Abstract
In order to investigate trophic interactions, the diets of peacock bass (Cichla kelberi) and dogfish (Galeocharax knerii) were studied in the Corumbá Reservoir between 1997 and 2000. This dietary study was performed to assess the niche breadth of each species and to determine the degree of niche overlap during different phases of reservoir colonization. During Period I, peacock bass were absent or recorded only in low numbers; during Periods II and III, peacock bass reached high abundances in the reservoir. Interactions between the species were weak during period I, but, during Periods II and III, they were found to interact intensively. The diet overlap was highest during Period II. The niche breadth fluctuated for both species in the different phases. Greater niche breadth was observed for dogfish during periods of low peacock abundance (i.e., Period I), and the lowest niche breadth value was observed during Period II. During the same period, the peacock bass exhibited a wide foraging niche. During Period III, the dogfish showed an increase of its niche breadth, while for the peacock bass a simultaneous decrease in the niche breadth, caused by increasing rates of cannibalism, was recorded. These results show that the presence of peacock bass induces changes in the diet of dogfish, probably due to a restricted number of prey items.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduction of an alien species has been considered among the most challenging problems in the conservation of Neotropical fish biodiversity in recent decades, particularly in inland waters (Rodriguez, 2001; Agostinho et al., 2005a, b). Introduction of alien species is considered the second largest cause of species extinctions, exceeded only by habitat degradation (Fuller et al., 1999; Simberloff, 2003). The impacts of alien introductions have been widely studied in several aquatic ecosystems, including rivers (Scoppettone, 1993), reservoirs (Fontenele & Peixoto, 1979; Santos et al., 1994), lakes, and lagoons (Zaret & Paine, 1973; Hughes, 1986; Godinho et al., 1994; Latini & Petrere, 2004).
The peacock bass (Cichla kelberi Kullander & Ferreira, 2006), a native species to the Amazon basin, is widely distributed in bodies of water throughout Brazil because of its value in both commercial and sport fishing. Though exactly when and where this species was introduced to the upper Paraná Basin is unknown, however, the peacock bass is now common in the basin, and, together with several native Amazonian species, it successfully colonized several habitats (Agostinho et al., 2005b). Since the peacock bass is a piscivorous species with wide feeding spectrum (Novaes et al., 2004), its introduction has exerted negative impacts on native biodiversity in several habitats (Zaret & Paine, 1973; Godinho et al., 1994; Santos et al., 1994; Latini & Petrere, 2004).
Due to the complexity of species introduction, we still have an incomplete understanding of the variables involved in this process, and our ability to make predictions regarding the success and various impacts of colonization is limited (Agostinho et al., 2005a). In order to integrate into a new community, a species must overcome the demographic (number of propagules), biotic and abiotic restrictions, and must be able to interact with the native biota, adjusting its behavior and niche and promoting alterations in the local taxocenoses and in the environment, in such a way to guarantee long term survival (Vermeij, 1996).
Introductions are generally the most deleterious for biodiversity when the introduced species are piscivorous (Moyle & Cech, 1996) due to their voracity and high rates of successful colonization. Due to their prominence in sport fishing and relative large size, predator species are common in legal or illegal stocking programs. Previous studies of the impact of peacock bass introductions on the fish assemblages have focused on predatory interactions, but the fish must also integrate into the new assemblage and adjustment its behavior and niche to the native piscivorous species.
Although the peacock bass is now widely distributed in the upper Paraná Basin, a survey conducted in the region of the Corumbá Reservoir before and during the first 8 months of its operation showed that this species was absent. Despite the lack of concrete information about the origin of this species in the reservoir, one possible source is from flooded fish farms in the area. Prior to the introduction of the peacock bass, the dogfish Galeocharax knerii (Steindachner, 1875), a species native to the Paraná Basin, was the dominant piscivorous fish in this area. Following the first year of operation, the peacock bass became abundant, but it apparently did not affect dogfish abundance.
The peacock bass and dogfish share several common features in feeding ecology such as piscivorous habits and a wide feeding spectrum (Hahn et al., 1998; Luz-Agostinho et al., 2006). In addition, both occur in the surface layers of the littoral regions of this reservoir (R. Fugi, unpublished data). It may be expected that interactions between species with similar feeding habits will include competition, ultimately resulting in altered resource utilization (Bohn & Amundsen 2001). Although interactive segregation (Nilsson, 1967), in which one species limits use of resources by another (Edds et al., 2002), is often easily demonstrated in the field, proving that this behavior is a direct result of a species interaction is difficult (Nilsson, 1967). In this study, our aim was to investigate the feeding interaction between an introduced piscivorous (peacock bass) and the main native piscivorous (dogfish) during the colonization of the Corumbá Reservoir.
Materials and methods
Study area
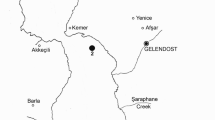
The Corumbá River (Góias State - Brazil) is the main tributary at the right bank of the Paranaíba River that, together with the Grande River, derives the Paraná River. Its drainage basin has an area of 34,000 km2, consisting predominantly of scrubland in the Cerrado Biome. It is an upland river, and the largest part of its course is narrow, with a rocky substrate and steep surrounding riverbanks (Paiva, 1982). The Corumbá River was dammed in September 1996, forming the Corumbá Hydroelectric Reservoir (Fig. 1). The Corumbá Reservoir has a surface area of 65 km2, a total volume of 1,500 × 106m3, an average depth of 23 m, and a hydraulic retention time of 40 days (Bonecker & Aoyagui, 2005).
Sampling and data analyses
Fish were sampled using gillnets with a wide range of mesh sizes (mesh size between 2.4 and 16.0 cm opposite knots) left in open and littoral areas for 24 h (checked at 8:00, 16:00, and 22:00). Sampling was conducted monthly during a four-year study period from March 1996 to August 1996 (river phase), September 1996 to February 1997 (filling phase), and March 1997 to February 2000 (operation phase) at four sampling stations.
The abundance of G. knerii and C. kelberi was expressed by capture per unit of effort (CPUE; individuals per 1,000 square meter of gillnets exposed during 24 h total sampling time). Immediately after capture, all fish were identified and measured (standard length—SL) and a numerical scale was used to quantify the degree of stomach’s fullness in C. kelberi and G. knerii: 0 = empty stomach, 1 = 0.25 full, 2 = 0.5–0.75 full, and 3 = completely full. Stomachs classified as 2 or 3, representative of all seasons and periods, were preserved in 4% formalin for diet analyses. A total of 5,390 stomachs of G. knerii were examined, from which only 20% were classified as degree 2 or 3. Of the 713 stomachs of C. kelberi, 30% were classified as either degree 2 or 3.
Diet was assessed only in the operation phase (March 1997 to February 2000). During this phase, three periods were distinct: Period I (March 1997 to January 1998), when peacock bass were absent or recorded in very low abundances, Period II (February 1998 to February 1999), and Period III (March 1999 to February 2000), when peacock bass reached high abundances in the reservoir. Interactions between the two species were weak during Period I, but, during Periods II and III, the species interacted intensively. The stomach samples were taken from individuals larger than 12 cm standard length, the size at which both dogfish and peacock consume only fish, and a total of 830 stomachs were analyzed for content (714 to dog-fish and 116 peacock bass). Of that sample, however, 199 of them (191 dog-fish and 8 peacock bass) presented highly digested prey and were not considered in the diet analysis. Thus, the diet analysis for G. knerii was based on 136 (13.0 to 22.6 cm SL) stomach contents in Period I, 179 in Period II (12.1–24.2 cm SL), and 108 in Period III (12.1–26.0 cm SL). Likewise, the analysis for C. kelberi consisted of 75 in Period II (12.2–38.0 cm SL) and 33 in Period III (12.6–26.8 cm SL). Thus, in addition to covering different seasons, we analyzed stomachs for both species representing a wide range of sizes.
Diet was assessed by the volumetric method (Hyslop, 1980), which expresses the relationship between the volume of a given species of prey and the total volume of all the prey species as a percentage. The volume of each prey was obtained using graduated test tubes (obtained by water displacement). Since no relevant change in the main food species was identified, data were pooled according period.
Diet overlap between the peacock bass and the dogfish was determined by the Pianka index (see Pianka, 1974; Gotelli & Entsminger, 2001). Overlap values range from 0 (no overlap) to 1 (complete overlap). The significance of the overlap was tested using randomization procedures in ECOSIM 7.0 (Gotelli & Entsminger, 2001).
The foraging niche breadth was calculated using the Levins index (Krebs, 1998), which assumes that the diet breadth can be estimated by the measurement of the uniformity of the distribution of the items among the several feeding resources. Breadth values range from 1.0 to n, with larger values representing a wider breadth of resource exploitation. The feeding items were also arranged according to their degree of importance (“rank-abundance curves”) to better simultaneously assess the prey richness and equitability.
Results
Abundance
Before the impoundment of the Corumbá Reservoir, the dogfish was one of the most abundant species in the area, and it became even more abundant after the reservoir’s formation (Fig. 2). On the other hand, the peacock bass was not sampled during the river and filling phases of collection. This species was first caught during the first year of reservoir operation (Period I), and it became abundant during Periods II and III (Fig. 2). There was considerable monthly fluctuation in peacock bass abundance, and a low CPUE was recorded during the winter periods (May to August).
Diet
During periods of low peacock bass abundance, the diet of dogfish (Period I) was composed of 15 fish prey species, with Pimelodus maculatus as the most important prey, representing about 40% of the diet (Fig. 3A). Astyanax altiparanae (16.2%) and Gymnotus carapo (14.1%) were also important dogfish food sources during this period.
In the second year of reservoir operation (Period II), when dogfish and peacock bass were in sympatry, the dogfish diet changed markedly from the period before peacock bass invasion (Period I) (Fig. 3B). During this period, ten species of fish were recorded in dogfish diet, with a predominance of P. maculatus, representing approximately 60% of the ingested food (by volume). Together with A. altiparanae, these two species represented 83% of the ingested food. Contrasting with the dogfish, the peacock bass consumed 13 fish species and did not show any particular dominance of species in its diet. In order of importance the preys most consumed were G. knerii (19.7%), Cichlasoma paranaense (17.9%), A. altiparanae (15.6%), C. kelberi (13.7%), and P. maculatus (12.3%). The overlap between the peacock bass and dogfish was relatively high (0.52), although it was not statistically significant at P = 0.05 level (P (observed ≥ expected) = 0.09).
During the third year of the reservoir’s operation (Period III), consistent with the previous years, P. maculatus dominated the dogfish diet (44.0%), along with A. altiparanae (19.2%) and C. kelberi (10.8%) (Fig. 3C). Also during this period, Apteronotus brasilienses and Pimelodus fur, which were not previously consumed, together composed approximately 20% of the total diet. The peacock bass diet changed drastically during this period, and it showed a high degree of cannibalism (41% of the diet; n = 33 stomachs). Another obvious change in the diet of peacock bass was the complete absence of G. knerii, the main prey in the previous period, and of P. maculatus. Furthermore, Apareiodon began to be consumed at high levels (21.1%) along with P. fur (12.3%), which was not recorded previously in the diet of the peacock bass. Astyanax altiparanae remained an important prey for peacock bass (15.8%). The diet overlap between the species was low (0.34) in this phase (P (observed ≥ expected) = 0.42).
Niche breadth fluctuated for both species during the different periods (Fig. 4). Greater niche breadth for dogfish was observed during period of low peacock bass abundance (Period I) (B = 4.7), when this species showed the greatest alimentary spectrum and the smallest dominance (Fig. 4A). An inverse tendency was observed in the first year in which both species coexisted (period II), when the dogfish niche breadth decreased (B = 2.4), with the lower number of prey species and with high dominance in the diet (Fig. 4B). During this period, however, the peacock bass exhibited a wide foraging niche (B = 7.3) with a high equitability and low dominance. In Period III, the dogfish showed an increase of its niche breadth (B = 3.8) and a decreased dominance of prey species, though the alimentary spectrum remained low (Fig. 4C). For peacock bass, an inverse tendency was recorded, with a clear decrease in the niche breadth (B = 3.9) and a high dominance, caused by widespread cannibalism (41% of prey were other peacock bass), and reduction in the number of prey species.
Discussion
Information about the exact time when a population was subjected to a new set of circumstances is a great advantage for the study of interactions, and the best example is the deliberate introduction of new species (Nilsson, 1967). During the colonization of the Corumbá Reservoir by fish fauna, the expansion of the peacock bass population in a community dominated by another piscivore (dogfish) provided an excellent opportunity to assess the effects of this introduced species on the previously dominant predator.
During periods of low abundance of peacock bass (Period I), the dogfish diet encompassed several prey species, and the niche breadth was large, indicating that this species behaved as a generalist piscivore. The consumption of a large number of prey species is common throughout piscivorous species (Almeida et al., 1997; L´Abeé-Lund et al., 2002; Kahilainen & Lehtonen, 2003; Hahn et al., 2004). Large populations of several species of fish (including P. maculatus, A. altiparanae, and A. fasciatus—R. Fugi unpublished data) potentially preyed upon by dogfish in the beginning of the Corumbá Reservoir formation, and the fact that dogfish was the only abundant piscivore during this phase enabled the dogfish to consume a wide variety of prey species. It contributed to the success of this species in the beginning of the reservoir formation. Since the dogfish was the main top predator during this period, this species may have been able to forage a large area of the reservoir. This may parsimoniously explain the success of the dogfish soon after the reservoir’s formation.
During Period II, the peacock bass was already present in high numbers, and, in some months, it had become even more abundant than the dogfish. Despite the large seasonal variation in bass captures—possibly because the low movements of this species during winter months, preventing gillnet capture during this period—the results show that the bass successfully colonized the reservoir in less than 1 year. The feeding plasticity of peacock bass was likely the primary reason for this species’ successful colonization of another Center-western Brazilian reservoir (Serra da Mesa Reservoir, Novaes et al., 2004). Parental care behavior increases the competitiveness of peacock bass (Latini & Petrere, 2004), and, along with feeding plasticity could be important for successful colonization of lentic habitat. The lentic characteristics of reservoirs cause considerable water transparency and facilitate predation (Agostinho et al., 2002; Thys & Hoffmann, 2005) by diurnal piscivores like the peacock bass (Novaes et al., 2004). Furthermore, abundant prey during the colonization phase (Agostinho et al., 1999) may also aid in the success of this species.
When peacock bass were first observed in great abundance (Period II), predation between the species was common. During this period, the dogfish was the main prey item of peacock bass, and the peacock bass was the third most common prey item of dogfish. There was a relatively large overlap in diet between these two species (0.52), showing the consumption of common resources, mainly P. maculatus and A. altiparanae. Although it was not statistically significant at the 5% level, notice that it was at P = 0.09. The dogfish diet changed immediately following the introduction of the peacock bass. Compared with the pre-invasion period, it began exploiting a smaller number of prey species, concentrating its diet upon the two most important species consumed previously (P. maculatus and A. altiparanae). The dogfish niche breadth decreased markedly during coexistence. The peacock bass diet, however, comprised several prey species, and its niche breadth was large.
Despite the difficulties in showing competition between species in a natural system, our data indicate an intense interaction between these two species. The most important change in the dogfish diet when coexisting with peacock bass was not the change of the main food resource (P. maculatus and A. altiparanae, which were also the main items during this period) but the reduction in the number of prey (from 15 to 10 prey species) and the high dominance of P. maculatus (from 40% to 60%). These results suggest that the interaction with peacock bass limited the number of prey species consumed by dogfish. The increased predation of peacock bass upon dogfish during the first year in which the species coexisted was probably the main cause of the change in dogfish diet. The lower number of prey species consumed by the dogfish may have been caused directly by the consequent reduction of foraging areas, avoiding habitats used by peacock bass, which decreased the risk of predation. This hypothesis is supported by the observation that dogfish consumed during this period mainly P. maculatus, a species that, in contrast to the peacock bass, prefers low light conditions (Dei Tos et al., 2002) found in deep waters. In fact, the competition between similar species may limit the use of habitats (Edds et al., 2002), which is reflected in their diets. Therefore, when the best habitat for foraging is also the most dangerous (i.e., when it contains a predator), prey must balance between the energy gain of feeding in a dangerous area and the risk of being eaten in that area (Lima & Dill, 1990). For example, the blue catfish might limit the habitat use of channel catfish through resource exploitation or by some mode of aggressive interference (Edds et al., 2002).
In the second year of coexistence (Period III), the dogfish diet was similar to that of the first year (Period II); however, the breadth of the niche increased. In contrast, the diet of peacock bass changed drastically, and this species showed a high degree of cannibalism (40%) as it no longer fed upon its main prey (the dogfish). Cannibalism has been recorded for many fish species, (Specziár & Biro, 2003; Katunzi et al., 2006) including species of Cichla (Jepsen et al., 1997; Santos et al., 2001; Gomiero & Braga, 2004), and it is often induced by decreasing prey availability (Gomiero & Braga, 2004). Such was probably not the case in the Corumbá Reservoir, where prey availability is high (and includes several species of Astyanax), which, according to Zaret (1977), should prevent cannibalism. In addition, its recent introduction into the Corumbá Reservoir would not allow a rapid decrease of the fish-prey populations. In Serra da Mesa Reservoir (Brazil), cannibalism was also observed for peacock bass during the initial stages of the colonization, but it represented only 8% of the total diet (Novaes et al., 2004). High levels of cannibalism were recorded in Lages Reservoir (Southeast Brazil), but the peacock bass had been introduced for ca. 50 years in this environment, and the cannibalism was probably a response to the low availability of native prey due to predation by peacock bass (Santos et al., 2004). The lack of macrophytes in the Corumbá Reservoir may explain the high cannibalism rates immediately following the colonization by the peacock bass since this species use the littoral vegetation as refuge until they reach 18–20 cm, and the lack of refuge may enable a high degree of predation (Santos et al., 2001; Gomiero & Braga, 2004), including predation by its own species.
In summary, our results show that the native piscivore diet changed in the presence of peacock bass probably due to a restricted number of prey items. This finding is consistent with the interactive segregation hypothesis (Nilsson, 1967). According to this hypothesis, alterations in resource utilization may be due to interspecific interactions, in this case, competition and predation. Although manipulated experiments are necessary to directly test this hypothesis, it is probable that changes we recorded in the foraging niche of the native species can be largely attributed to predation in the Corumbá Reservoir. This conclusion is supported by the fact that, during the second year of co-existence between the two predators, when elevated cannibalism was evident and peacock bass did not prey upon the dogfish, the foraging niche breadth of the native species increased, and the overlap in resources between the two species decreased.
References
Agostinho, A. A., L. C. Gomes, D. R. Fernandes & H. I. Suzuki, 2002. Efficiency of fish ladders for Neotropical ichthyofauna. River Research and Application 18: 299–306.
Agostinho, A. A., L. E. Miranda, L. M. Bini, L. C. Gomes, S. M. Thomaz & H. I. Suzuki, 1999. Patterns of colonization in neotropical reservoirs, prognoses on aging. In Tundisi J. G. & M. S. Straskraba (eds), Theoretical Reservoir Ecology and its application. IIE—International Institute of Ecology, São Carlos: 227–265.
Agostinho, A. A., F. M. Pelicice & H. F. Julio Jr, 2005a. Introdução de espécies de peixes em águas continentais brasileiras: uma síntese. In Rocha, O., E. L. G. Espindola, N. Fenerich-Verani, J. R. Verani & A. C. Rietzler (eds), Espécies Invasoras de Águas Doces: estudo de caso e propostas de manejo. Editora da Universidade Federal de São Carlos, São Carlos: 13–23.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2005b. Conservation of the biodiversity of Brazil’s inland waters. Conservation Biology 19: 646–652.
Almeida, V. L. L., N. S. Hahn & A. E. A. M. Vazzoler, 1997. Feeding patterns in five predatory fishes of the high Paraná river floodplain (PR, Brazil). Ecology Freshwater Fishes 6: 123–133.
Bohn, T. & P. Amundsen, 2001. The competitive edge of an invading specialist. Ecology 82: 2150–2163.
Bonecker, C. C. & A. S. M. Aoyagui, 2005. Relationships between rotifers, phytoplankton and bacterioplankton in the Corumbá reservoir, Góias State, Brazil. Hydrobiologia 546: 415–421.
Dei Tos, C., G. Barbieri, A. A. Agostinho, L. C. Gomes & H. I. Suzuki, 2002. Ecology of Pimelodus maculatus (Siluriformes) in the Corumbá Reservoir, Brazil. Cybium 26: 275–282.
Edds, D. R., W. J. Mattheus & F. P. Gelwick, 2002. Resource use by large catfishes in a reservoir: is there evidence for interactive segregation and innate differences? Journal of Fish Biology 60: 739–750.
Fontenele, O. & J. T. Peixoto, 1979. Apreciação sobre os resultados da introdução do tucunaré comum Cichla ocellaris (Bloch & Schneider, 1801), nos açudes do Nordeste Brasileiro, através da pesca comercial. Boletim Técnico do Departamento Nacional de Obras Contra Seca 37: 109–134.
Fuller, P. L., L. G. Nico & J. D. Williams, 1999. Nonindigenous Fishes Introduced into Inland Waters of the United States. American Fisheries Society, Bethesda, Maryland.
Godinho, A. L., M. T. Fonseca & L. M. Araújo, 1994. The ecology of predator fish introduction: the case of Rio Doce Valley lakes. In Pinto-Coelho, R. M., A. Giani & E. Sperling (eds), Ecology and Human Impact on Lakes and Reservoirs in Minas Gerais with Special Reference to Future Development Strategies. Segrac, Belo Horizonte: 77–83.
Gomiero, L. M. & F. M. S. Braga, 2004. Cannibalism as the main feeding behaviour of tucunares introduced in Southeast Brazil. Brazilian Journal of Biology 64: 625–632.
Gotelli, N. J. & Entsminger, G. L. 2001. ECOSIM: null models software for ecology (version 7.0).
Hahn, N. S., A. A. Agostinho, L. C. Gomes & L. M Bini, 1998. Estrutura trófica da ictiofauna do reservatório de Itaipu (Paraná-Brasil) nos primeiros anos de sua formação. Interciencia 23, 299–305.
Hahn, N. S., R. Fugi & I. F. Andrian, 2004. Trophic ecology of the fish assemblages. In Thomaz S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 247–259.
Hughes, N. F., 1986. Changes in the feeding biology of Nile perch Lates niloticus (L) (Pisces: Centropomidae) in Lake Victoria East Africa, since its introduction in 1960, and its impact on the native fish community of the Nyanza Gulf. Journal of Fish Biology 29: 541–548.
Hyslop, E. J., 1980. Stomach contents analysis, a review of methods and their application. Journal of Fish Biology 17: 411–429.
Jepsen, D. B., K. O. Winemiller & D. C. Taphorn, 1997. Temporal patterns of resource partitioning among Cichla species in a Venezuelan blackwater river. Journal of Fish Biology 51: 1085–1108.
Kahilainen, K. & H. Lehtonen, 2003. Piscivory and prey selection of four predator species in a whitefish dominated subarctic lake. Journal of Fish Biology 63: 659–672.
Katunzi, E. F. B., W. L. T. Densen, J. H. Wanink & F. Witte, 2006. Spatial and seasonal patterns in the feeding habits of juvenile Lates niloticus (L.), in the Mwanza Gulf of Lake Voctoria. Hydrobiologia 568: 121–133.
Krebs, C. J., 1998. Ecological Methodology, 2nd ed. Menlo Park: Benjamin/Cummings.
L’Abeé-Lund, J. H., P. Aass & H. Saegrov, 2002. Long-term variation in piscivory in a brown trout population: effects of changes in available prey organisms. Ecology of Freshwater Fishes 11: 260–269.
Latini, A. O. & M. Petrere, 2004. Reduction of a native fish fauna by alien species: an example from Brazilian freshwater tropical lakes. Fisheries Management and Ecology 11: 71–79.
Lima, S. L. & L. M. Dill, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68: 619–640.
Luz-Agostinho, K. D., L. M. Bini, R. Fugi, A. A. Agostinho & H. F. Julio, 2006. Food spectrum and trophic structure of the ichthyofauna of Corumbá reservoir, Paraná river Basin, Brazil. Neotropical Ichthyology 4: 61–68.
Moyle, P. B. & J. J. Cech, 1996. Fishes: An Introduction to Ichthyology. Englewood Cliffs: Printice Hall.
Nilsson, N., 1967. Interactive segregation between fish species. In Gerking, S. D. (ed.), The Biological Basis of Freshwater Fish Production. Blackwell Scientific, Oxford: 295–313.
Novaes, J. L. C., E. P. Caramaschi & K. O. Winemiller, 2004. Feeding of Cichla monoculus Spix, 1829 (Teleostei: Cichlidae) during and after reservoir formation in the Tocantins River. Acta Limnologica Brasiliensia 16: 41–49.
Paiva, M. P., 1982. Grandes Represas do Brasil. Editerra, Brasília.
Pianka, E. R., 1974. Niche overlap and diffuse competition. Proceedings of the National Academy of Sciences U.S.A. 71: 2141–2145.
Rodriguez, J. P., 2001. Exotic species introductions into South America: an understimated threat? Biodiversity and Conservation 10: 1983–1996.
Santos, L. N., A. F. Gonzáles & F. G. Araújo, 2001. Dieta do tucunaré-amarelo Cichla monoculus (Bloch & Schneider) (Osteichthyes, Cichlidae), no reservatório de Lajes, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia 18: 191–204.
Santos, G. B., P. M. Maia Barbosa, F. Vieira & C. M. Lopes, 1994. Fish and zooplankton community structure in reservoirs of southeastern Brazil: effects of the introduction of exotic predatory fish. In Pinto-Coelho, R. M., A. Giani & E. Sperling (eds), Ecology and Human Impact on Lakes and Reservoirs in Minas Gerais. Segrac, Belo Horizonte: 115–132.
Santos, A. F. G. N., L. N. Santos, C. C. Andrade, R. N. Santos & F. G. Araújo, 2004. Alimentação de duas espécies carnívoras no reservatório de Lajes. Revista da Universidade Rural 24: 161–168.
Scoppettone, G. G., 1993. Interaction between native and non-native fishes of the upper Muddy River, Nevada. Transaction of the American Fisheries Society 122: 599–608.
Simberloff, D., 2003. Confronting introduced species: a form of xenophobia? Biological Invasions 5: 179–192.
Specziar, A. & P. Biro, 2003. Population structure and feeding characteristics of Volga pikeperch, Sander volgensis (Pisces, Percidae), in a Lake Balaton. Hydrobiologia 506: 503–510.
Thys, I. & L. Hoffmann, 2005. Diverse responses of planktonic crustaceans to fish predation by shifts in depth selection and size at maturity. Hydrobiologia 551: 87–98.
Vermeij, G. J., 1996. An agend for invasion biology. Biological Conservation 78: 3–9.
Zaret, T. M., 1977. Inhibition of cannibalism in Cichla ocellaris and hypothesis of predator mimicry among South Americam fishes. Evolution 31: 421–437.
Zaret, T. M. & R. T. Paine, 1973. Species introduction in a tropical lakes. Science 182: 449–455.
Acknowledgments
We are grateful to Nupélia/UEM and Furnas Centrais Elétricas for supporting this study. We wish to thank Dr. Sidinei M. Thomaz (Universidade Estadual de Maringá) for the manuscript review and suggestions, and CNPq for the grants. We thank Amy Shaw (Mississippi State University) for reviewing the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. Martens
Rights and permissions
About this article
Cite this article
Fugi, R., Luz-Agostinho, K.D.G. & Agostinho, A.A. Trophic interaction between an introduced (peacock bass) and a native (dogfish) piscivorous fish in a Neotropical impounded river. Hydrobiologia 607, 143–150 (2008). https://doi.org/10.1007/s10750-008-9384-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9384-2