Abstract
The invasive ctenophore Mnemiopsis leidyi was accidentally introduced into the Black Sea in the early 1980s and it was first sighted in the Aegean Sea (Eastern Mediterranean) in the early 1990s. This article presents a first attempt to develop a predictive spatial model based on M. leidyi presence data and satellite environmental data from the Aegean Sea during early summer, in order to identify those areas in the Greek Seas and the entire Mediterranean basin that could serve as potential habitat for the species. Generalized additive models (GAM) were applied. The final GAM model indicated higher probability of finding M. leidyi present in depths of 65–135 m and sea surface temperature values of 21–25°C. Furthermore, the significant interaction between photosynthetically active radiation (PAR) and sea level anomaly (SLA) indicated a higher probability of M. leidyi presence in low values of PAR and SLA. In the next step, the final GAM was applied in a prediction grid of mean monthly satellite values for June 2004–2006 in order to estimate probability of M. leidyi presence in the Hellenic Seas and the whole Mediterranean basin at a GIS resolution of 4 km. In the Aegean Sea, species potential habitat included areas influenced by the Black Sea Water (e.g. Thracian Sea, Limnos-Imvros plateau), gulfs that are affected by river runoffs, such as the Thermaikos, Strymonikos and Patraikos gulfs, or areas with strong anthropogenic influence such as the Saronikos gulf. Areas with the same environmental conditions as those in Aegean Sea have been indicated in certain spots of the Levantine Sea as well as in coastal waters of Egypt and Libya, although their spatial extent varied largely among years examined. However, the occurrence of conditions that are linked to high probability of M. leidyi presence does not necessarily mean that these areas can support successful reproduction, high population or bloom levels, since these depend on a combination of temperature, salinity, food availability and the abundance of predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ctenophore Mnemiopsis leidyi A. Agassiz 1865 is a voracious zooplanktivorous species and its native habitat is found in estuaries and coastal regions along the eastern coast of North and South America (Javidpour et al., 2006). It was accidentally introduced into the Black Sea in the early 1980s (Vinogradov et al., 1989) possibly with ballast water from ship coming from the NW Atlantic coastal region. It has exhibited an explosive mass development there since 1988, and has expanded to the Azov, Marmara and Eastern Mediterranean Seas (Studenikina et al., 1991; Shiganova, 1993; Shiganova et al., 2001a).
This ctenophore is a polymorphic species with wide tolerance to environmental factors and high phenotypic variability (Javidpour et al., 2006) and it has been included in the International Union for Conservation of Nature (IUCN) list of 100 ‘World’s Worst’ invaders. Mnemiopsis leidyi strongly affected all levels of ecosystems and fishery in the Black, Azov and Caspian Seas (Shiganova et al., 2001a, 2003, 2004a, b). Mnemiopsis leidyi is one of the most carnivorous species among comb jellies and it is well adapted for rapid population growth, presenting high feeding, growth and reproduction rates (Finenko et al., 1995). As most planktonic ctenophores, M. leidyi is a simultaneous hermaphrodite and capable of self-fertilization (Planka, 1974; Hirota, 1972; Reeve & Walter, 1976). It exhibits a totally planktonic life stage with early stages utilizing protozoa and microzooplankton while adults feed primarily on crustaceans (often copepods and cladocera), mollusc larvae, eggs and young fish larvae (Rapoza et al., 2005). In the Black Sea a sharp decline in icthyo- and mesozooplankton abundance was observed, followed by a change in species composition (Vinogradov et al., 1989, 1992; Shiganova, 1997; Konsulov & Kamburska, 1998; Kovalev et al., 1998) and an abrupt decline in the biomass of zooplanktivorous fish (Volovik et al., 1993; Prodanov et al., 1997; Shiganova, 1997, 1998) such as the Black Sea anchovy and the Mediterranean horse mackerel. These species spawn during summer and suffered from decreased zooplankton abundance (Shiganova et al., 2001a, b). By the late 1980s and early 1990s the pelagic ecosystem of the Black Sea had become a dead-end gelatinous food web and the reduction of M. leidyi populations in the Black Sea occurred after one of its predators, the ctenophore Beroe ovata, was introduced in the region (Rapoza et al., 2005).
The first occurrence M. leidyi in the Aegean Sea was recorded during late spring-summer 1990 in Saronikos Gulf (45–75 individuals/m2, Shiganova et al., 2001b). After 1991, M. leidyi swarms were observed in several coastal areas of the northern Aegean and few specimens were collected in offshore waters (Shiganova et al., 2001a). It is believed that the flow of Black Sea water mass to the northern Aegean Sea contributes to the dispersal of M. leidyi in the area. Further east, M. leidyi appeared in Mersin Bay in spring 1992 (Kideys & Niermann, 1994; Uysal & Mutlu, 1993), and in Syrian coastal waters in October 1993 (Shiganova, 1997). The individuals from these areas as well as from Saronikos gulf were probably introduced with ballast waters from ships because they were found near Latakia port (Mersin Bay) and Piraeus port (Saronikos gulf) (Shiganova et al., 2001a). In 1999, M. leidyi reached the Caspian Sea, where it is currently expanding at an even more rapid rate than in the Black Sea (Shiganova et al., 2001b). It has been recently found in the western Baltic Sea (Javidpour et al., 2006).
In Aegean Sea, M. leidyi has also been found in Lesvos island in the NE Aegean Sea in 1995 and in several coastal areas of the Aegean Sea (Skyros, Limnos and Alonissos islands and Halkidiki Peninsula) between 1991 and 1996 (Shiganova et al., 2001a). It is believed that the continuous flow of Black Sea water mass to the northern Aegean Sea and the dominant local circulation pattern has resulted in the dispersal of M. leidyi individuals to this area (Shiganova et al., 2004a). However no decrease in mesozooplankton biomass has been observed in the Aegean Sea after the appearance of M. leidyi in the area (Shiganova et al., 2001a).
The majority of the studies on the presence of M. leidyi in the Mediterranean and the Black Sea are referring mainly to species occurrence, life cycle and reproduction studies (Rapoza et al., 2005), population dynamics (Shiganova et al., 2001a, b, 2004a, b) and the associated changes in the pelagic community structure (Shiganova, 1998; Shiganova et al., 2001a, 2004a, b). Although M. leidyi is a rapidly expanded invader with negative impacts in different ecosystems, there are no attempts to define the potential areas that could favour species presence. The prediction of an invasive species potential habitat has particular interest in a closed region such as the Mediterranean basin, which is characterized by strong anthropogenic influence and ecosystem degradation that could enhance the impact of the species into the ecosystem.
Spatial modelling of a species distribution in relation to environmental parameters appears to be a prospective approach to define areas with certain environmental characteristics that could potentially support species presence (Guisan & Zimmermann, 2000; Riou et al., 2001; Francis et al., 2005). It is increasingly becoming an effective tool for understanding the processes that affect the interannual variability in species distribution and provide essential information for management purposes. In addition, satellite environmental data may be used for modelling as proxies to infer spatial variations of environmental factors, allowing estimations in various years, periods and regions. Furthermore, new powerful statistical techniques and GIS tools have been created during the last decade and the use of such tools in the development of habitat distribution models has rapidly increased in ecology (Guisan & Zimmermann, 2000; Riou et al., 2001; Francis et al., 2005).
Thus, the present work is a first approach to develop a spatial model based on North Aegean Sea satellite data to determine the environmental conditions that characterize areas where M. leidyi is present and, based on this environmental interaction, to identify other potential areas that could support species presence in the Hellenic and Mediterranean Seas.
Materials and methods
Study area
The northern Aegean Sea is characterized by high hydrological complexity mostly related to the Black Sea waters (BSW) that enter the Aegean Sea through the Dardanelles strait as a surface current (Zervakis & Georgopoulos, 2002). Two anticyclonic systems dominate the area: one in the Samothraki plateau (the Samothraki gyre) and another one in the Strymonikos Gulf (Somarakis et al., 2002; Fig. 1). These gyres are considered permanent features in the area during early summer and are coupled with a cyclonic system located south of the island of Thasos. The overall circulation is mainly determined by the presence of the Limnos-Imvros stream (LIS), which carries waters of Black Sea origin into the Samothraki plateau (Somarakis et al., 2002; Fig. 1). The thickness of the BSW exiting the Dardanelles is less than 40 m; characterized by its very low salinity (<30 psu), in relation to the Aegean Sea waters (Zervakis et al., 2000). The outflow of BSW enhances local productivity and its advection in the Aegean Sea induces high hydrological and biological complexity (Isari et al., 2006, 2007; Somarakis & Nikolioudakis, 2007). The BSW layer undergoes modification of its characteristics by air-sea interaction and vertical diffusion through mixing with the underlying waters reaching gradually a salinity of 38 psu in the region of the Sporades Islands (central and western Aegean Sea).
The extent of the BSW plume is controlled by the prevailing wind conditions (Vlasenko et al., 1996). The wind field over the Aegean Sea is strong and its direction is largely controlled by orography. Semi-enclosed water bodies, common in Greek waters (many islands, irregular coastline, semi- or enclosed gulfs), are often highly influenced by wind patterns, producing in many cases local water movements that can favour bottom-up and top-down processes. Etesians (strong summer north-westerly winds) dominate the Aegean from mid-July to mid-September (Hyder et al., 2002). Furthermore, six main rivers discharge into the northern Aegean Sea (Axios, Aliakmon, Pinios, Strymon, Nestos and Evros) that end in large, semi-closed gulfs such as Thermaikos and Strymonikos Gulfs (Stergiou et al., 1997; Isari et al., 2006).
Sampling
Plankton specimens were collected during three research surveys during early summer in the northern Aegean Sea in June 2004–2006. Sampling design was based on a grid of stations spaced on parallel transects that were approximately 10 nautical miles apart (Fig. 1). A total of 205 stations were located at five nautical mile intervals on each transect. Standard vertical plankton tows were carried out at each station, by a WP2 sampler (mouth opening: 0.255 m2, mesh-size: 0.200-mm). Mnemiopsis leidyi specimens were identified and counted on board while zooplankton samples were preserved in 4% buffered formalin. Qualitative and quantitative analyses of ichthyoplankton samples were performed in the laboratory under a binocular microscope. The total number of M. leidyi and the total number of anchovy eggs and larvae were determined and counted for each sample station.
Environmental data
The area is well monitored in terms of monthly satellite imagery. Sea surface temperature distribution (SST in °C) is available through the German Aerospace Agency’s (DLR) satellite data archive (EOWEB), while sea surface chlorophyll concentration (CHLO in mg/m³) and photosynthetically active radiation (PAR in Ein/m2/day) are downloaded through Oceancolor Web, NASA’s online Distributed Active Archive Centre. PAR is defined as the quantum energy flux from the sun in the spectral range 400–700 nm. Sea surface salinity distribution (SSS in psu from CMA BCC GODAS model, Liu et al., 2005) is available through the International Research Institute for Climate and Society (IRI—University of Columbia) online data distribution archive. Bathymetry is calculated through processing (kriging) of a point dataset derived from a blending of depth soundings collected from ships with detailed gravity anomaly information obtained from the Geosat and ERS-1 satellite altimetry missions (Smith & Sandwell, 1997). Sea level anomaly (SLA in cm) is available through AVISO website using their Live Access Server. These aforementioned parameters might be important either as a direct influence on the distribution of anchovy or as a proxy for other factors (Bellido et al., 2001). For example, SLA describes ocean processes, such as gyres, meanders and eddies (Larnicol et al., 2002; Pujol & Larnicol, 2005), which enhance productivity and often function as physical barriers differentiating the distribution of species or species life stages, whereas PAR, representing the amount of solar radiation useable for plants to photosynthesize (Frouin et al., 2003), might be indicative of the extent of the euphotic zone, with its lower limit defined as the depth to which PAR values are reduced to 0.1% of the surface measurements (Hader et al., 1994). The mean monthly values for June 2004–2006 of satellite imagery were estimated for all these variables (Valavanis et al., 2004).
Model estimation
Generalized Additive Models (GAMs) were used in order to define the set of the environmental parameters that describe the areas of M. leidyi presence in northern Aegean Sea in June 2004–2006. A GAM (Hastie & Tibshirani, 1990) is a generalized linear model with a linear predictor involving a sum of smooth functions of covariates (Wood, 2006). The main advantage of GAMs over traditional regression methods is their capability to model non-linearities using non-parametric smoothers (Hastie & Tibshirani, 1990; Wood, 2006). The selection of the GAM model followed the methodology proposed by Wood & Augustin (2002), using the ‘mgcv’ library in the R statistical software (R Development Core Team, 2005).
As response variable (y), we used the binary presence/absence of M. leidyi. Independent variables included the cubic root of the depth, the natural logarithm of CHLO, the SST, the SSS, the SLA and the PAR. Bottom depth and CHLO presented high variability in their original values, and thus transformation was necessary to achieve uniform distribution for GAM development (Hastie & Tibshirani, 1990). The appropriate type of transformation was based on the inspection of Quantile–Quantile plots (QQ-plots) to check if variables under certain transformations follow the normal distribution. Environmental variables were ranked according to the above criteria and the best model was chosen based on a stepwise forward selection method. In addition, besides the environmental variables, the binary presence/absence of anchovy eggs and anchovy larvae was tested. For the construction of such a model that could successfully estimate probabilities of the M. leidyi presence, data collected over a wide range of environmental parameters should be collated. Therefore, pooled data from all three examined years were used, in order to obtain more possible observed conditions and ensure potentiality (ICES, 2005).
The output of the GAMs is smoothed fits for each environmental predictor. The individual models cannot be tested for significance using the P-values provided by ‘mgcv’ library since the true number of degrees of freedom is unknown. Therefore, the best GAM model was chosen based on a stepwise forward selection method that reduces the collinearity problem starting from a simple initial model with few explanatory variables (Sacau et al., 2005; Zuur personal communication). Specifically, models were compared based on the level of deviance explained (0–100%; the higher the better), the Akaike Information Criterion (AIC) and the Un-biased Risk estimator (UBRE, the lower the better). Furthermore, the degree of smoothing of each parameter was chosen based on the observed data and the Generalized Cross Validation method suggested by Wood (2006) and incorporated in the ‘mgcv’ library. A binomial error distribution and the natural cubic spline smoother were chosen as appropriate for the model. All first order interactions of the parameters included in the final model were tested.
In the next step, the final GAM was applied in a predictive mode in order to estimate the probability of M. leidyi presence to each point of the area used for modelling for June 2004–2006, respectively. Therefore, a specific set of satellite conditions was attributed to a specific probability of M. leidyi presence. Moreover, the final GAM was applied in a prediction grid of mean monthly satellite values for June 2004–2006, estimated for the entire Hellenic Seas as well as for the whole Mediterranean basin, at a GIS resolution of 4 km. The areas with a specific set of satellite conditions corresponding to different probabilities (i.e. 25, 50 and 75%) of M. leidyi presence were plotted. The Surfer v8.0 of the Golden Software Inc. software was used for mapping.
Model validation
The predictive accuracy of the final model was tested by comparing M. leidyi presence and the results of the final GAM model (i.e. the predicted probabilities) concerning the northern Aegean Sea, region used for modelling for each examined year. For this purpose, the Receiver Operating Characteristic (ROC)-plots (Fieldings & Bell, 1997; Guisan and Zimmermann 2000) and the area under the Receiver Operating Characteristic Curve (AUC) were estimated. AUC has been used extensively in the species’ distribution modelling literature, measuring the ability of a model to discriminate between those sites where a species is present and those where it is absent (Hanley & McNeil, 1982). It provides an indication of the usefulness of the models for indicating areas in terms of their relative importance as habitat for the particular species. The values of AUC ranges from 0 to 1, where a score of 1 indicates perfect discrimination, a score of 0.5 implies predictive discrimination that is no better than a random guess and values <0.5 indicate performance worse than random (Boyce et al., 2002; Elith et al., 2006). Estimations were made with the presence/absence library of the R statistical software.
Results
M. leidyi distribution in northern Aegean Sea
The distribution of M. leidyi in northern Aegean Sea (including both larvae and adult specimens) is shown in Fig. 2. Although its abundance and distribution shows a large degree of interannual variability, the highest abundances were observed in Thermaikos and Strymonikos gulfs where large rivers outflow. The lowest abundances were recorded in June 2005 (3–50 ind/m2), whereas the highest in June 2004 (4–188 ind/m2). The wider and most southern distribution (up to N. Evoikos Gulf) of the species was observed in 2006 compared to the previous years. A comparison of the distribution of M. leidyi and the distribution of anchovy eggs, in the northern Aegean Sea showed contrasting spatial patterns (Fig. 2), with higher abundances of M. leidyi in areas presenting low abundances of anchovy eggs. This was more pronounced in Thermaikos gulf in 2004–2006, Strymonikos gulf in 2004 and N. Evoikos gulf in 2006.
Model estimation
The final selected GAM model based on satellite data included as main effects: SST, Depth and the interactive effect of PAR and SLA, and they are described in Table 1. All variables selected in the final model were statistically significant. Despite the contrasting spatial pattern, possibly because it was not consistent in all areas and years, the entry in the final GAM model of the term “anchovy eggs presence” was found non-significant. The entry of the term “anchovy larvae presence” resulted in a minor reduction of the AIC (less than 0.5%), which implied that the inclusion of the latter term practically did not improve the final GAM model. Therefore these results a not included in Table 1.
The results of the final GAM model are shown as plots of the best-fitting smooths for the effect of the environmental parameters on M. leidyi presence (Fig. 3). The 95% confidence intervals were also plotted around the best-fitting smooths for the main effects. Interaction effects are shown as a perspective plot without error bounds. The y-axis reflects the relative importance of each parameter of the model and for the interaction effects this is presented on the z-axis (Fig. 3). It should be noted that the effect of each variable is the conditional effect, i.e. the effect of the variable given that all other variables are included in the model. Plots indicated higher probability of M. leidyi presence in depths of 65–135 m and SST of 21–25°C (the maximum temperature available). The interaction plot between PAR and SLA also indicated a higher probability of M. leidyi presence in the lower values of PAR combined with the lower values of SLA.
Coefficients of the generalized additive models (GAMs) for M. leidyi against (a) SST, (b) Bottom depth and (c) the interaction plot of PAR and SLA. Black thick lines indicate the value of GAMs coefficient, dotted lines represent the confidence intervals at P = 0.05. The rug under the single variable effects plots indicates the density of points for different variable values
Model validation
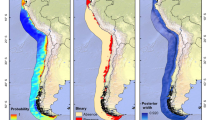
This final model that was based on pooled data from all years was tested for accuracy with the calculation of AUC value for each ROC plot corresponding to satellite data from each separate year. Results indicated good discrimination ability of the model according to Elith et al. (2006), as it exceeded 0.75 in all cases (Table 2). The model was applied in a predictive mode for the entire Hellenic Seas at a GIS resolution of 4 km, in order to map the areas that present environmental conditions similar to those found in northern Aegean Sea. Similar maps were drawn for the entire Mediterranean Sea (Figs. 4–6). The resulted maps indicated areas with environmental conditions that are associated with specific probability values i.e. 0–25, 25–50, 50–75 and 75–100% probability of M. leidyi presence in northern Aegean Sea.
Discussion
Results show that the abundance of M. leidyi in the northern Aegean Sea exhibits a high degree of interannual variability and the highest abundances were generally observed in gulfs influenced by river runoffs such as Thermaikos and Strymonikos gulf (Figs. 1 and 2). Rivers outflows bring terrestrial input with subsequent increase in nutrients and local productivity, thus allowing the ecosystem to support higher abundance of zooplankton populations. Experiments by Shiganova et al. (2004a) have shown that the species can feed and reproduce successfully in the environmental conditions of the northern Aegean Sea. Satellite environmental data describe sea surface conditions rather than describing the actual environment in which the species exists. However, according to the estimated GAM model there was a higher probability of M. leidyi presence in 60–140 m, 21–25°C, and low levels of PAR and SLA. Although difficult to interpret, this interaction effect is likely to indicate an association with waters of ‘shallower’ euphotic zone (possibly indicating more stratified water masses) combined with downward water movement. This could indicate that there is a positive effect of anticyclonic gyres in the northern Aegean Sea (known as retention areas for zooplankton and ichthyoplankton) as well as a summer effect, implied by the higher probability of M. leidyi presence in warmer, more stratified waters.
Furthermore, there is an indication for contrasting spatial occupation of M. leidyi and anchovy eggs (i.e. higher abundance of M. leidyi coinciding with low abundances of anchovy eggs and vice versa) in the northern Aegean Sea (Fig. 2). However, this was not consistent in all areas and years, being more pronounced during June 2004 and in Thermaikos gulf during June 2005. Pelagic cnidarians and ctenophores may compete with zooplanktivorous fish and fish larvae. In addition, M. leidyi is known to strongly compete with larval anchovy for food in Black Sea (Gordina et al., 2005) being, at the same time, a prominent predator of anchovy eggs and yolk-sac larvae (Shiganova et al., 2001a). This might partly explain the observed contrasting spatial patterns. However, because this contrasting spatial pattern in anchovy eggs and M. leidyi presence was not consistent in areas and years due to the spatio-temporal variability in the abundance of both species, the entry of this relationship into the GAM model was found non-significant.
Modelling the relationship of M. leidyi presence with satellite environmental data from the northern Aegean Sea provided a map of these areas in the Hellenic Seas and the entire Mediterranean basin with certain environmental conditions related to certain probabilities of M. leidyi presence. Areas indicated in the Hellenic Seas varied in their spatial extent annually, depending on the environmental conditions. In the Aegean Sea, areas that have been indicated were characterized by BSW influence (e.g. Thracian Sea, Limnos–Imvros plateau), including gulfs that are affected by river runoffs such as Thermaikos and Strymonikos gulf or have strong anthropogenic influence such as Saronikos gulf (Fig. 4). In the Ionian Sea, the main areas were Patraikos gulf and the coastal waters between the islands and the mainland, areas characterized by shallow, productive waters and river runoffs. Areas have also been indicated in the Turkish coastal waters of the Aegean Sea that are strongly associated with the BSW, such as the Saros Bay and the area around the island of Imvros as well as areas like the Edremit gulf and the gulf of Izmir (Figs. 4 and 5). Known records of the species in Saronikos Gulf since late spring-summer 1990 (Shiganova et al., 2001a) as well as from other coastal areas of the Aegean Sea (Skyros, Limnos and Alonissos islands, Halkidiki Peninsula) between 1991 and 1996 (Shiganova et al., 2001a) and Izmir Gulf (Kideys & Niermann, 1994) generally confirm the areas indicated by the model as potential M. leidyi habitat.
Maps for the entire Mediterranean Sea (Figs. 5 and 6) indicated areas with similar environmental characteristics to those in the Aegean Sea. It is well understood that the spatial and seasonal extent of these areas is affected by the variability in climate and the environment, thus estimation was restricted to early summer (June) and estimated for three different years (Figs. 5 and 6). In the Eastern Mediterranean basin, areas have been indicated in the Levantine: the Syrian and the Lebanese coastal waters, the Turkish southeast coastal waters, such as the Gulf of Antalya and the Gulf of Mersin, and offshore eastern Cyprus. Areas have also been indicated in the coastal waters of Egypt and Libya, although their spatial extent varied largely from year to year. Data from the Eastern Mediterranean confirm the presence of M. leidyi in Mersin Bay in spring 1992 (Uysal & Mutlu, 1993; Kideys & Niermann, 1994) and in the Syrian coastal waters in October 1993 (Shiganova, 1997; Shiganova et al., 2001a, b), but it is unknown if there is a persistent, reproducing, local population.
Although the model explains only a small fraction of total deviance (26.70%), the areas indicated are shallow, productive areas, influenced by river runoffs, being under strong anthropogenic influence (e.g. big commercial ports) or by the BSW mass, which most likely contributes to the dispersal of M. leidyi to the northern Aegean Sea. Although there was a lack of adequate data, areas indicated by the model are mostly areas of known presence of M. leidyi. The model could be further improved by adding data from other areas like the Black Sea region and by taking into account the temporal variation in the environmental parameters (e.g. weekly differences), the abundance of anchovy eggs as well as the abundance of competitor species and predators. The seasonal abundance of M. leidyi largely depends on the combination of favourable conditions regarding temperature, salinity, food availability and predators’ abundance; therefore the occurrence of M. leidyi in the Aegean Sea and the eastern Mediterranean does not necessarily mean that the species in the area can reproduce, reach high populations or bloom levels, causing ecosystem effects similar to the ones occurred in the Black Sea. For example, the occurrence of high temperatures during the winter period is very important to M. leidyi’s successful reproduction (Shiganova et al., 2001a).
The case of the western Mediterranean Sea presents particular interest because, as of this study, there are no reported records of the species presence. Mapping indicated those regions that present environmental characteristics similar to those where the species occurs in the northern Aegean Sea. Derived areas include the Adriatic Sea, the coastal waters of the Ligurian and the Tyrrhenian seas, the straits of Sicily, coastal areas in Tunisia and Libya (e.g. the Gulf of Benghazi, the Gulf of Gabes and the Gulf of Shirte, Figs. 5 and 6), as well as the Gulf of Lions and the Catalan Sea. Future plans of establishment of oil pipe lines in the region (i.e. Adriatic Sea) could increase the risk of the invasion of M. leidyi with ballast waters to the western Mediterranean. It should be noted that all these areas seem to have environmental conditions that potentially, but not necessarily, could support the species presence, such that the species can both successfully reproduce and reach high populations or bloom levels.
The areas indicated in the western Mediterranean coincide with the major spawning grounds and fishing grounds of small pelagics and especially anchovy (i.e. the Adriatic Sea, the Gulf of Lions, the Catalan Sea and the Gulf of Tunis, see García Lafuente et al., 2002; Cuttitta et al., 2003; Sabates et al., 2007; Palomera et al., 2007). Since experience from the Black Sea has shown that high abundances of M. leidyi (Shiganova et al., 2001a) involve a high risk for alterations in the local food web and in the abundance of zooplanktivorous fish populations, such as anchovy (Volovik et al., 1993; Prodanov et al., 1997; Shiganova, 1997; 1998), it is important to examine through future work the possible dispersion pathways of M. leidyi to the western Mediterranean.
Furthermore, future work may involve the examination of whether local conditions (e.g. temperature, salinity, prey’s availability and/or predators’ abundance) in the Mediterranean Sea could favour M. leidyi’s successful reproduction, high populations or bloom levels during all year round that might possibly lead to an alteration of the food web in a similar way to the case of the Black Sea.
References
Bellido, J. M., G. J. Pierce & J. Wang, 2001. Modelling intra-annual variation in abundance of squid Loligo forbesi in Scottish waters using generalised additive models. Fisheries Research 52: 23–39.
Boyce, M. S., P. R. Vernier, S. E. Nielsen & F. K. A. Schmiegelow, 2002. Evaluating resource selection functions. Ecological Modelling 157: 281–300.
Cuttitta, A., V. Carini, B. Patti, A. Bonanno, G. Basilone, S. Mazzola, J. Garcia Lafuente, A. Garcia, G. Buscaino, L. Aguzzi, L. Rollandi, G. Morizzo & C. Cavalcante, 2003. Anchovy egg and larval distribution in relation to biological and physical oceanography in the strait of Sicily. Hydrobiologia 503: 117–120.
Elith, J., C. H. Graham, R. P. Anderson, M. Dudik, S. Ferrier, A. Guisan, R. J. Hijmans, F. Huettmann, J. R. Leathwick, A. Lehmann, J. Li, L. G. Lohmann, B. A. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. Mc. C. Overton, A. T. Peterson, S. J. Phillips, K. S. Richardson, R. Scachetti-Pereira, R. E. Schapire, J. Soberon, S. Williams, M. S. Wisz & N. E. Zimmermann, 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151.
Fieldings, A. H. & J. F. Bell, 1997. A review of methods for the assessment of prediction errors in conservation presence: absence models. Environmental Conservation 24: 38–49.
Finenko, G. A., G. I. Abolmasova & Z. A. Romanova, 1995. Intensity of the nutrition, respiration and growth of Mnemiopsis mccradyi in relation to grazing conditions. Biologia Morya 21: 315–320.
Francis, M. P., M. A. Morrison, J. Leathwick, C. Walsh & C. Middleton, 2005. Predictive models of small fish presence and abundance in northern New Zealand harbours. Estuarine Coastal and Shelf Science 64: 419–435.
Frouin, R., B. A. Franz & P. J. Werdell, 2003. The SeaWiFS PAR product. In Hooker, S. B. & E. R. Firestone (eds), Algorithm Updates for the Fourth SeaWiFS Data Reprocessing, NASA/TM 2003–206892 22: 46–50.
García Lafuente, J., A. García, S. Mazzola, L. Quintanilla, A. Delgado, A. Cuttitta & B. Patti, 2002. Hydrographic phenomena influencing early life stages of the Sicilian Channel anchovy. Fisheries Oceanography 11: 31–44.
Gordina, A. D., J. A. Zagorodnyaya, A. E. Kideys, L. Bat & H. H. Satilmis, 2005. Summer ichthyoplankton, food supply of fish larvae and impact of invasive ctenophores on the nutrition of fish larvae in the Black Sea during 2000 and 2001. Journal of Marine Biological Association of the United Kingdom 85: 537–548.
Guisan, A. & N. E. Zimmermann, 2000. Predictive habitat distribution models in ecology. Ecological Modelling 135: 147–186.
Hader, D. P., R. C. Worrest, H. D. Kumar & R. C. Smith, 1994. Effects of increased solar ultraviolet radiation on aquatic ecosystems. United Nations Environment Programme, Environmental Effects of Ozone Depletion, 1994 Assessment. http://sedac.ciesin.org/ozone/UNEP/UNEP94toc.html
Hanley, J. A. & B. J. McNeil, 1982. The meaning and use of the area under a Receiver Operating Characteristic (ROC) curve. Radiology 143: 29–36.
Hastie, T. & R. Tibshirani, 1990. Generalized Additive Models. Chapman and Hall, London.
Hirota, J., 1972. Laboratory culture and metabolism of the ctenophore Pleurabrachia bachei A. Agassiz. In Takenouti, A. Y. (ed.), Biological Oceanography of the Northern North Pacific Ocean. Idemitsu Shoten: 465–484.
Hyder, P. W., J. H. Simpson, S. Christopoulos & Y. Krestenitis, 2002. The seasonal cycles of stratification and circulation in the Thermaikos Gulf Region of Freshwater Influence (ROFI), north-west Aegean. Continental Shelf Research 22: 2573–2597.
ICES, 2005. Report on the Study Group on Regional Scale Ecology of Small Pelagic Fish (SGRESP). ICES CM/2005/G:06.
Isari, S., S. Psarra, P. Pitta, P. Mara, M. O. Tomprou, A. Ramfos, S. Somarakis, A. Tselepides, C. Koutsikopoulos & N. Fragopoulu, 2007. Differential patterns of mesozooplankters’ distribution in relation to physical and biological variables of the Northeastern Aegean Sea (eastern Mediterranean). Marine Biology 151: 1035–1050.
Isari, S., A. Ramfos, S. Somarakis, C. Koutsikopoulos, A. Kallianiotis & N. Fragopoulu, 2006. Mesozooplankton distribution in relation to hydrology of the Northeastern Aegean Sea, eastern Mediterranean. Journal of Plankton Research 28: 241–255.
Javidpour, J., U. Sommer & T. Shiganova, 2006. First record of Mnemiopsis leidyi A. Agassiz 1865 in the Baltic Sea. Aquatic Invasions 1: 299–302.
Kideys, A. E. & U. Niermann, 1994. Occurrence of Mnemiopsis along the Turkish coast. ICES Journal of Marine Science 51: 423–427.
Konsulov, A. S. & L. T. Kamburska, 1998. Ecological determination of the new Ctenophora – Beroe ovata invasion in the Black Sea. Proceedings of the Institute of Oceanology of Varna 2: 195–198.
Kovalev, A. V., S. Besiktepe, J. Zagorodnyaya & A. Kideys, 1998. Mediterraneanization of the Black Sea zooplankton is continuing. In Ivanov, L. & T. Oguz (eds), Ecosystem Modeling as a Management Tool for the Black Sea. Kluwer Academic Publishers, The Netherlands.
Larnicol, G., N. Ayoub & P. Y. Le Traon, 2002. Major changes in Mediterranean Sea level variability from 7 years of TOPEX/Poseidon and ERS-1/2 data. Journal of Marine Systems 33: 63–89.
Liu, Y., R. Zhang, Y. Yin & T. Niu, 2005. The application of ARGO data to the global ocean data assimilation operational system of NCC. Acta Meteorological Sinica 19: 355–365.
Palomera, I., M. P. Olivar, J. Salat, A. Sabates, M. Coll, A. Garcia & B. Morales-Nin, 2007. Small pelagic in the NW Mediterranean Sea: an ecological review. Progress in Oceanography 74: 377–396.
Planka, H. D., 1974. Ctenophora. Reproduction of Marine Invertebrates 1: 201–265.
Prodanov, K., K. Mikhailov, G. Daskalov, K. Maxim, A. Chashchin, A. Arkhipov, V. Shlyakov & E. Ozdamar, 1997. Environmental Impact on Fish Resources in the Black Sea. In Ozsoy, E. & A. Mikaelyan (eds), Sensitivity of North Sea. Baltic Sea and Black Sea to Anthropogenic and Climatic Changes. Kluwer, Dordrecht: 163–181.
Pujol, M. I. & G. Larnicol, 2005. Mediterranean sea eddy kinetic energy variability from 11 years of altimetric data. Journal of Marine Systems 58: 121–142.
R Development Core Team 2005. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org
Rapoza, R., D. Novak & J. H. Costello, 2005. Life-stage dependent, in situ dietary patterns of the lobate ctenophore Mnemiopsis leidyi Agassiz 1865. Journal of Plankton Research 27: 951–956.
Reeve, M. R. & M. A. Walter, 1976. A large-scale experiment on the growth and predation potencial of ctenophore populations. In Mackie, G. O. (ed.), Coelenterate Ecology and Behavior. Plenum: 187–199.
Riou, P., O. Le Pape & S. I. Rogers, 2001. Relative contributions of different sole and plaice nurseries to the adult population in the Eastern Channel: application of a combined method using generalized linear models and a geographic information system. Aquatic Living Resources 14: 125–135.
Sabates, A., M. P. Olivar, J. Salat, I. Palomera & F. Alemany, 2007. Physical and biological processes controlling the distribution of fish larvae in the NW Mediterranean. Progress in Oceanography 74: 355–376.
Sacau, M., G. J. Pierce, J. Wang, A. I. Arkhipkin, J. Portela, P. Brickle, M. B. Santos, A. F. Zuur & X. Cardoso, 2005. The spatio-temporal pattern of Argentine shortfin squid Illex argentinus abundance in the southwest Atlantic. Aquatic Living Resources 18: 361–372.
Shiganova, T. A., 1993. Ctenophore M. leidyi and ichthyoplankton in the Sea of Marmara in October of 1992. Oceanology 33: 900–903.
Shiganova, T. A., 1997. M. leidyi abundance in the Black Sea and its impact on the pelagic community. In Ozsoy, E. & A. Mikaelyan (eds), Sensitivity of North Sea, Baltic Sea and Black Sea to anthropogenic and climatic changes. Kluwer Academic Publishers, Dordrecht: 117–130.
Shiganova, T. A., 1998. Invasion of the Black Sea by the ctenophore M. leidyi and recent changes in pelagic community structure. Fisheries Oceanography 7: 175–390.
Shiganova, T. A., Y. V. Bulgakova, E. I. Musaeva, Z. A. Mirzoyan & M. L. Martynyuk, 2003. Ctenophore invaders Mnemiopsis leidyi (A. Agassiz) and Beroe ovata Mayer 1912 and their effect on pelagic ecosystem of northeastern Black Sea. Biological Bulletin N3.
Shiganova, T. A., E. D. Christou, J. V. Bulgakova, I. Siokou-Frangou, S. Zervoudaki & A. Siapatis, 2004a. Distribution and biology of the invader M. leidyi in the Northern Aegean Sea, comparison with indigenous species Bolinopsis vitrea. In Dumont, H., T. Shiganova & U. Niermann (eds), Aquatic Invasions in the Black, Caspian and Mediterranean Seas. NATO Science Series 4. Earth and Environmental Sciences. Kluwer Academic Publishers: 113–135.
Shiganova, T. A., H. J. D. Dumont, A. Mikaelyan, D. Glazov, M. Y. V. Bulgakova, E. I. Musaeva, P. Y. Sorokin, L. A. Pautova, Z. A. Mirzoyan & E. I. Studenikina, 2004b. Interaction between the invading ctenophores Mnemiopsis leidyi (A. Agassiz) and Beroe ovata (Mayer 1912), and their influence on the pelagic ecosystem of the Northeastern Black Sea. In Dumont, H., T. Shiganova & U. Niermann (eds), The Ctenophore Mnemiopsis leidyi in the Black, Caspian and Mediterranean Seas and Other aquatic Invasions. NATO Science Series 2. Environment. Kluwer Academic Publishers: 33–70.
Shiganova, T. A., A. Kamakin, D. P. Zhukova, V. B. Ushivtzhev, A. B. Dulimov & E. I. Musaeva, 2001a. New invader in the Caspian Sea - ctenophore Mnemiopsis and its initial effect on the pelagic ecosystem. Oceanology 41: 542–549.
Shiganova, T. A., Z. A. Mirzoyan, E. A. Studenikina, S. P. Volovik, I. Siokou-Frangou, S. Zervoudaki, E. D. Christou, A. Y. Skirta & H. J. Dumont, 2001b. Population development of the invader ctenophore Mnemiopsis leidyi in the Black Sea and other seas of the Mediterranean basin. Marine Biology 139: 431–445.
Smith, W. H. F. & D. T. Sandwell, 1997. Global sea floor topography from satellite altimetry and ship depth soundings. Science 277: 1956–1962.
Somarakis, S., P. Drakopoulos & V. Filippou, 2002. Distribution and abundance of larval fishes in the northern Aegean Sea—eastern Mediterranean—in relation to early summer oceanographic conditions. Journal of Plankton Research 24: 339–357.
Somarakis, S. & N. Nikolioudakis, 2007. Oceanographic habitat, growth and mortality of larval anchovy (Engraulis encrasicolus) in the northern Aegean Sea (eastern Mediterranean). Marine Biology 152: 1143–1158.
Stergiou, K. I., E. D. Christou, D. Georgopoulos, A. Zenetos & C. Souvermezoglou, 1997. The Hellenic Seas: physics, chemistry, biology and fisheries. Oceanography and Marine Biology: An Annual Review 35: 415–538.
Studenikina, E. I., S. P. Volovik, I. A. Miryozan & G. I. Luts, 1991. The ctenophore M. leidyi in the Sea of Azov. Oceanology 3: 722–725.
Uysal, Z. & E. Mutlu, 1993. Preliminary note on the occurrence and biometry of ctenophore Mnemiopsis leidyi finally invaded Mersin Bay. Turkish Journal Zoology 17: 229–239.
Valavanis, V. D., S. Georgakarakos, A. Kapantagakis, A. Palialexis & I. Katara, 2004. A GIS environmental modeling approach to Essential Fish Habitat designation. Ecological Modelling 178: 417–427.
Vinogradov, M. E., V. V. Sapozhnikov & E. A. Shushkina, 1992. The Black Sea ecosystem. Moscow. Russia. Nauka: 112.
Vinogradov, M. E., E. A. Shushkina, E. I. Musaeva & P. Y. Sorokin, 1989. Ctenophore M. leidyi (A. Agassiz) (Ctenophora: Lobata) - new settler in the Black Sea. Oceanology 29: 293–298.
Vlasenko, V. I., N. N. Stashchuk, V. A. Ivanov, E. G. Nikolaenko, O. Uslu & H. Benli, 1996. Influence of the water exchange through Dardanelles on the thermohaline structure of the Aegean Sea. Bulletin de l’Institut Oceanographique. Monaco 17: 147–165.
Volovik, S. P., I. A. Mirzoyan & G. S. Volovik, 1993. M. leidyi: biology, population dynamics, impact to the ecosystem and fisheries. ICES Biological Oceanography Committee 69: 1–11.
Wood, S. N., 2006. Generalized Additive Models. An Introduction with R. Chapman & Hall, New York.
Wood, S. N. & N. H. Augustin, 2002. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecological Modelling 157: 157–177.
Zervakis, V. & D. Georgopoulos, 2002. Hydrology and circulation in the Northern Aegean Sea throughout 1997 and 1998. Mediterranean Marine Science 3: 5–19.
Zervakis, V., D. Georgopoulos & P. G. Drakopoulos, 2000. The role of the North Aegean in triggering the recent Eastern Mediterranean climatic changes. Journal of Geophysical Research 105: 26103–26116.
Acknowledgements
The study was partially financed by the Commission of the European Union (EnviEFH-FP6-22466: Environmental Approach to Essential Fish Habitat Designation). Authors thank the captain and the crew of RV “Philia” for their assistance during the surveys and Alain F. Zuur (Highland Statistics, Ltd.) for his help and statistical advice regarding data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editor: V. D. Valavanis
Essential Fish Habitat Mapping in the Mediterranean
Rights and permissions
About this article
Cite this article
Siapatis, A., Giannoulaki, M., Valavanis, V.D. et al. Modelling potential habitat of the invasive ctenophore Mnemiopsis leidyi in Aegean Sea. Hydrobiologia 612, 281–295 (2008). https://doi.org/10.1007/s10750-008-9497-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9497-7