seems to be more important to nitrifier activity.
Total PCB content was positively correlated with water content and organic content, consistent with studies that found PCBs bind strongly to fine sediments (Zaranke et al., 1997). Contrary to our hypothesis, there was no relationship between total PCB content and potential nitrification despite high concentrations of PCBs at some sites (Ch1, Ch3, Ch4, and R3). One possible explanation for the differences among high potential nitrification rates at Ch3, Ch4, and R3 compared to low rates at Ch1 is the relative stability of environmental conditions at the sites. Ch3 and Ch4 are marine sites, R3 is a freshwater site, but Ch1 is located at a brackish/freshwater interface and is subject to variable salinity stress. Thus, Ch1 is influenced by multiple stressors (including PCB contamination and changing salinity), which may account for lower potential nitrification rates.
Variable salinities, as in Ch1, have been shown to affect potential nitrification rates. A short term study with Nitrosomonas demonstrated that this species had optimum activity in the 5–10 PSU range, activity that rapidly decreased within 24 h of being exposed to either lower or higher salinities (Jones & Hood, 1980). Nitrifiers may need a lag period in order to acclimate to new salinities (Finstein & Bitsky, 1972). Helder & de Vries (1983) demonstrated that a marine Nitrosomonas species exposed to different salinities required 12 days to return to initial rates. Although a preferred salinity of nitrifiers has not been determined, estuarine field samples have been found to exhibit optimal activity at low or intermediate salinities (Nijhof & Bovendeur, 1990; Caffrey et al., 2003). However, nitrifiers can adapt to the salinity prevailing in their environment (Caffrey et al., 2003; Canfield et al., 2005). In Escambia Bay, nitrifiers from site R1 were unable to nitrify when exposed to salinities of 10 PSU or higher (Smith, 2006). In contrast, nitrifiers from Ch3 had the highest nitrification rates at 20 PSU, intermediate rates at 10 and 30 PSU, but were unable to nitrify at 0 PSU (Smith, 2006). This may be why potential nitrification rates do not seem to be affected by high, but consistent, salinity in Ch3 and Ch4.
Nutrient cycling in intact sediments
In contrast to potential nitrification, which is a measure of the activity of nitrifying bacteria under optimal conditions, benthic fluxes reflect bacterial activity in intact sediments at in situ temperatures. Benthic fluxes of nutrients and oxygen are driven by the concentration gradient between the overlying water and porewater (Hall et al., 1989). Many of the same factors that control nitrification rates can also control benthic flux rates. Benthic fluxes of nutrients are an important factor in sustaining high productivity of any system (Friedrich et al., 2002).
NO3 − + NO2 − fluxes represent the balance between NO3 − production by nitrification and NO3 − removal by denitrification, dissimilatory NO3 − reduction to NH4 + and anaerobic ammonium oxidation (anammox). When fluxes are positive, this suggests nitrification is enhanced over removal processes. S1 and S6 were the only sites in Escambia Bay where a significant release of NO3 − + NO2 − from the sediment to the overlying water occurred. Where temperature and DO concentrations were high, as with S1 (30.5°C and 6.1 mg l−1, respectively), high rates of nitrification lead to high NO3 − release rates (Cowan et al., 1996). Bottom water NO3 − + NO2 − concentrations were negatively correlated with NO3 − + NO2 − fluxes in Escambia Bay as has been observed in previous studies (Henriksen, 1980; Jensen et al., 1990; Niencheski & Jahnke, 2002). Sites with lower bottom water NO3 − + NO2 − concentration, such as S1 (1.5 μM), had significant NO3 − + NO2 − release from the sediment into the overlying water column. At low NO3 − + NO2 − concentrations in overlying water, fluxes are most likely driven by the balance between nitrification and denitrification (Twilley et al. 1999). Sites with high bottom water NO3 − + NO2 − concentrations (>6 μM) had significant uptake of NO3 − + NO2 − from the water column into the sediment (Table 5). These sites, S2, R2, and Ch1, also had low potential nitrification rates, which suggesting that direct denitrification from NO3 − uptake occurred at these sites. Initial oxygen concentrations in the cores were also significantly positively correlated with NO3 − + NO2 − fluxes. Thus, high DO can enhance nitrification leading to positive NO3 − fluxes, while low DO would enhance anaerobic processes like denitrification, which leads to negative NO3 − fluxes (Twilley et al., 1999).
Mineralization and decomposition can be a significant source of NH4 + for nitrification. Sediment oxygen consumption was used to estimate NH4 + production using the Redfield ratio of 6.6 C:N and assuming that oxygen consumption is equal to dissolved inorganic carbon (DIC) production. All of the measured fluxes were found to be lower than the predicted NH4 + production (Table 5). This analysis suggests that coupled nitrification–denitrification may be occurring at sites like S2, R2, Ch1, and Ch4 that also have significant NO3 − + NO2 − uptake. This potential for coupled nitrification-denitrification is supported by the high potential nitrification rates seen at sites such as Ch3 and Ch4. This has been observed in other estuaries and coastal systems (Henriksen et al., 1993; Caffrey et al., 2003; Tobias et al., 2003). NH4 + fluxes were negatively correlated with the DO concentration in the overlying water of the cores. Hypoxia in bottom waters can result in higher fluxes of NH4 + from the sediments to the overlying water, promoting a positive feedback loop that can contribute to poor water quality conditions (Kemp et al., 1990). This is apparent in Ch4, one of the most hypoxic sites (0.08 mg l−1) with the highest bottom water concentration of NO3 − + NO2 −. Ch4 also had some of the finest grain sediment and NH4 + fluxes can also be highest at finer-grained, silty sediments (Niencheski & Jahnke, 2002). Ch1 was the only site with significant uptake of NH4 +.
Mineralization of phosphorus is also important in this system, which is periodically phosphorus limited (Murrell et al., 2002). Bottom water temperature, oxygen, and NO3 − + NO2 − concentrations, as well as sediment Fe content have been noted as the most important factors controlling the release of DIP from sediments in previous studies (Boström et al., 1988; Jensen & Andersen, 1992; Smolders et al., 2001). S4, Ch2, Ch3, and R1 were the only sites in Escambia Bay with DIP fluxes that were significantly different than zero. S4 and Ch3 had small, positive fluxes where DIP was released from the sediment to the overlying water column. Enhanced release of phosphorus has been seen under anoxic conditions (Jensen et al., 1995) and this appeared to be happening in Ch3 (bottom water DO 0.9 mg l−1). Periods of high temperatures and increased rates of microbial metabolism can reduce bottom water oxygen and NO3 − + NO2 − levels, enhancing Fe reduction and the release of Fe bound phosphorus (Jensen & Andersen, 1992). R1 was the only site where a large significant uptake of DIP occurred. Ripl (1976) hypothesized that high levels of bottom water NO3 − + NO2 − can inhibit Fe reduction and prevent DIP release from the sediment. The high bottom water NO3 − + NO2 − concentrations at R1 (10.59 μM) are consistent with this idea. Fundamental differences in the phosphorus cycle between freshwater and marine environments may also explain why R1 behaves differently than the other sites from Escambia Bay. Phosphate binds more strongly with particles in fresh and brackish water due to decreased competition for adsorption sites by other ions (particularly sulfate) in the water (Caraco et al., 1989). The River site also receives agricultural runoff from upstream. Phosphorus can reach undesirable concentrations in stormwater, agricultural, or industrial runoff and lead to eutrophication. R1 may act as a phosphorus sink, maintaining water quality during periods of moderate and high river flow when the site remains fresh.
Role of benthic microalgae
Benthic microalgae, mainly diatoms, are abundant in the shallow sediments of Escambia Bay based on the high chlorophyll a concentrations. The lowest concentrations of chlorophyll a in Escambia Bay were seen at the deepest sites. Secchi disk depth values indicated that 10–20% of the incident light could reach the bottom at the shallow sites (Table 1). However, the lowest potential nitrification rates were seen at the shallower sites. Benthic algae present at these sites may have used up NH4 + faster than the nitrifiers. Because nitrifying bacteria grow slowly, other organisms such as benthic microalgae using nitrogen can outcompete nitrifiers (Risgaard-Petersen, 2003; Risgaard-Petersen et al., 2005). This is consistent with the low NH4 + fluxes at the shoal sites in this study and in Murrell et al. (2009). However, the effects of benthic microalgae on nutrient fluxes in Pensacola Bay are muted compared to other systems, with few differences between light and dark benthic flux measurements (Murrell et al., 2009).
Analysis of recent changes and meteorological impacts within Escambia Bay
This study examined pre- and post-storm differences in Escambia Bay’s benthic fluxes. Instead of sampling right before and right after a storm, Escambia Bay’s 2005 fluxes were compared to flux data from three different years (2000, 2003, and 2004) before the storms. Our sites were sampled in 2005 one year after Hurricane Ivan and 1–2 months after Hurricane Dennis. Rather than looking at average bay-wide comparisons over the years, we made direct comparisons between the same sites. It is important to note that no other papers have examined benthic fluxes following storm events.
There are two aspects of meteorological events that are important to sediment processes. The first is the physical aspect. Any major storm event will cause resuspension of sediments. Between sampling in 2004 and sampling in 2005, two major hurricanes (Ivan and Dennis) and record breaking rain (March 2005 and April 2005) and wind affected Escambia Bay [NOAA public communication (a)]. The evidence of resuspension is seen in significantly lower porewater NH4 + concentrations at Ch3 in 2005 compared to 2004. The storm resuspension stripped NH4 + out of the porewater, although NH4 + fluxes were higher in 2005 compared to previous years at Ch3. After Hurricane Ivan, wind, resuspension, and tidal surge also flushed away any diatoms present (Hagy et al., 2006). The second important aspect of meteorological events is the runoff that follows precipitation from a storm event. The nutrients from stormwater runoff cause an increase in chlorophyll a levels, which can lead to phytoplankton blooms. In fact, there was a phytoplankton bloom with chlorophyll a concentrations reaching 20 μg l−1 one month following Hurricane Ivan (Hagy et al., 2006). Once the blooms die off, they sink to the bottom of the estuary floor and cause increased mineralization. This, in turn, enhances nitrogen cycling leading to higher NH4 + fluxes, DIP fluxes, NO3 − + NO2 − fluxes, and higher potential nitrification rates.
Our data show little evidence of enhanced nutrient recycling in Escambia Bay as a whole following the extreme weather events of 2004 and 2005. For the most part, NO3 − + NO2 − fluxes were generally lower and SOC, NH4 +, and DIP fluxes from 2005 were more variable than in previous years (Fig. 7). However, two sites, S4 and Ch1, did have significantly higher potential nitrification rates in 2005 (after the storms) compared to 2004 data for S4 and 2003 data for Ch1 (Fig. 7). On the other hand, deeper sites, Ch3 and Ch4, did not have significantly different rates. This could be because deep sites may not be directly affected by enhanced storm wave activity, while shallow sites may see considerably more disturbance and runoff due to storms.
In Escambia Bay as a whole, extreme weather events did not result in any bay-wide differences, although individual differences were seen at some sites when compared to previous years. There were no significant effects on benthic processes from meteorological events.
Conclusions
This study of benthic nutrient fluxes and potential nitrification rates in Escambia Bay occurred after a period of extreme rain and wind. Extreme weather events did not result in any bay-wide differences, although individual differences were seen at some sites. There is little evidence of enhanced nutrient recycling in Escambia Bay due to storm events. NO3 − + NO2 − fluxes were generally lower and SOC, NH4 +, and DIP fluxes from 2005 were more variable than in previous years. Only a few shoal sites demonstrated significantly higher potential nitrification rates in 2005 after the storms, which could be because shallow sites see considerably more disturbance and runoff than deeper sites during storms.
Potential nitrification in Escambia Bay was driven by the combination of water content, organic content, extractable NH4 +, and extractable PO4 3−. The highest rates were seen at the deep, organic rich sites, which is unusual since deep sites tend to have low oxygen conditions. Nitrifiers may not be as sensitive to oxygen levels as was once thought since the nitrifiers in Escambia Bay appear to be active even under low oxygen conditions. High PCB content alone did not appear to inhibit potential nitrification rates. However, the combination of PCB contamination with stressors such as variable salinity and low DO levels (seen at Ch1) may have had a negative impact on potential nitrification rates.
Areas of Escambia Bay are still contaminated with PCBs almost 40 years after an episodic spill. Further toxicological studies should be done to clarify whether current levels of PCBs affect the ecological functionality of Escambia Bay since PCBs are still present. Sediment nutrient dynamics in Escambia Bay appear to be resilient to extreme climatic events, since there were no significant effects on sediment processes in the Bay as a whole when performing inter-site comparisons between the 2005 data and data from 2000, 2003, and 2004. In fact, Escambia Bay seemed to resume to pre-storm conditions within several months after the storms.
References
Ammerman, J., 2003. Determination of Orthophosphate by Flow Injection Analysis. QuikChem Method 31-115-01-1-I for Lachat Instruments, Milwaukee, WI.
ATSDR (Agency for Toxic Substances and Disease Registry), 1995. Toxicological Profile for Polychlorinated Biphenyls. Draft for Public Comment (Update). Prepared by Research Triangle Institute, under Contract No. 205-93-0606 for ATSDR, Public Health Service, U.S. Department of Health and Human Services.
Blackburn, T. H., 1988. Benthic mineralization and bacterial production. In Blackburn, T. H. & J. Sorensen (eds), Nitrogen Cycling in Marine Sediments. Wiley, New York: 175–190.
Boström, B., J. M. Andersen, S. Fleischer & M. Jansson, 1988. Exchange of phosphorous across the sediment-water interface. Hydrobiologia 170: 229–244.
Burkholder, J. M., 1998. Implication of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecological Applications 8: S37–S62.
Caffrey, J. M. & W. M. Kemp, 1990. Nitrogen cycling in sediments with estuarine populations of Potamogeton perfoliatus and Zostera marina. Marine Ecological Progress Series 66: 147–160.
Caffrey, J. M., N. P. Sloth, H. F. Kaspar & T. H. Blackburn, 1993. Effects of organic loading on nitrification and denitrification in a marine sediment microcosm. FEMS Microbiology Ecology 12: 159–167.
Caffrey, J. M., D. E. Hammond, J. S. Kuwabara, L. G. Miller & R. R. Twilley, 1996. Benthic processes in South San Francisco Bay: the role of organic inputs and bioturbation. In Hollibaugh, J. T. (ed.), San Francisco Bay: The Ecosystem. Pacific Division/American Association for the Advancement of Science, San Francisco: 425–442.
Caffrey, J. M., N. E. Harrington, I. Solem & B. B. Ward, 2003. Biogeochemical processes in a small California Estuary: 2. nitrification activity, community structure and role in nitrogen budgets. Marine Ecology Progress Series 248: 27–40.
Campos, J. L., J. M. Garrido, R. Mendez & J. M. Lema, 2001. Effect of two broad-spectrum antibiotics on activity and stability of continuous nitrifying system. Applied Biochemistry and Biotechnology 95: 1–10.
Canfield, D. E., B. Thamdrup & E. Kristensen, 2005. Aquatic geomicrobiology. Elsevier Academic Press, London.
Caraco, N. F., J. J. Cole & G. E. Likens, 1989. Evidence for sulphate-controlled phosphorus release from sediments of aquatic systems. Nature 341: 316–318.
Cowan, J. L. W., J. R. Pennock & W. R. Boynton, 1996. Seasonal and interannual patterns of sediment-water nutrient and oxygen fluxes in Mobile Bay, Alabama (USA): regulating factors and ecological significance. Marine Ecology Progress Series 141: 229–245.
Diamond, D. H., 2003. Determination of Nitrate in Brackish or Seawater by Flow Injection Analysis. QuikChem Method 31-107-04-1-A for Lachat Instruments, Milwaukee, WI.
Diaz, R. J. & R. Rosenberg, 1995. Marine benthic hypoxia: a review of the ecological effects on the behavioural responses of benthic macrofauna. Oceanography and Marine Biology. Annual Review 33: 245–303.
DiDonato, G. T., E. M. Lores, M. C. Murrell, L. S. Smith & J. M. Caffrey, 2006. Benthic nutrient flux in a small estuary in northwestern Florida (USA). Gulf and Caribbean Research 18: 15–25.
Duke, T. W., J. I. Lowe & A. J. Wilson Jr., 1970. A polychlorinated biphenyl (Aroclor® 1254) in the water, sediment, and biota of Escambia Bay, Florida. Bulletin of Environmental Contamination and Toxicology 5: 171.
Dušek, L., 1995. Activity of nitrifying populations in grassland soil polluted by polychlorinated biphenyls (PCBs). Plant and Soil 176: 273–282.
Finstein, M. S. & M. R. Bitsky, 1972. Relationship of autotrophic ammonium-oxidizing bacteria to marine salts. Water Resources 6: 31–40.
Fogel, M. L., C. Aguilar, R. Cuhel, D. J. Hollander, J. D. Willey & H. W. Paerl, 1999. Biological and isotopic changes in coastal waters induced by Hurricane Gordon. Limnology and Oceanography 44: 1359–1369.
Folk, R. L., 1974. Petrology of Sedimentary Rocks. Hempell’s, Austin, TX.
Friedrich, J., C. Dinkel, G. Friedl, N. Pimenov, J. Wijsman, M. T. Gomoiu, A. Cociasu, L. Popa & B. Wehrli, 2002. Benthic nutrient cycling and diagenetic pathways in the North-western Black Sea. Estuarine Coastal Shelf Science 54: 369–383.
Goreau, T. J., W. A. Kaplan, S. C. Wofsy, M. B. McElroy, F. W. Valois & S. W. Watson, 1980. Production of NO2 − and N2O by nitrifying bacteria at reduced concentrations of oxygen. Applied and Environmental Microbiology 40: 526–532.
Hagy III, J. D. & M. C. Murrell, 2007. Susceptibility of a Gulf of Mexico estuary to hypoxia: an analysis using box models. Estuarine, Coastal and Shelf Science 74: 239–253.
Hagy III, J. D., J. C. Lehrter & M. C. Murrell, 2006. Effects of Hurricane Ivan on water quality in Pensacola Bay, FL, USA. Estuaries and Coasts 29: 919–925.
Hall, P. O. J., L. G. Andersson, M. M. Rutgers van der Loeff, B. Sundby & S. F. G. Westerlund, 1989. Oxygen uptake kinetics in the benthic boundary layer of sediments. Limnology and Oceanography 34: 734–736.
Hansen, J. I., 1980. Potential Nitrification in Marine Sediments. M.Sc. Thesis, University of Aarhus, Denmark.
Helder, W. & R. T. P. de Vries, 1983. Estuarine nitrite maxima and nitrifying bacteria (Ems-Dollard Estuary). Netherlands Journal of Sea Research 17: 1–18.
Henriksen, K., 1980. Measurement of in situ rates of nitrification in sediment. Microbiology Ecology 6: 329–337.
Henriksen, K. & W. M. Kemp, 1988. Nitrification in estuarine and coastal marine sediments. In Blackburn, T. H. & J. Sørensen (eds), Nitrogen cycling in coastal marine environments. Wiley, Chichester: 207–249.
Henriksen, K., J. I. Hansen & T. H. Blackburn, 1981. Rates of nitrification, distribution of nitrifying bacteria, and nitrate fluxes in different types of sediment from Danish waters. Marine Biology 61: 299–304.
Henriksen, K., M. B. Rasmussen & A. Jensen, 1983. Effect of bioturbation on microbial nitrogen transformations in the sediment and fluxes of ammonium and nitrate to the overlying water. Ecological Bulletin 35: 193–205.
Henriksen, K., T. H. Blackburn, B. A. Lomstein & C. P. McRoy, 1993. Rates of nitrification, distribution of nitrifying bacteria and inorganic N fluxes in the northern Bering-Chukchi shelf sediments. Continental Shelf Research 13: 629–651.
Herbert, R. A., 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiology Review 23: 563–590.
Holmes, R. M., A. Aminot, R. Kerouel, B. A. Hooker & B. J. Peterson, 1999. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 56: 1801–1808.
Jaynes, M. L. & S. R. Carpenter, 1986. Effects of vascular and nonvascular macrophytes on sediment redox and solute dynamics. Ecology 67: 875–882.
Jensen, H. S. & F. Ø. Andersen, 1992. Importance of temperature, nitrate, and pH for phosphate release from anaerobic sediments of 4 shallow, eutrophic lakes. Limnology and Oceanography 37: 577–589.
Jensen, M. H., E. Lomstein & J. Sørensen, 1990. Benthic NH4 + and NO3 − flux following sedimentation of a spring phytoplankton bloom in Aarhus Bight, Denmark. Marine Ecological Progress Series 61: 87–96.
Jensen, H. S., P. B. Mortensen, F. Ø. Andersen, E. Rasmussen & A. Jensen, 1995. Phosphorous cycling in a coastal marine sediment, Aarhus Bay, Denmark. Limnology and Oceanography 40: 908–917.
Jones, R. D. & M. A. Hood, 1980. Effects of temperature, pH, salinity and inorganic nitrogen on the rate of ammonium oxidation by nitrifiers isolated from wetland environments. Microbiology Ecology 6: 339–347.
Kemp, W. M., R. L. Wetzel, W. R. Boynton, C. F. D’Elia & J. C. Stevenson, 1982. Nitrogen cycling in estuarine interfaces. In Kennedy, V. S. (ed.), Estuarine Comparisons. Academic Press, New York: 209–230.
Kemp, W. M., P. Sampou, J. Caffrey, M. Mayer, K. Henriksen & W. R. Boynton, 1990. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnology and Oceanography 35: 1545–1563.
Kennish, M. J., 1992. Ecology of Estuaries: Anthropogenic Effects. CRC Press, Boca Raton, Florida.
Keppler, C. J., J. Hoguet, K. Smith, A. H. Ringwood & A. J. Lewitus, 2005. Sublethal effects of the toxic alga Heterosigma akashiwo on the southeastern oyster (Crassostrea virginica). Harmful Algae 4: 275–285.
Landsberg, J. H., 2002. The effects of harmful algal blooms on aquatic organisms. Source Reviews in Fisheries Science 10: 113–390.
Liao, N., 2003. Determination of Ammonia in Brackish or Seawater by Flow Injection Analysis. Quickchem Method 31-107-06-1-B for Lachat Instruments, Milwaukee, WI.
Livingston, R. J., 1999. Ecology and trophic organization, in Pensacola Bay System environmental study, section 5A. Unpublished report from Champion International Corporation: 1–125.
Lohse, L., J. F. P. Maschaert, C. P. Slomp, W. Helder & W. van Raaphorst, 1993. Nitrogen cycling in North Sea sediments: interaction of denitrification and nitrification in offshore and coastal areas. Marine Ecology Progress Series 101: 283–296.
MacFarlene, G. T. & R. A. Herbert, 1984. Effect of oxygen tension, salinity, temperature, and organic matter concentration on the growth and nitrifying activity of an estuarine strain of Nitrosomonas. FEMS Microbiology Letters 23: 107–111.
Mallin, M. A., M. H. Posey, G. C. Shank, M. R. McIver, S. H. Ensign & T. D. Alphin, 1999. Hurricane effects on water quality and benthos in the Cape Fear watershed: natural and anthropogenic impacts. Ecological Applications 9: 350–362.
Maruya, K. A., B. G. Loganathan, K. Kannan, S. McCumber-Kahn & R. F. Lee, 1997. Organic and organometallic compounds in estuarine sediments from the Gulf of Mexico (1993–1994). Estuaries 20: 700–709.
Meehl, G. A., T. F. Stocker, W. D. Collins, P. Friedlingstein, A. T. Gaye, J. M. Gregory, A. Kitoh, R. Knutti, J. M. Murphy, A. Noda, S. C. B. Raper, G. Watterson, A. J. Weaver & Z. C. Zhao, 2007. Global climate projections. In Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller (eds), Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Miller, J. L., M. A. Sardo, T. L. Thompson & R. M. Miller, 1996. Effect of application solvents on heterotrophic and nitrifying populations in soil microcosms. Journal of Environmental Toxicology and Chemistry 16: 447–451.
Murrell, M. C., R. S. Stanley, E. M. Lores, G. T. Didonato, L. M. Smith & D. A. Flemer, 2002. Evidence that phosphorus limits phytoplankton growth in a Gulf of Mexico Estuary: Pensacola Bay, Florida, USA. Bulletin of Marine Science 70: 155–167.
Murrell, M. C., J. Campbell, J. D. Hagy III & J. M. Caffrey, 2009. Effects of irradiance on benthic and water column processes in a Gulf of Mexico estuary: Pensacola Bay, Florida, USA. Estuarine Coastal and Shelf Science. doi:10.1016/j.ecss.2008.12.002.
National Oceanic and Atmospheric Administration (NOAA). Public communication (a). http://weather.noaa.gov/weather/current/KPNS.html.
National Oceanic and Atmospheric Administration (NOAA). Public communication (b). http://response.restoration.noaa.gov/book_shelf/122_squirt_cards.pdf.
National Research Council, 2000. Clean Coastal Waters: Understanding and Reducing the Effects of Nutrient Pollution. National Academy Press, Washington, DC.
Niencheski, L. F. & R. A. Jahnke, 2002. Benthic respiration and inorganic nutrient fluxes in the estuarine region of Patos Lagoon (Brazil). Aquatic Geochemistry 8: 135–152.
Nijhof, M. & J. Bovendeur, 1990. Fixed film nitrification characteristics in sea-water recirculation fish culture systems. Aquaculture 87: 133–143. doi:10.1016/0044-8486(90)90270-W.
Nimmo, D. R., R. R. Blackman, A. J. Wilson Jr. & J. Forester, 1971. Toxicity and distribution of Aroclor® 1254 in the pink shrimp Paneaeus duorarum. Marine Biology 11: 191. doi:10.1007/BF00401266.
Nimmo, D. R., D. J. Hansen, J. A. Couch, N. R. Cooley, P. R. Parrish & J. I. Lowe, 1975. Toxicity of Aroclor®1254 and its physiological activity in several estuarine organisms. Environmental Contamination and Toxicology 3: 22–39.
Olsson, M., B. Karlsson & E. Ahnland, 1992. Seals and seal protection: a presentation of a Swedish research project. Ambio 21: 494–496.
Olsson, M., B. Karlsson & E. Ahnland, 1994. Diseases and environmental contaminants in seals from the Baltic and Swedish west coast. Science of the Total Environment 154: 217–227.
Paerl, H. W., J. D. Bales, L. W. Ausley, C. P. Buzzelli, L. B. Crowder, L. A. Eby, J. M. Fear, M. Go, B. J. Peierls, T. L. Richardson & J. S. Ramus, 2001. Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. Proceedings of the National Academy of Sciences 98: 5655–5660.
Parsons, T. R., Y. Maita & C. M. Lalli, 1984. A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press, Oxford.
Ripl, W., 1976. Biochemical oxidation of polluted lake sediment with nitrate—a new lake restoration method. Ambio 3: 132–135.
Risgaard-Petersen, N., 2003. Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: on the influence of benthic microalgae. Limnology and Oceanography 48: 93–105.
Risgaard-Petersen, N., R. L. Meyer & N. P. Revsbech, 2005. Denitrification and anaerobic ammonium oxidation in sediments: effects of microphtyobenthos and NO3 −. Aquatic Microbial Ecology 40: 67–76.
Rysgaard, P., T. Thastum, T. Dalsgaard, P. B. Christensen & N. P. Sloth, 1999. Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22: 21–30.
Sayler, G. S., M. P. Shiaris, W. Beck & S. Held, 1982. Effects of polychlorinated biphenyls and environmental biotransformation products on aquatic nitrification. Applied and Environmental Microbiology 43: 949–952.
Seitzinger, S. P., 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S. P., S. W. Nixon & M. E. Q. Pilson, 1984. Denitrification and nitrous oxide production in a coastal marine ecosystem. Limnology and Oceanography 29: 73–83.
Shumway, S. E., H. P. van Egmond, J. W. Hurst & L. L. Bean, 1995. Management of shellfish resources. In Hallengraeff, G. M., D. M. Anderson & A. Cambella (eds), Manual on Harmful Marine Microalgae. UNESCO, Paris: 433–461.
Smith, K., 2006. Effects of Salinity on Potential Nitrification. Masters Thesis, University of West Florida.
Smolders, A. J. P., L. P. M. Lamers, M. Moonen, K. Zwaga & J. G. M. Roelofs, 2001. Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry 54: 219–228.
Thorpe, P., R. Bartel, P. Ryan, K. Albertson, T. Pratt & D. Cairns, 1997. The Pensacola Bay System Surface Water Improvement and Management Plan: A Comprehensive Plan for the Restoration and Preservation of the Pensacola Bay System. Program Development Series 97-2. Northwest Florida Water Management District, Havana.
Tobias, C., A. Giblin, J. McClelland, J. Tucker & B. Peterson, 2003. Sediment DIN fluxes and prefential recycling of benthic microalgal nitrogen in a shallow macrotidal estuary. Marine Ecology Progress Series 257: 25–36.
Twilley, R. R., J. Cowan, T. Miller-Way, P. A. Montagna & B. Mortazavi, 1999. Benthic nutrient fluxes in selected estuaries in the Gulf of Mexico. In Bianchi, T. S., J. R. Pennock & R. R. Twilley (eds), Biogeochemistry of Gulf of Mexico Estuaries. Wiley, New York, NY, USA: 163–209.
USAF (U.S. Air Force), 1989. The Installation Restoration Program Toxicology Guide, Vol. 3. Aerospace Medical Division, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio: 52-1–52-68.
U.S. Environmental Protection Agency (USEPA), 1999. Method 1668, revision A: chlorinated biphenyl congeners in water, soil, sediment, and tissue by HRGC/HRMS. EPA No. EPA-821-R-00-002.
U.S. Environmental Protection Agency (USEPA), 2004. The Ecological Condition of the Pensacola Bay System, Northwest Florida (1994–2001). U.S. Environmental Protection Agency, Office of Research and Development, National Health and Ecological Effects Research Laboratory, Gulf Ecology Division, Gulf Breeze, Florida.
Vitousek, P. M., J. D. Aber, R. W. Howarth, G. E. Likens, P. A. Matson, D. W. Schindler, W. H. Schlesinger & D. G. Tilman, 1997. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 7: 737–750.
Voytek, M. A. & B. B. Ward, 1995. Detection of ammonia-oxidizing bacteria of the beta-subclass of the class proteobacteria in aquatic samples with the PCR. Applied and Environmental Microbiology 61: 2811.
Welschmeyer, N. A., 1994. Fluorometric analysis of chlorophyll-a in the presence of chlorophyll-b and phaeopigments. Limnology and Oceanography 39: 1985–1992.
Wilson, A. J. & J. Forester, 1978. Persistence of Aroclor® 1254 in a contaminated estuary. Environmental Contamination and Toxicology 19: 637–640.
Zaranke, D. T., R. W. Griffiths & N. K. Kaushik, 1997. Biomagnification of polychlorinated biphenyls through a riverine food web. Environmental Toxicology and Chemistry 16: 1463–1471.
Acknowledgements
This work was funded by a National Science Foundation grant (OCE-0352221) to J. M. Caffrey. We thank R. Snyder, W. Jeffrey, R. Deveraux, M. Murrell, C. Mohrherr, T. Martin, M. Wagner, E. Gaige, and A. Ren for their field and lab assistance. We thank M. Lewis, M. Murrell, and two anonymous reviewers for comments on an earlier version of this manuscript. We also thank University of West Florida Marine Services and the University of West Florida Wetlands Research Laboratory for their assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: P. Viaroli
Rights and permissions
About this article
Cite this article
Smith, K.A., Caffrey, J.M. The effects of human activities and extreme meteorological events on sediment nitrogen dynamics in an urban estuary, Escambia Bay, Florida, USA. Hydrobiologia 627, 67–85 (2009). https://doi.org/10.1007/s10750-009-9716-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9716-x