Abstract
Taxonomic classification is a method for recognizing and registering the world’s organism diversity, in the context of continual changing knowledge about evolutionary (genetic) and ecological relations and phenotype variation. The present system of cyanobacteria must be modified according to combined markers, in which molecular data (as an indisputable genetic basis) should be correlated with biochemical, ultrastructural, phenotypic and ecological data. New data are necessary in order to correct or up date the system; thus, the classification must continually be revised and supplemented. The greatest problem is to transfer all modern data derived from molecular investigations to experimental research and establish the necessary and correct nomenclatural rules for scientific practice. The molecular approach must be the baseline for the reorganisation of our knowledge; however, it should explain and be in agreement with morphological and ecological variation of cyanobacterial genotypes. The present article summarizes the main conclusions, derived from modern cyanobacterial diversity studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Application of molecular methods into cyanobacterial taxonomy enhanced several important principles for cyanobacterial systematics:
-
(i)
Morphological characters of various taxonomic units should concur with their phylogenetic position. The first molecular analyses were potential but not very conclusive, regarding this principle. The reason was that many sequenced strains were designated by incorrect names; however, the clusters obtained characterized well the natural taxonomic units after the nomenclatural revision and reconstruction of phylogenetic trees (e.g., cf. Wilmotte & Golubić, 1991; Turner, 1997; Komárek & Kaštovský, 2003a).
-
(ii)
Wide genetic diversity was found particularly in coccoid and simple filamentous and pseudofilamentous types. As a result of combined molecular and phenotypic analyses, phylogenetic relationships among simple cyanobacteria were found to be non-linear and few parallel clades were recognized. Several distinct features are in agreement with these clades, e.g. ultrastructural patterns (basic position of thylakoids in cells), type of cell division, structure of cell wall, size of cells or differentiation of the thallus, etc. (Komárek & Kaštovský, 2003a; Turicchia et al., 2009).
-
(iii)
The taxonomic value of morphological features has to be re-evaluated. Several distinct traditional characters, used for classification of generic and suprageneric units, did not appear to be quite compatible with the molecular results, e.g. presence/absence of sheaths, size of cells (in distinct limits), false and true branching, etc. These features probably have only ecotypic value within each genotype and their mode of variation may have a genetic basis which is not presently sequenced. The morphological markers which are compatible with natural molecular clusters have to be defined.
-
(iv)
Numerous morphologically derived taxa, particularly on the generic level, were based only on one or a few widely conceived morphological characters. The hierarchy of characters according to genetic importance has to be revised and applied to the system.
-
(v)
The cluster of heterocytous types was found to be monophyletic, but for classification inside this group (families, genera) the system has been revised in light of new phylogenetic investigations (Castenholz, 2001; Lyra et al., 2001; Gugger et al., 2002; Iteman et al., 2002; Gugger & Hoffmann, 2004; Hrouzek et al., 2005; Rajaniemi et al., 2005; Willame et al., 2006).
The necessity for re-evaluating criteria used for the concept of taxonomic units (genera, species) and their characterization is therefore indispensable, following the application of molecular methods for taxonomic re-evaluation in cyanobacteria. The more or less standardized molecular method used is 16S rRNA gene sequencing, particularly for the genus level.
Generic concept
The clusters resulting from 16S rRNA gene sequencing correspond more or less to the traditional cyanobacterial genera, defined by distinct phenotypic characters. A similarity limit of 95% was established as being intergeneric between cyanobacterial genera (Wayne et al., 1987; Stackebrand & Goebel, 1994). This sharp limit cannot be used as a standard key criterion for separating biological taxa quite obligatorily, but it can be an important and the first marker for generic differentiation. From the last few years of use it follows that the characterization of genera should be based on molecular separation (about 95% or less genetic similarity) combined with at least one diacritical autapomorphic cytomorphological character. Genera, defined in this way, belong usually also to one ecophysiological type (Garcia-Pichel et al., 1998).
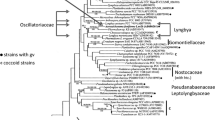
Almost all the traditional (Geitlerian) cyanobacterial genera have been confirmed by molecular methods, including the newly established ones on the basis of revised morphological characters after modern morphological and ecological revisions, over the past decades of the 20th century (Rippka & Cohen-Bazire, 1983; Anagnostidis & Komárek, 1985, 1988; Komárek & Anagnostidis, 1989, 2005; Cyanothece, Limnothrix, Planktothrix, Cyanobacterium and others). Of course, the traditional genera were (and must be) only revised on the bases of the type species. From this practice it follows that the modern approach to the cyanobacteria systematics leads to the division of some existing genera into more generic units, characterized both genetically and morphologically. It is remarkable that several of these ‘newly discovered’ genera correspond to taxa originally described by old, ‘pre-starting-point’ authors (but later connected to other genera on the basis of one prominent character by classical authorities; Bornet & Flahault, 1886–1888; Gomont, 1892; Geitler, 1932; Elenkin, 1936–1949; Bourrelly, 1970; and others). Such old and recently justified genetic and generic units include Arthrospira, Trichormus, Hassallia and others. New generic taxa, which were separated from the traditional genera, include, e.g. Brasilonema, Cylindrospermopsis, Cyanothece, Phormidesmis, Halothece/Euhalothece complex, Thermosynechococcus and Geminocystis. In-depth studies of some of Earths sparsely investigated habitats also lead to the discovery of new genetically and morphologically well defined genera. Spirirestis or Rexia from the nostocalean cyanobacteria are such genera, while a few others are prepared for validation. A review of the recently revised nostocalean genera is included in Table 1.
Examples of recently revised generic entities
Synechococcus
Simple and unicellular cyanobacteria with more or less rod-like cells are usually classified into this genus. They are commonly distributed, easily grown in cultures and many of strains have been isolated. Several of them were used as important experimental strains, but sometimes under incorrect names (‘Anacystis nidulans’ = Synechococcus nidulans). Many strains have already been sequenced and at least 12 clusters were separated, but their differences did not always reach 95% of similarity. A few of them were described as separate genera, e.g. Cyanobium (Rippka & Cohen-Bazire, 1983), which probably now should include also the picoplanktic ‘oceanic Synechococcus-types’. However, the name ‘Cyanobium’ is not commonly used in recent literature. On the other hand, there appears to be a thermophilic genus Thermosynechococcus used in the experimental literature, but this is used without any valid description. The type species is designated ‘Thermosynechococcus (Synechococcus) elongatus’ (Katoh et al., 2001) but Synechococcus elongatus is the type species of the original genus ‘Synechococcus’, which is not thermophilic. Several thermophilic species were described (S. lividus, S. vulcanus, S. bigranulatus and others) and ‘Thermosynechococcus’ is based probably on the strain of the species Synechococcus bigranulatus (=‘thermophilic S. elongatus’). Synechococcus should therefore be divided into several generic units, but the whole taxonomy must be evaluated and characterized using correctly selected type species and strains (Fig. 1).
Part of a phylogenetic tree of the traditional genus Synechoccocus (derived from Castenholz, 2001). Few different clusters were classified in several genera. The EM photos illustrate particularly the position of thylakoids, characteristic for various genera
Synechocystis
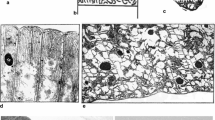
PCC 6803 of ‘Synechocystis sp.’ is one of the main experimental strains at present. It was also the first strain which has had its entire genome described. Strain PCC 6803 really corresponds very closely to the type species of the genus Synechocystis (S. aquatilis) using all markers. The genus Synechocystis therefore represents a clearly separate genetic cluster with characteristic morphology and cytology. The problem is, that in Bergey’s Manual (Castenholz, 2001), not the strain PCC 6803 but PCC 6308 was designated as a type for Synechocystis. But this strain is very far from typical strain PCC 6803 of Synechocystis both genetically and morphologically–cytologically, occurs in other collection and experimental papers also under designations ATCC 27150, CCAP 143011, UTEX 1598, CALU 743 and others and was recently separated into a new genus Geminocystis (G. herdmanii, Korelusová et al., 2009; Fig. 2). The reference strain of Synechocystis must be therefore determined correctly as PCC 6803. For differences of both types see Fig. 2.
Heterogeneity of the genus Synechocystis. The strain PCC 6308 is clearly different both genetically and cytologically from typical cluster of Synechocystis. The tree after Korelusová et al. (2009); A cluster corresponding to new genus Geminocystis, B cluster correcponding to the genus Cyanobacterium, C Synechocystis cluster with the reference strain PCC 6803
Aphanothece
The genus Aphanothece is evidently heterogeneous, even morphologically, and must be revised together with the genus Gloeothece. It contains 4–5 different clusters. At least two distinct and separate clusters have been defined recently genetically, morphologically and ecologically (Fig. 3): (i) a typical benthic, subaerophytic and metaphytic genus Aphanothece (with cells 2.5–8 μm wide) and (ii) typical planktic morphotypes (cells 0.3–2 μm wide; Jezberová, 2006). Both of these clusters have distant positions in the phylogenetic tree, different sizes of cells, structure of mucilage and very different ultrastructure (the second cluster of planktic morphotypes has more characters resembling the genus Cyanobium).
Heterogeneity of the traditional genus Aphanothece. The planktic types (Anathece) are clearly separated from typical cluster of Aphanothece, based on A. microscopica. Phylogenetic tree according to Jezberová (2006)
Cyanothece
This genus was originally separated from the genus Synechococcus on the basis of morphological and cytological markers (Komárek, 1976). Later, this separation was supported by molecular analyses (Rudi et al., 1997). However, smaller unicellular types with oval cells were later falsely designated as Cyanothece by Rippka & Cohen-Bazire (1983). Several of these were later used as important models in biochemical and molecular laboratories. Their genetic position and morphological features are far from the type species C. aeruginosa. However, the name Cyanothece is commonly used in this incorrect sense. The first steps to correct this error can be found in the studies of Garcia-Pichel et al. (1998), Margheri et al. (1999) and Komárek et al. (2004). The names Euhalothece, Halothece and Cyanobacterium (the last one, a generic name introduced by Rippka & Cohen-Bazire, 1983) should be used to correct this taxonomic confusion; (Fig. 4).
Basic review of Cyanothece like cyanobacteria: A Phylogenetic tree with isolated position of the type species (reference strain) of Cyanothece aeruginosa (type species of the genus Cyanothece) among filamentous cyanobacteria. The phylogenetic tree according to Rudi et al. (1997), photos from Komárek et al. (2004). B Review of strains designated as the genera Halothece, Euhalothece, Cyanobacterium and Cyanothece, from Komárek et al. (2004); 1–4 clusters classified to different genera. The phylogenetic tree from Margheri et al. (1999)
Phormidium
Another very heterogeneous genus is Phormidium, which contains several morphologically recognized genetic clusters. Revision of this genus is just beginning and recently a special genus Phormidesmis was already distinctly separated. It appears to be more related both genetically and ultrastructurally by markers closer to pseudanabaenacean than to phormidiacean genera (Komárek et al., 2009; Turicchia et al., 2009).
Nostocales
Many genera in nostocalean cyanobacteria are evidently heterogeneous, especially Anabaena, Aphanizomenon, Trichormus, Nostoc and others (Gugger et al., 2002; Iteman et al., 2002; Hrouzek et al., 2005; Rajaniemi et al., 2005; Willame et al., 2006; etc.). The results are included partly in another review (Komárek, 2009).
Examples of new described genera
Modern investigation of various biotopes has yielded significant new data concerning cyanobacterial diversity. New genera and morphotypes were discovered using new molecular methods combined with precise morphological analyses, particularly among taxa from extreme and tropical habitats. These morphotypes cannot be included in any previously known and/or genetically revised genotypes. Three methodological procedures are used to describe new genera in recent papers:
-
(1)
New genera can be described according to only morphological characters (based on botanical nomenclatoric rules) if they cannot be included by their phenotype into any known and genetically confirmed genus. In the future, they must be confirmed by molecular methods, and this support is very feasible or likely. Up to now, all generic entities, defined by revised morphological markers, have been found to be genetically separated clusters (Cylindrospermopsis, Planktothrix, Arthrospira, Limnothrix, Tychonema, Trichormus, etc.). Two new genera, Catenula Joosten (2006) from planktic habitats in the Netherlands and the tropical genus Macrospermum Komárek (2008), were recently described using this method.
-
(2)
New generic names appeared in prominent molecular or biochemical studies based on strains, which started to be used as important models and experimental organisms. However, these did not have valid descriptions or proper morphological and ecological comparison with other genera. Such genera are possibly acceptable, but they must be validly described with all pertinent characteristics, and their nomenclature must be resolved. Ignoring nomenclature leads to confusion. Such genera include, e.g. Thermosynechococcus Katoh et al. (2001; Fig. 1) or Crocosphaera Zehr et al. (2001).
-
(3)
Acceptably, there appear to be more and more descriptions of new genera in the literature satisfying all the modern criteria. The authors use combined molecular and phenotype markers and guidelines incorporating both botanical and bacteriological nomenclatoric rules to avoid questions and doubts about validity. Examples of such recently published genera are, e.g. Spirirestis Flechtner et al. (2002) (Fig. 5), Acaryochloris Miyashita et al. (2003), Chlorogloeocystis Brown et al. (2005), Rexia Casamatta et al. (2006) or Brasilonema Fiore et al. (2007).
Fig. 5 Example of the new described genus according to polyphasic method (combined molecular, morphological and ecological markers). The clade 1 contains the genera classified into the family Microchaetaceae. Phylogenetic tree and photos after Flechtner et al. (2002)
Species and strain concept
While sequencing by 16S rRNA gene can be used as a standard genetic approach to delimitate cyanobacterial genera, a similar method is problematic for populations and species. It is evident that ecologically and morphologically stable and recurring (in various localities and over time) morpho- and ecotypes occur within various genera, but their diversification process was evidently different within generic clusters from different habitats. All populations of cyanobacteria are continually changing and evolving. The horizontal exchange of genetic material (Rudi et al., 1998; Barker et al., 1999; Hayes et al., 2006) and rapid adaptation to changing environmental factors (Kohl & Nicklisch, 1981; Erdmann & Hagemann, 2001; Hagemann, 2002; Komárek & Kaštovský, 2003b) result in various speciation within various generic clades. This variability in adaptation combined with mutation process complicate the unique criteria for species delimitation.
Different subgeneric clusters (traditional species) are characterized by more or less stable and recognizable ecological, morphological or biochemical modifications (over a certain time period), which are important particularly for ecological scientists (in problematics of different toxicity, primary production, activity of fixation of gaseous nitrogen, etc. in strains and species). However, there are problems in delimitation of such subgeneric clusters. Several examples of various speciation:
-
Pigment types: Several genera produce different pigment mutants (green, aeruginose, olive-green, orange-yellow, red, etc.) in one population, which can stabilize in different habitats or cultures. Classification of such types is unclear. Similar situations were described, e.g. by Kohl & Nicklisch (1981) in Limnothrix redekei, Skulberg & Skulberg (1985) and Suda et al. (2002) in Planktothrix, Waterbury et al. (1979), Six et al. (2007) and Dufresne et al. (2008) in oceanic Synechococcus and Prochlorococcus etc. Sometimes such stabilized pigment mutants are classified traditionally like species (Planktothrix agardhii/rubescens), but the taxonomic evaluation of such differentiation has been still commonly unclear. In several cases, we can isolate a wide spectrum of differently coloured and in cultures stabilized strains (Six et al., 2007).
-
Several genera are clearly separated genetically, but subgeneric units (species) cannot be recognized by 16S rRNA gene sequencing methods. However, they contain a stable number of distinct morphotypes and ecotypes, which differ also according to secondary metabolites. Their easy recognition in natural populations is important for ecological and applied hydrobiological studies. For example, planktic and toxin-producing genera include Microcystis (Fig. 6) and Planktothrix, for which identification of various morphospecies is necessary for ecological research.
-
Different genetic clusters and subclusters, separated by 16S rRNA gene sequencing method may contain morphologically very similar types, which differ only ecologically or can be scarcely distinguished based on slight phenotypic variation. Such types, usually designated as cryptospecies (Sáez & Lozano, 2005; Johansen & Casamatta, 2005; Turicchia et al., 2009) are not identifiable according to morphological markers (sometimes only as ecological species), and their status also needs further discussion for taxonomic classification.
-
Another case is where the genetic differences between distinct morphospecies, classified traditionally in one and the same genus, are so large that they corresponded to genetic clusters separated by more than 95% similarity from each other, but their morphology does not allow them to be separated into various genera. The characteristic example is the genus Halospirulina Nübel et al. (2000), which comprises one sub-cluster within the traditional genus Spirulina (Fig. 7). If we accept Halospirulina, we must divide the whole genus Spirulina into more units. Almost all traditional morphospecies of Spirulina have the similar isolated position like Halospirulina and they could be therefore all classified as distinct genera. The type species of Halospirulina (H. tapeticola) does not differ phenotypically from Spirulina subsalsa.
-
The species concept is complicated also by the fact that almost all populations and strains differ slightly one from another. Two exactly identical strains do not exist. This situation supports the idea that only strains within genera can be designated by a number or symbol, but not just the species. However, the diversity is more complicated, intrageneric clusters are very diverse, many of them must be identifiable and recognizable for ecological and experimental studies, and designation only by symbols is not acceptable.
For cyanobacterial subgeneric diversity it follows that, according to current knowledge, the category ‘species’ is useful, but its concept or definition can be probably only a conventional measure, with different criteria in different genera. The most appropriate present definition is as follows:
“Group of populations (+strains) which belong to one and the same genotype (genus), characterized by stabilized phenotypic features (definable and recognizable, with distinct limits of variation) and having the same ecological criteria. They occur repetitively (in time) in a variety of ecologically similar localities”.
Nomenclature
The Latin names of taxa are not only formal designations, but they symbolize the complex set of characteristics of different taxa, including genetic, biochemical and ecophysiological markers and morphological plasticity. Cyanobacteria were traditionally identified by the Botanical Nomenclature Code, but since their bacteriological nature was stressed, the tendency to apply the Bacteriological Code was intensely promoted (Stanier et al., 1978; Oren, 2004; Oren & Tindall 2005). Because the respective nomenclatural committees did not propose any compromise or come up with a new convenient proposal, many authors use combined instructions and recommendations of both Codes for descriptions of new taxa (e.g., Fiore et al., 2007; etc.). However, new taxa appear that do not adhere or respect either Code. These often appear in experimentally oriented articles. Such studies sometimes have high scientific value, but the ignorance of formal taxonomic prescriptions substantially devalues these investigations and creates a lot of confusions. Examples of such insufficiently described taxa are Anacystis nidulans, Thermosynechococcus, Prochlorococcum, Crocosphaera, etc.
The origin of cyanobacteria and their basic cytological structure is, of course, bacterial, but their ecological, biological and morphological features are very precise. Cyanobateria play a distinct role as phototrophic primary producers in natural ecosystems, just like other microscopic algae. They have both photosystems, thus a plant-like photosynthetic system, and many types form morphologically diversified, multicellular individuals with specialized cells, not known in other bacteria. In the last few years, efforts of nomenclatorists have tended towards attempting to establish a unified nomenclatural system for all groups of organisms, but the idea of creating special nomenclatural rules for cyanobacteria (respecting demands of both, botanical and bacteriological Codes) seems to be more logical and practical. In agreement with this conclusion, a special nomenclatural guide was initiated from International Association for Cyanophyta/Cyanobacteria Systematics and Ecology (IAC) symposium in 1989 and designed and published (Komárek & Golubić, 2005; www.cyanodb.cz). Unfortunately, it has been a priori rejected by nomenclatural specialists from both communities and thus it is not yet used by specialists in cyanobacterial taxonomy.
Proposals to completely eliminate the names of taxa from cyanobacterial research were particularly apparent in bacteriological circles. In principle, there is no good a reason to not accept another convenient system for the registration of cyanobacterial taxa, based on numbering or various other coding. But it is possible only if this system is at least as convenient as the traditional system of names, is applicable to the entire diversity of cyanobacteria from both natural habitats and cultures, and has potential for all users of a cyanobacterial classification system, including especially ecologists. In case that such a system will be designed, it can surely be accepted, but it must be improved in all aspects, emphasizing the knowledge of cyanobacterial diversity. Various chips and molecular arrays for precise coding of cyanobacterial genotypes certainly will be increasingly used. However, replacement of the present nomenclatural system by an inconvenient system, which does not express all cyanobacterial diversity, is premature and irresponsible. A good example is the problematics of strains PCC 6803 and PCC 6308 of Synechocystis described above (Fig. 2). The correct taxonomy and designation of strains without the help of binomial nomenclature is still now hardly possible. Nomenclatural practice is connected also with the problem of arbitrary use of names in experimental works. Many model strains are identified quite arbitrarily, unfortunately even those which were already sequenced. The incorrect names are still often used in the Database of Sequences (GenBank), as well as in biochemical and molecular studies. Of course, taxonomic methods evolve and the taxonomist must continually update to use the most modern methods. This practice results in many necessary nomenclatural changes, which are an integral and indispensable part of modern taxonomic work, and ideally should be continually applied to all strain collections (strain designations) and experimental studies. But there does not exist any authority, which can implement such changes, and experimental workers completely undervalue such revisions (e.g., Anacystis nidulans = Synechococcus nidulans, Anabaena variabilis = Trichormus variabilis, omitting such genera as Cyanobium, etc.). Ignorance of corrections and use of revised and valid names is the same error as the use of incorrect application of chemical or physical symbols, or use of old-fashioned methods.
Concluding remarks
Taxonomic classification is the only way to evaluate the diversity of all natural populations and strains on a genetic (molecular) background, combined with stable cytomorphological and ecophysiological markers. The use of computers and databases is the most progressive method for future taxonomic registration.
The modern system of cyanobacteria must be based on the molecular definition of genotypes (= basic clusters with a similarity index of ±95% or less using the 16S rRNA gene sequencing, considered the standard method), which correspond to the traditional taxonomic category of ‘genus’. Obligatory separation of genera must be done also by at least one diacritical phenotypic character (or autapomorphic set of characters) and ecological, ecophysiological, ultrastructural and biochemical characteristics, which are included as an integral part of the generic definition.
This revised system confirms in principle the traditional cyanobacterial genera, which must be continually corrected and updated (Hoffmann et al. 2005). This revised system justifies the definition of numerous new generic entities, which have arisen through genetic (and morphological) separation of existing genera, and/or are described from newly studied habitats, extreme ecosystems or by revision of cultured material.
The species concept is not uniform and must be modernized according to the diverse nature of various genera. The characterization of species can then be appreciated rather conventionally within different genera.
Use of Linnean (binomial) nomenclature is still quite indispensable for the characterization and understanding of the entire (both natural and cultivated–experimental) diversity of cyanobacteria. No other convenient system exists. However, the demand for transferring confirmed taxonomic and nomenclatural revision into ecological and particularly experimental studies is highly desirable. Molecular cyanobacteriologists pay attention to the use of molecular methods for taxonomic articles, but unfortunately they often do not accept the results of modern investigations into cyanobacterial diversity in their studies and strain collections.
References
Anagnostidis, K. & J. Komárek, 1985. Modern approach to the classification system of Cyanophytes 1 – introduction. Algological Studies 38–39: 291–302.
Anagnostidis, K. & J. Komárek, 1988. Modern approach to the classification system of cyanophytes 3 – Oscillatoriales. Algological Studies 50–53: 327–472.
Barker, G. L. A., P. K. Hayes, S. L. O’Mahony, P. Vacharapiyasophon & A. E. Walsby, 1999. A molecular and phenotypic analysis of Nodularia (Cyanobacteria) from the Baltic Sea. Journal of Phycology 35: 931–937.
Bornet, E. & C. Flahault, 1886–1888. Révision des Nostocacées hétérocystées. Annales des Sciences Naturelles, Serie 7, Botanique 3: 323–381, 4: 343–373, 5: 51–129, 7: 171–262.
Bourrelly, P., 1970. Les algues d’eau douce III. N. Boubée and Cie, Paris: 512 pp.
Brown, I., D. Mummey & K. E. Cooksey, 2005. A novel cyanobacterium exhibiting an elevated tolerance for iron. FEMS Microbiology Ecology 52: 307–314.
Casamatta, D. A., S. R. Gomez & J. R. Johansen, 2006. Rexia erecta gen. et sp. nov. and Capsosira lowei sp. nov., two newly described cyanobacterial taxa from the Great Smoky Mountains National Park (USA). Hydrobiologia 561: 13–26.
Castenholz, R. W., 2001. Oxygenic photosynthetic bacteria. In Boone, D. R. & R. W. Castenholz (eds), Bergey’s Manual of Systematic Bacteriology, Vol. 1, 2nd ed. Springer-Verlag, New York: 473–600.
Dufresne, A., M. Ostrowski, D. J. Scanlan, L. Garczarek, W. R. Hess & F. Partensky, 2008. The role of lateral gene transfer in niche adaptation of marine Synechococcus. 7th European Workshop on Molecular Biology of Cyanobacteria (Abstracts), p. 96.
Elenkin, A. A., 1936, 1938, 1949. Monographia algarum cyanophycearum aguidulcium et terrestrium in finibus USSR inventarum. [Sinezelenye vodorosli SSSR]. 1,2(1-2): 1-1908, Izd. AN SSSR, Moskva-Leningrad.
Erdmann, N. & M. Hagemann, 2001. Salt acclimation of algae and cyanobacteria: a comparison. In Rai, L. C. & J. P. Gaur (eds), Algal Adaptation to Environmental Stresses. Springer, Heidelberg: 323–362.
Fiore, M. F., C. L. Sant’Anna, M. T. P. Azevedo, J. Komárek, J. Kaštovský, J. Sulek & A. S. Lorenzi, 2007. The cyanobacterial genus Brasilonema – molecular and phenotype evaluation. Journal of Phycology 43: 789–798.
Flechtner, V. R., S. L. Boyer, J. R. Johansen & M. L. DeNoble, 2002. Spirirestis rafaelensis gen. et sp. nov. (Cyanophyceae), a new cyanobacterial genus from arid soils. Nova Hedwigia 74: 1–24.
Garcia-Pichel, F., U. Nübel & G. Muyzer, 1998. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Archives of Microbiology 169: 469–482.
Geitler, L., 1932. Cyanophyceae. In Rabenhorst’s Kryptogamenflora von Deutschland, Österreich und der Schweiz 14: 1–1196, Akad. Verlagsges, Leipzig.
Gomont, M., 1892. Monographie des Ocillatoriées (Nostocacées homocystées). Annales des Sciences Naturelles, Serie 7, Botanique 15: 263–368, 16: 91–264.
Gugger, M. & L. Hoffmann, 2004. Polyphyly of true branching cyanobacteria (Stigonematales). International Journal of Systematic and Evolutionary Microbiology 54: 349–357.
Gugger, M., C. Lyra, P. Henriksen, A. Couté, J.-F. Humbert & K. Sivonen, 2002. Phylogenetic comparison of the cyanobacterial genera Anabaena and Aphanizomenon. International Journal of Systematic and Evolutionary Microbiology 52: 1–14.
Hagemann, M., 2002. Environmental stress, signalling and basic acclimation reactions. In Solheim, R. et al. (eds), Cyanobacteria and Nitrogen Fixation in Extreme Environments. European Science Foundation CYANOFIX: 24 (Abstract).
Hayes, P. K., J. Batley & C. Jenkins, 2006. Gene exchange within populations of Nodularia spumigena in the Baltic Sea. Program and Abstract, 12th International Symposium of Phototrophic Prokaryotes, Pau: 87 (Abstract).
Hoffmann, L., J. Komárek & J. Kaštovský, 2005. System of cyanoprokaryotes (cyanobacteria) – state in 2004. Algological Studies (Cyanobacterial Research 6) 117: 95–115.
Hrouzek, P., S. Ventura, A. Lukešová, M. A. Mugnai, S. Turicchia & J. Komárek, 2005. Diversity of soil Nostoc strains: phylogenetic and morphological variability. Algological Studies (Cyanobacterial Research 6) 117: 251–264.
Iteman, I., R. Rippka, N. Tandeau de Marsac & M. Herdman, 2002. rDNA analyses of planktonic heterocystous cyanobacteria, including members of the genera Anabaenopsis and Cyanospira. Microbiology 148: 481–496.
Jezberová, J., 2006. Phenotypic diversity and phylogeny of picocyanobacteria in mesotrophic and euthrophic freshwater reservoirs investigated by a cultivation-dependent polyphasic approach. PhD Thesis, Fac. Biol. Sci, Univ., South Bohemia: 76pp.
Johansen, J. R. & D. A. Casamatta, 2005. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algological Studies (Cyanobacterial Research 6) 117: 71–93.
Joosten, A. M. T., 2006. Flora of the blue-green algae of the Netherlands. 1. The non-filamentous species of inland waters. KNNV Publ.Utrecht: 239 pp.
Katoh, H., S. Itoh, J.-R. Shen & M. Ikeuchi, 2001. Functional analysis of psbV and a novel c-type cytochrome gene psbV2 of the thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant and Cell Physiology 42: 599–607.
Kohl, J.-G. & A. Nicklisch, 1981. Chromatic adaptation of the planktic blue-green alga Oscillatoria redekei Van Goor and its ecological significance. Internationale Revue der gesamten Hydrobiologie 66: 83–94.
Komárek, J., 1976. Taxonomic review of the genera Synechocystis SAUV. 1892, Synechococcus NÄG. 1849 and Cyanothece gen. nov. (Cyanophyceae). Archiv für Protistenkunde 118: 119–179.
Komárek, J., 2008. The cyanobacterial genus Macrospermum. Fottea (Olomouc) 8: 79–86.
Komárek, J., 2009. Modern taxonomic revision of planktic nostocacean cyanobacteria: a short review of genera. Hydrobiologia 30. doi:10.1007/s10750-009-0030-4.
Komárek, J. & K. Anagnostidis, 1989. Modern approach to the classification system of cyanophytes 4 – Nostocales. Algological Studies 56: 247–345.
Komárek, J. & K. Anagnostidis, 2005. Cyanoprokaryota-2. Teil/2nd Part: oscillatoriales. In Büdel B., Krienitz L., Gärtner G. & Schagerl M. (eds), Süsswasserflora von Mitteleuropa 19/2. Elsevier/Spektrum, Heidelberg: 759 pp.
Komárek, J. & S. Golubić, 2005. Proposal for unified nomenclatural rules for Cyanobacteria vs. Cyanophytes: “Cyano-Guide”. In Hoffmann, L. (ed.), Nomenclature of Cyanophyta/Cyanobacteria: Roundtable on the Unification of the Nomenclature Under the Botanical and Bacteriological Codes. Algological Studies (Cyanobacterial Research 6) 117: 17–18.
Komárek, J. & J. Kaštovský, 2003a. Coincidences of structural and molecular characters in evolutionary lines of cyanobacteria. Algological Studies (Cyanobacterial Research 4) 109: 305–325.
Komárek, J. & J. Kaštovský, 2003b. Adaptability in diversification processes of cyanobacteria; the example of Synechococcus bigranulatus. Algological Studies (Cyanobacterial Research 4) 109: 299–304.
Komárek, J., V. Cepák, J. Kaštovský & J. Sulek, 2004. What are the cyanobacterial genera Cyanothece and Cyanobacterium? Contribution to the combined molecular and phenotype taxonomic evaluation of cyanobacterial diversity. Algological Studies (Cyanobacterial Ressearch 5) 113: 1–36.
Komárek, J., J. Kaštovský, S. Ventura, S. Turicchia & J. Šmarda, 2009. The cyanobacterial genus Phormidesmis. Algological Studies 129(1): 41–59.
Korelusová, J., J. Kaštovský & J. Komárek, 2009. Diversity of the cyanobacterial genus Synechocystis Sauvegeau and Geminocystis genus nova. Journal of Phycology 45: 928–937.
Lyra, C., S. Suomalainen, M. Gugger, C. Vezie, P. Sundman, L. Paulin & K. Sivonen, 2001. Molecular characterization of planktic cyanobacteria of Anabaena, Aphanizomenon, Microcystis and Planktothrix genera. International Journal of Systematic and Evolutionary Microbiology 51: 513–526.
Margheri, M. C., M. Bosco, L. Giovannetti & S. Ventura, 1999. Assessment of the genetic diversity of halotolerant coccoid cyanobacteria using amplified 16S rDNA restriction analysis. FEMS Microbiological Letters 173: 9–16.
Miyashita, H., H. Ikemoto, N. Kurano & S. Miachi, 2003. Acaryochloris marina gen. et sp. nov. (Cyanobacteria), an oxygenic photosynthetic prokaryote containing chl d as a major pigment. Journal of Phycology 39: 1247–1253.
Nübel, U., F. Garcia-Pichel & G. Muyzer, 2000. The halotolerance and phylogeny of cyanobacteria with tightly coiled trichomes (Spirulina Turpin) and the description of Halospirulina tapeticola gen. nov., sp. nov. International Journal of Systematic and Evolutionary Microbiology 50: 1265–1277.
Oren, A., 2004. A proposal for further integration of the cyanobacteria under the Bacteriological Code. International Journal of Systematic and Evolutionary Microbiology 54: 1895–1902.
Oren, A. & B. J. Tindall, 2005. Nomenclature of the cyanophyta/cyanobacteria/cyanoprokaryotes under the International Code of nomenclature of Prokaryotes. Algological Studies (Cyanobacterial Research 6) 117: 39–52.
Rajaniemi, P., J. Komárek, R. Willame, P. Hrouzek, K. Kaštovská, L. Hoffmann & K. Sivonen, 2005. Taxonomic consequences from the combined molecular and phenotype evaluation of selected Anabaena and Aphanizomenon strains. Algological Studies 117 (Cyanobacterial Research 6): 371–391.
Rippka, R. & G. Cohen-Bazire, 1983. The Cyanobacteriales: a legitimate order based on the type strain Cyanobacterium stanieri? Annales de Microbiologie 134B: 21–36.
Rudi, K., O. M. Skulberg, F. Larsen & K. S. Jakobsen, 1997. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7 and V8. Applied and Environmental Microbiology 63: 2593–2599.
Rudi, K., O. M. Skulberg & K. S. Jakobsen, 1998. Evolution of cyanobacteria by exchange of genetic material among phyletically related strains. Journal of Bacteriology 180: 3453–3461.
Sáez, A. G. & E. Lozano, 2005. Body doubles. Nature 433: 111.
Six, C., J.-C. Thomas, L. Garczarek, M. Ostrowski, A. Dufresne, N. Blot, D. J. Scanlan & F. Partensky, 2007. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: a comparative genomics study. Genome Biology 8(12): R259.1–R25922.
Skulberg, O. M. & R. Skulberg, 1985. Planktic species of Oscillatoria (Cyanophyceae) from Norway. Characterization and classification. Algological Studies 38(39): 157–174.
Stackebrand, E. & B. M. Goebel, 1994. Taxonomic Note: a for place for DNA–DNA reassociationand 16S rRNA sequence analysis in the present species definition in Bacteriology. International Journal of Systematic and Evolutionary Microbiology 44: 846–849.
Stanier, R. Y., W. R. Sistrom, T. A. Hansen, B. A. Whitton, R. W. Castenholz, N. Pfennig, V. N. Gorlenko, E. N. Kondratieva, K. E. Eimhjellen, R. Whittenbury, R. L. Gherna & H. G. Trüper, 1978. Proposal to place the nomenclature of the cyanobacteria (blue–green algae) under the rules of the International Code of Nomenclature of Bacteria. International Journal of Systematic and Evolutionary Microbiology 28: 335–336.
Suda, S., M. M. Watanabe, S. Otsuka, A. Mahakahant, W. Yongmanitchai, N. Nopartnaraporn, Y. Liu & J. G. Day, 2002. Taxonomic revision of water bloom-forming species of oscillatorioid cyanobacteria. International Journal of Systematic and Evolutionary Microbiology 52: 1577–1595.
Turicchia, S., S. Ventura, J. Komárek, E. Soldati & J. Komárková-Legnerová, 2009. Molecular and phenotype evaluation of oscillatorialean cyanobacteria from alkaline marshes of Northern Belize. Nova Hedwigia 89(1–2): 165–200.
Turner, S., 1997. Molecular systematics of oxygenic photosynthetic bacteria. Plant Systematics and Evolution 11: 13–52.
Waterbury, J. B., S. W. Watson, R. R. L. Guillard & L. E. Brand, 1979. Wide spread occurrence of a unicellular, marine, planktonic cyanobacterium. Nature 277(5694): 293–294.
Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, W. E. C. Moore, R. G. E. Murray, E. Stackebrand, M. P. Starr & H. G. Trüper, 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. International Journal of Systematic and Evolutionary Microbiology 37: 463–464.
Willame, R., C. Boutte, S. Grubisic, A. Wilmotte, J. Komárek & L. Hoffmann, 2006. Morphological and molecular characterisation of planktonic cyanobacteria from Belgium and Luxembourg. Journal of Phycology 42: 1312–1332.
Wilmotte, A. & S. Golubić, 1991. Morphological and genetic criteria in the taxonomy of Cyanophyta/Cyanobacteria. Algological Studies 64: 1–24.
Zehr, J. P., J. B. Waterbury, P. J. Turner, J. P. Montoya, E. Omoregie, G. F. Steward, A. Hansen & D. M. Karl, 2001. Unicellular Cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature 421: 635–638.
Acknowledgements
This work was supported by grant GA AVCR No. IAA600050704. The author is thankful to all colleagues who are interested in the reorganization of the modern taxonomic system of cyanobacteria. This work was presented as an invited paper at the Bat Sheva de Rothschild seminar on Phytoplankton in the Physical Environment—The 15th Workshop of the International Association of Phytoplankton Taxonomy and Ecology, IAP (Israel).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Zohary, J. Padisák & L. Naselli-Flores / Phytoplankton in the Physical Environment: Papers from the 15th Workshop of the International Association for Phytoplankton Taxonomy and Ecology (IAP), held at the Ramot Holiday Resort on the Golan Heights, Israel, 23–30 November 2008
Rights and permissions
About this article
Cite this article
Komárek, J. Recent changes (2008) in cyanobacteria taxonomy based on a combination of molecular background with phenotype and ecological consequences (genus and species concept). Hydrobiologia 639, 245–259 (2010). https://doi.org/10.1007/s10750-009-0031-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0031-3