Abstract
Reservoir hydrodynamics may create heterogeneity in nitrogen (N) fixation along the riverine–transition–lacustrine gradient. In particular, N fixation may be highest in reservoir transition zones where phytoplankton biomass is also expected to be relatively high. We investigated spatial patterns of N fixation in three Texas (USA) reservoirs of varying trophic state. We sampled 6–9 stations along the longitudinal axes of the major inflows and measured N fixation using the acetylene reduction method. Total N, total phosphorus (P), and algal biomass (as chlorophyll-a) were also measured at each sample location. Measurable N fixation was observed in all reservoirs and was light-dependent. Nitrogen fixation was consistently low in the riverine zone, highest in the transition zone, and low in lacustrine zone of all reservoirs. The absolute magnitude of N fixation was similar in two relatively unproductive reservoirs and an order of magnitude higher in the eutrophic reservoir. A similar pattern was observed in mean nutrient and chlorophyll-a concentrations among reservoirs. However, chlorophyll-a concentrations were highest in the riverine zone of each reservoir and exhibited a monotonic decrease in the downstream direction. Maximum chlorophyll-a concentrations did not coincide with maximum N fixation rates. Results of our study indicate that reservoir transition zones can be biogeochemical hot spots for planktonic N fixation, regardless of trophic state. Therefore, transition zones may be the most at risk locations for water quality degradation associated with increased reservoir productivity. Water quality managers and aquatic scientists should consider the spatial heterogeneity imposed by unique hydrodynamic controls in reservoir ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
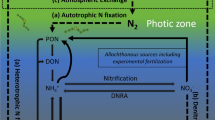
Nitrogen (N) fixation, the conversion of atmospheric N2 to ammonium (NH4 +), can be an important source of N to aquatic ecosystems (Howarth et al., 1988) and is a process that can structure the phytoplankton community (Schindler, 1977; Smith, 1983). In lakes and reservoirs, the organisms most responsible for N fixation are generally heterocystous cyanobacteria because they out compete other phytoplankton groups when the N to phosphorus (P) ratio is low and N becomes limiting (Levine & Schindler, 1999). Nitrogen fixation rates are generally greatest in eutrophic ecosystems because elevated nutrient concentrations support higher phytoplankton biomass and because the N:P ratio of nutrients supplied to eutrophic systems is decidedly lower than the N:P of nutrients entering more oligotrophic systems (Downing & McCauley, 1992). Nevertheless, N fixation can be an important source of N in oligotrophic systems also, as demonstrated in the world’s oceans (Kolber, 2006).
Reservoirs are created ecosystems that behave as “hybrids” of river and lake ecosystems. Kimmel & Groeger (1984) and later Thornton et al. (1990) described a heuristic model for reservoir limnology which described spatially explicit patterns in morphological characteristics, environmental variation, and ecological responses along riverine, transition, and lacustrine zones within reservoirs. This model has become widely accepted and is included in some major limnology texts (Wetzel, 2001; Kalff, 2002). However, reservoirs continue to be understudied in contrast to other aquatic ecosystems (Kennedy et al., 2003), and reservoir gradient models have not been extended to include diverse ecological processes such as N fixation.
In a recent study, we demonstrated that N fixation in a eutrophic reservoir was initiated seasonally by the depletion of nitrate from the water column (Scott et al., 2008a). We also demonstrated that coarse spatial patterns of N fixation in this reservoir were linked to anthropogenic activity in its watershed. In another study, we demonstrated that patterns of N fixation across reservoirs in a large geographic area were related to physical factors in reservoirs and their watersheds (Forbes et al., 2008). However, fine scale spatial variability of N fixation within reservoir ecosystems has not been well studied, and it is unclear whether the heuristic model proposed by Kimmel & Groeger (1984) and Thornton et al. (1990) may be useful for understanding spatial patterns in reservoir N fixation.
In this study, we report data on planktonic N fixation along the riverine–transition–lacustrine gradient in three reservoirs of varying trophic state. We also report seasonal trends in dissolved N availability from our study reservoirs using long-term monitoring data. Overall, our goal was to determine if spatially explicit patterns in N fixation were similar among reservoirs, and to identify if “hot spots” of N fixation occurred within reservoirs regardless of trophic state. The objectives of the study were to (1) characterize spatial patterns in N fixation within each reservoir, (2) compare spatial patterns within each reservoir among the three reservoirs, (3) compare the magnitude of N fixation rates in reservoirs of varying trophic state, and (4) characterize the likelihood that N fixation regularly occurs in reservoirs of varying trophic state using long-term nitrate concentration data. We hypothesized that N fixation would be greatest in reservoir transition zones where phytoplankton biomass should be highest (Thornton et al., 1990). We also hypothesized that N fixation would occur in all reservoirs and that transition zone N fixation maxima would occur in reservoirs of all trophic states.
Materials and methods
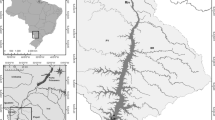
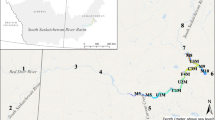
We sampled three reservoirs in central Texas, USA, along the longitudinal axis of their major inflows (Fig. 1). We positioned the upper-most sampling stations as far up as easily accessible by boat within the riverine zone of each reservoir. The location of this station varied between 5 and 20% of the total reservoir length. Additional stations were positioned down the riverine–transition–lacustrine gradient. We sampled at least one station in the lacustrine zone of each reservoir, although most of our stations would likely have been considered riverine or transition zone stations. The three reservoirs were similar in surface area but differed somewhat in mean depth, volume, annual hydraulic loads (USGS, 2005a, b, c), and hydraulic residence time (Table 1). We sampled Waco Reservoir on July 30, 2003, Stillhouse Hollow Reservoir on August 9, 2006, and Canyon Reservoir on August 16, 2006. We collected 1 l of water from the near-surface at each station within the reservoirs, maintained the sample at ambient temperature in dark, and transferred it to the laboratory within 4 h of collection.
In the laboratory, we transferred six-30 ml sub-samples from each sample into separate glass syringes for estimation of N fixation rates using the acetylene reduction method (Flett et al., 1976). We inoculated syringes with 5 ml acetylene gas that was generated from the dissolution of calcium carbide in deionized water. We gently agitated syringes until acetylene was completely dissolved. We incubated three syringes in the dark and three others under light (PAR = 350–450 μmol m−2 s−1). We ran incubations for 10–16 h and held temperatures constant at the level of average ambient water temperature recorded during sampling.
Following incubation, we measured ethylene concentrations on a Carle® AGC Series gas chromatograph equipped with a flame ionization detector and a 1.8-m stainless steel column packed with 80% Porapack N and 20% Porapack Q (80/100 mesh). We held the GC column temperature at 70°C, used helium as the carrier gas, and calibrated the instrument daily with 10-ppm ethylene standards. We converted ethylene production rates to N fixation rates using a conversion of 3 μmols ethylene generated per 1 μmol N2 fixed (Flett et al., 1976). We determined volumetric N fixation rates (expressed as μg N l−1 h−1) for samples incubated in dark and light conditions. Since N fixation was only determined in near-surface samples using laboratory incubation, the measured rates actually represent potential N fixation. However, incubation times were relatively long which could cause an underestimation of N fixation, so our method likely yielded conservative estimates overall. In addition to N fixation assays, we determined the amount of algal biomass (as chlorophyll-a) in all samples and measured the concentrations of total nitrogen (TN) and total phosphorus (TP) from three stations in Waco Reservoir and all stations in Stillhouse Hollow and Canyon Reservoirs (APHA, 1998).
Long-term nitrate data on the three study reservoirs was provided by the Texas Commission on Environmental Quality (TCEQ, 2007) and the Texas Institute for Applied Environmental Research (TIAER, 2007). TCEQ provided nitrate data from 1972 to 2007 on Stillhouse Hollow and Canyon Reservoirs that varied in frequency from monthly to quarterly sampling. TIAER provided monthly nitrate data for Waco Reservoir from 1996 to 2005. In most cases these samples were collected near the dam in each reservoir. We calculated average monthly nitrate concentrations across all years and report these values plus or minus the standard error of the mean.
Results
Mean TN and TP concentrations from reservoirs confirmed that both Stillhouse Hollow and Canyon were relatively oligotrophic–mesotrophic systems and that Waco was eutrophic (Table 2). Stillhouse Hollow exhibited disproportionally high, mean chlorophyll-a concentration (Table 2), but this was primarily influenced by high algal biomass in the most upstream reaches of the reservoir (Fig. 2). In all three reservoirs, algal biomass was greatest in the most upstream regions and monotonically decreased along the longitudinal axis of the major inflow (Fig. 2).
Spatial patterns of N fixation (determined from light incubations) and chlorophyll-a concentration along longitudinal axes of major reservoir inflows. Values on x-axis represent the location of sampling stations as a percentage of the total distance between reservoir inflow (0%) and outflow (100%). Note differences in scale for N fixation values among reservoirs
Measurable N fixation was observed in all three reservoirs for samples incubated under light. Samples incubated in dark had little, if any, N fixation. The magnitude of N fixation rates were similar in Stillhouse Hollow and Canyon Reservoirs, but were approximately 10 times higher in Waco Reservoir. Nitrogen fixation also varied spatially within our study reservoirs along the riverine–transition–lacustrine gradient (Fig. 2). In all three reservoirs, N fixation was relatively low in the most upstream reaches of the reservoir (riverine zone), highest in mid-reservoir (transition zone), and relatively low in the most downstream reaches (lacustrine zone). Maximum N fixation rates occurred at approximately 30% of the total reservoir length in Stillhouse Hollow and Canyon Reservoirs and at approximately 50% of the total reservoir length in Waco Reservoir (Fig. 2).
Mean nitrate concentrations from long-term monitoring data ranged from 25 to 140 μg N l−1 in Stillhouse Hollow Reservoir, from 80 to 360 μg N l−1 in Canyon Reservoir, and from 30 to 1,400 μg N l−1 in Waco Reservoir. Highest seasonal nitrate concentration occurred in late winter and early spring (February to March) and lowest seasonal nitrate concentration occurred in mid-summer (July to August) in all reservoirs (Fig. 3). Although the annual depletion of nitrate was particularly pronounced in Waco Reservoir from July through September, it was also apparent in Stillhouse Hollow and Canyon Reservoirs, particularly in the month of August.
Seasonal patterns in nitrate concentrations in the study reservoirs calculated from long-term monitoring data provided by the Texas Commission on Environmental Quality (TCEQ, 2007) and the Texas Institute for Applied Environmental Research (TIAER, 2007). See text for details. Note differences in scale among reservoirs
Discussion
Our study indicates that N fixation can vary substantially along the riverine–transition–lacustrine gradient in river impoundment reservoirs. Although other studies have found spatial differences in the nutrient limiting phytoplankton production in reservoirs (e.g., Bukaveckas & Crain, 2002), we believe our study is the first to demonstrate a spatial pattern in N fixation along the longitudinal axes of reservoirs. Nitrogen fixation rates in reservoir transition zones were 25–60 times higher than N fixation rates in riverine and lacustrine areas. Thus, reservoir transition zones can be particularly important locations for cyanobacterial N fixation and perhaps other biogeochemical processes.
Although the spatial pattern of N fixation was similar among reservoirs regardless of trophic state, the magnitude of N fixation increased with reservoir trophic status. A pattern of increasing N fixation with increased trophic state is consistent with the idea of higher N fixation in eutrophic systems (Howarth et al., 1988). In a 2-year study, N fixation in Waco Reservoir was only triggered when nitrate concentrations were strongly diminished during summer (Scott et al., 2008a). Since summer periods of nitrate deficiency appear common in all three reservoirs included in our study (Fig. 3), N fixation may also occur in these reservoirs during most summers, regardless of trophic state. What differs is the absolute magnitude of N fixation among reservoirs, with highest rates occurring in eutrophic systems capable of supporting greater cyanobacterial biomass and metabolism.
Interestingly, the pattern of increasing N fixation with increased phytoplankton biomass did not hold within reservoirs. In fact, none of the reservoirs exhibited a transition-zone maximum in algal biomass as proposed by Thornton et al., (1990). Rather, algal biomass was at its maximum in the riverine zone of all reservoirs, which is similar to a pattern of increased algal biomass and production observed along a depth gradient in a larger set of Texas reservoirs (Forbes et al., 2008).
Unfortunately, the extent of spatial pattern in N fixation cannot be completely inferred from our study because our sampling stations did not cover the complete spectrum of locations along the riverine–lacustrine gradient in all reservoirs. For example, considerable portions of Stillhouse Hollow (between 50 and 90% of total reservoir length) were not sampled. Also, the uppermost portion (0–20%) and much of the lower portion (70–90%) of Canyon reservoir were not sampled. We infer a linear decrease in N fixation from our last transition zone station to the lacustrine zone stations, but this trend could be an artifact of our limited data. Our sampling was also limited to a single event in one summer on each reservoir. In some of our previous work (Scott et al., 2008a), we found that N fixation exhibited inter-annual variability in Waco reservoir, but that coarse spatial patterns (between arms of reservoir) were consistent among years. Therefore, it seems possible that although the magnitude of N fixation may differ from year to year, the spatial pattern could remain similar.
The spatial pattern of N fixation along longitudinal reservoir axes observed in this study should be confirmed in future studies with more intensive sampling along the riverine–transition–lacustrine gradient. However, if this pattern is consistent among reservoirs as our study indicates, the next logical question is what mechanism(s) controls the pattern. Kimmel et al. (1990) suggested that phytoplankton production in reservoirs should follow a pattern similar to what we observed for N fixation. They suggested phytoplankton production would be relatively low in the riverine zone due to light limitation, highest in the transition zone where nutrient and light availability is highest, and low in the lacustrine zone due to nutrient limitation. Since cyanobacterial N fixation is inexorably linked with photosynthesis, these same factors could control the spatial pattern of N fixation in reservoirs. However, this explanation is complicated by the fact that relatively high N availability in the transition zone of reservoirs (compared with the lacustrine zone) should suppress N fixation. Furthermore, algal biomass was always greatest in riverine zones in our study reservoirs (Fig. 3).
Another possible explanation is that reservoir morphometry favors physical conditions that are conducive to cyanobacterial success in the river–reservoir transition zones (Forbes et al., 2008). For example, transition zones are broader, deeper, and have longer water residence times than riverine zones (Thornton et al., 1990), which would support slower growing phytoplankton such as cyanobacteria. Transition zones are also narrower and perhaps more sheltered than lacustrine zones, promoting greater water column stability. Increased stability, or “calm” conditions, can favor cyanobacteria via their ability to modify their vacuolation and control their location in the water column (Reynolds, 1972). Therefore, increased N fixation in the transition zones of reservoirs could be a function of light climate that is maximized by buoyancy-regulating cyanobacteria. Transition zones are also shallower than lacustrine zones and are therefore more nutrient rich. This potentially could support higher phytoplankton biomass and metabolism and possibly N fixation (Forbes et al., 2008). Again however, explanations involving increased nutrient availability confound the need for N fixation, and phytoplankton biomass was never highest in the transition zone of our study reservoirs.
A third explanation for the spatial pattern of N fixation in reservoirs is that biogeochemical N transformations along the flow path of water could alter the N:P ratio along the riverine–transition–lacustrine gradient. Recent work has demonstrated that denitrification lowered the ratio of N:P along the flow path of water in a created wetland (Scott et al., 2008b) which changed the nutrient limiting periphyton production from P to N, and stimulated substantial N fixation (Scott et al., 2005, 2007). Reservoirs can efficiently retain both N and P (Tomaszek & Koszelnik, 2003; Hejzlar et al., 2007), and may permanently remove a majority of N via denitrification (David et al., 2006). Therefore, even modest P release from reservoir sediments could cause an imbalance between N and P (Sen et al., 2007), and perhaps favor cyanobacteria in the phytoplankton community. A shift in phytoplankton community structure (favoring cyanobacteria) simultaneous with a decrease in phytoplankton biomass along the riverine–transition zone gradient could explain why N fixation rates were decoupled from algal biomass in our study. However, the importance of the mechanism would also depend on sediment N regeneration (Nowlin et al., 2005) and the relative importance of N regeneration versus N loss pathways such as denitrification. More work is needed to understand how internal nutrient cycling in reservoirs might affect the availability of N and P and potentially lower N:P ratio along the riverine–transition–lacustrine gradient.
Recent research has indicated that biogeochemical activity is distributed heterogeneously across the biosphere and that reservoir ecosystems are noteworthy hot spots. Dean & Gorham (1998) found that reservoirs bury an estimated 160 Tg C per year whereas the world’s oceans bury only 100 Tg C per year. This finding was particularly striking because reservoirs comprise only 0.11% of the Earth’s surface area while the oceans comprise 71%. Furthermore, reservoirs can retain significant quantities of N (Tomaszek & Koszelnik, 2003) and virtually all of this N can be lost back to the atmosphere via denitrification (David et al., 2006). Clearly, reservoirs are enormously important features on the landscape, not only from the perspective of human water use, but also from the perspective of global biogeochemical cycles. Results of our study suggest that N fixation can occur in reservoir ecosystems regardless of trophic status and, more importantly, that the spatial pattern of N fixation within reservoirs can be similar among reservoirs (Fig. 2). Thus, reservoirs themselves are far from homogeneous, and the transition zone may be an important biogeochemically active zone within these ecosystems.
In conclusion, our study indicates that cyanobacterial N fixation can be spatially heterogeneous within reservoirs. More importantly, spatial heterogeneity in N fixation can be similar among reservoirs of varying trophic state with rates in transition zones as much as 25–60 times higher than rates in riverine and lacustrine zones. Thus, reservoir transition zones appear to be noteworthy hot spots for N fixation and potentially other biogeochemical processes. This finding has important implications for managing nuisance cyanobacteria in reservoirs and understanding relationships between land use change, accelerated eutrophication, and phytoplankton community structure in reservoirs. The river–reservoir transition zone may be at highest risk of experiencing water quality problems (Lind et al., 1993). Our study indicates that N-fixing cyanobacteria may become problematic first in this reservoir zone as reservoir productivity increases. More work is needed to confirm the spatial patterns observed in this study and to investigate potential ecosystem processes that control the pattern.
References
APHA (American Public Health Association), 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. APHA and the American Public Health Association, Washington, DC, USA.
Bukaveckas, P. A. & A. S. Crain, 2002. Inter-annual, seasonal, and spatial variability in nutrient limitation of phytoplankton production in a river impoundment. Hydrobiologia 481: 19–31.
David, M. B., L. G. Wall, T. V. Royer & J. L. Tank, 2006. Denitrification and the nitrogen budget of a reservoir in an agricultural landscape. Ecological Applications 16: 2177–2190.
Dean, W. E. & E. Gorham, 1998. Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26: 535–538.
Downing, J. A. & E. McCauley, 1992. The nitrogen:phosphorus ratio in lakes. Limnology and Oceanography 37: 936–945.
Flett, R. J., R. D. Hamilton & N. E. R. Campbell, 1976. Aquatic acetylene reduction techniques: solutions to several problems. Canadian Journal of Microbiology 22: 43–51.
Forbes, M. G., R. D. Doyle, J. T. Scott, J. K. Stanley, H. Huang & B. W. Brooks, 2008. Physical factors control phytoplankton production and N2 fixation in eight Texas reservoirs. Ecosystems 11: 1181–1197.
Hejzlar, J., K. Šámalová, P. Boers & B. Kronvang, 2007. Modelling phosphorus retention in lakes and reservoirs. In Kronvang, B., J. Faganeli & N. Ogrinc (eds), The Interactions Between Sediment and Water. Springer, New York: 123–130.
Howarth, R. W., R. Marino, J. Lane & J. J. Cole, 1988. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnology and Oceanography 33: 669–687.
Kalff, J., 2002. Limnology. Prentice Hall, Upper Saddle River, NJ, USA.
Kennedy, R. H., J. G. Tundisi, V. Straškrábová, O. T. Lind & J. Hejzlar, 2003. Reservoirs and the limnologist’s growing role in sustainable water resource management. Hydrobiologia 504: xi–xii.
Kimmel, B. L. & A. W. Groeger, 1984. Factors controlling primary production in lakes and reservoirs. In Lake and Reservoir Management. Proc. North American Lake Management Society Symposium. EPA 440/5/84-001. U.S.E.P.A., Washington DC: 277–281, 604 pp.
Kimmel, B. L., O. T. Lind & L. J. Paulson, 1990. Reservoir primary production. In Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. Wiley, New York.
Kolber, Z. S., 2006. Getting a better picture of the ocean’s nitrogen budget. Science 312: 1479–1480.
Levine, S. N. & D. W. Schindler, 1999. Influence of nitrogen to phosphorus supply ratios and physicochemical conditions on cyanobacteria and phytoplankton species composition in the Experimental Lakes Area, Canada. Canadian Journal of Fisheries and Aquatic Science 56: 451–466.
Lind, O. T., T. T. Terrell & B. L. Kimmel, 1993. Problems in reservoir trophic-state classification and implications for reservoir management. In Straskraba, M., J. G. Tundisi & A. Duncan (eds), Comparative Reservoir Limnology and Water Quality Management. Kluwer, Netherlands: 57–67.
Nowlin, W. H., J. L. Evarts & M. J. Vanni, 2005. Release rates and potential fates of nitrogen and phosphorus from sediments in a eutrophic reservoir. Freshwater Biology 50: 301–322.
Reynolds, C. S., 1972. Growth, gas vacuolation and buoyancy in natural populations of blue-green alga. Freshwater Biology 2: 87–106.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 46: 260–262.
Scott, J. T., R. D. Doyle & C. T. Filstrup, 2005. Periphyton nutrient limitation and nitrogen fixation potential along a wetland nutrient-depletion gradient. Wetlands 25: 439–448.
Scott, J. T., R. D. Doyle, J. A. Back & S. I. Dworkin, 2007. The role of N2 fixation in alleviating N limitation in wetland metaphyton: enzymatic, isotopic, and elemental evidence. Biogeochemistry 84: 207–218.
Scott, J. T., R. D. Doyle, S. J. Prochnow & J. D. White, 2008a. Are watershed and lacustrine controls on planktonic N2 fixation hierarchichally structured? Ecological Applications 18: 805–819.
Scott, J. T., M. J. McCarthy, W. S. Gardner & R. D. Doyle, 2008b. Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry 87: 99–111.
Sen, S., B. E. Haggard, I. Chaubey, K. R. Brye, T. A. Costello & M. D. Matlock, 2007. Sediment phosphorus release at Beaver Reservoir, Northwest Arkansas, USA, 2002–2003; a preliminary investigation. Water, Air and Soil Pollution 179: 67–77.
Smith, V. H., 1983. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221: 669–671.
TCEQ (Texas Commission on Environmental Quality), 2007. Surface Water Quality Monitoring. Monitoring and Operations Division, TCEQ, Austin, Texas. Available from http://www.tceq.state.tx.us/assets/public/compliance/monops/water/wdma/dmrg/2007/2007dmrg_complete.pdf.
TIAER (Texas Institute for Applied Environmental Research), 2007. Semi-annual Water Quality Report for the North Bosque River Watershed. TIAER, Stephenville, Texas. Available from: http://tiaerweb.tarleton.edu/pdf/TR0601.pdf.
Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), 1990. Reservoir limnology: ecological perspectives. Wiley, New York.
Tomaszek, J. A. & P. Koszelnik, 2003. A simple model of N retention in reservoirs. Hydrobiologia 504: 51–58.
USGS (United States Geological Survey), 2005a. Water-Data Report TX-2005; 08103800 Lampasas River near Kempner, TX. USGS-Texas, Austin, Texas. Available from http://waterdata.usgs.gov/nwis/nwisman/?site_no=08103800.
USGS (United States Geological Survey), 2005b. Water-Data Report TX-2005; 08167500 Guadalupe River near Spring Branch, TX. USGS-Texas, Austin, Texas. Available from http://waterdata.usgs.gov/nwis/nwisman/?site_no=08167500.
USGS (United States Geological Survey), 2005c. Water-Data Report TX-2005; 08095200 North Bosque River at Valley Mills, TX. USGS-Texas, Austin, Texas. Available from http://waterdata.usgs.gov/nwis/nwisman/?site_no=08095200.
Wetzel, R. G., 2001. Limnology: Lake and River Ecosystems, 3rd ed. Academic Press, San Diego, CA, USA.
Acknowledgments
This work was supported by a grant from the City of Waco to R. Doyle and from the Texas Commission on Environmental Quality (TCEQ) to B. Brooks and R. Doyle. The Texas Institute for Applied Environmental Research (TIAER) and TCEQ provided long-term nitrate concentration data on reservoirs. The authors thank S. Williams, E. Martin, and B. Fulton for their assistance with field and laboratory efforts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Scott, J.T., Stanley, J.K., Doyle, R.D. et al. River–reservoir transition zones are nitrogen fixation hot spots regardless of ecosystem trophic state. Hydrobiologia 625, 61–68 (2009). https://doi.org/10.1007/s10750-008-9696-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9696-2