Abstract
As a result of increased anthropogenic nitrogen (N) loading in surface waters of agricultural watersheds, there is enhanced interest to understand and quantify N removal mechanisms. Denitrification, an important N removal mechanism in aquatic systems, may contribute to reducing N pollution in agricultural headwater streams. However, the key factors controlling this process in lotic systems remain unclear. The objective of our study was to examine the factors regulating rates of denitrification in the sediments of agricultural headwater streams in the mid-western USA. Denitrification rates were variable among streams and treatments (<0.1–28.0 μg N g AFDM−1 h−1) and on average, were higher than those reported for similar headwater streams. Carbon quantity and quality, and pH had no effect on denitrification, while temperature and nitrate ( \( {\text{NO}}^{ - }_{{\text{3}}} \)) concentrations had a positive effect on rates of denitrification. Specifically, \( {\text{NO}}^{ - }_{{\text{3}}} \) controlled denitrification following Michaelis-Menten kinetics. We calculated a value of km (1.0 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N L-1) that was comparable to other studies in aquatic sediments but was well below the median in-stream \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations (5.2–17.4 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N L−1) observed at the study sites. Despite high rates of denitrification, this removal mechanism is most likely \( {\text{NO}}^{ - }_{{\text{3}}} \) saturated in the agricultural headwater streams we examined, suggesting that these systems are not effective at removing in-stream N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the mid-western USA, fertilizer applications, particularly nitrogen (N) and phosphorus (P), have led to the eutrophication of surface waters and coastal hypoxia (Carpenter et al., 1998; Burkhart & James, 1999; Rabalais et al., 2002). Eutrophication has been linked to noxious algal blooms (Burkhart & James, 1999), cases of infant methemoglobinemia and non-Hodgkin’s lymphoma in humans (Ward et al., 1996), and loss of biodiversity (Galloway & Cowling, 2002). Agricultural states such as Iowa, Illinois, Indiana and Ohio are the dominant states contributing to the net export of nitrate (\( {\text{NO}}^{ - }_{{\text{3}}} \)) to the Mississippi River Basin, and this nutrient is a key contributor to the hypoxic zone in the Gulf of Mexico (Goolsby et al., 1999).
Due to concerns over hypoxia, attention in the scientific community has been focused on understanding and quantifying N removal mechanisms (Mitsch et al., 2001). In aquatic systems, denitrification is the dominant N removal mechanism, permanently removing \( {\text{NO}}^{ - }_{{\text{3}}} \) from a system via N2. Denitrification is a chemoheterotrophic reaction, mediated by facultative bacteria when dissolved oxygen is less than 0.5 mg l−1 (Piña-Ochoa & Álvarez-Cobelas, 2006). Levels of \( {\text{NO}}^{ - }_{{\text{3}}} \), organic carbon (C), and oxygen, and temperature, pH, and specific enzyme inhibitors have the ability to directly influence this microbial-mediated process (Knowles, 1982). Other factors have indirect effects on denitrification; for example, aquatic vegetation alters the availability of organic C and oxygen, and nitrification impacts the availability of \( {\text{NO}}^{ - }_{{\text{3}}} \) (Risgaard-Petersen & Jensen, 1997; Eriksson & Weisner, 1999; Risgaard-Petersen, 2003). However, the dominant factors that regulate denitrification rates in aquatic sediments, specifically in lotic ecosystems, are unclear (Boyer et al., 2006).
Headwater streams are thought to remove more N via denitrification than higher order streams and rivers (Alexander et al., 2000; Peterson et al., 2001). Studies have identified denitrification in various headwater streams as being pH inhibited (Baeseman et al., 2006), organic C limited (Pfenning & McMahon, 1996), \( {\text{NO}}^{ - }_{{\text{3}}} \) limited (Martin et al., 2001; Royer et al., 2004), and \( {\text{NO}}^{ - }_{{\text{3}}} \) saturated (Bernot et al., 2006). These were either field-based studies or limited laboratory experiments designed to test one or two factors. In order to provide a full understanding of what regulates denitrification in headwater streams, controlled laboratory studies should manipulate several factors. Also, confusion regarding co-limitations must be avoided by manipulating one factor at a time.
The objective of this study was to identify the factors that regulate rates of denitrification in the sediments of agricultural headwater streams. Identifying factors that limit denitrification will facilitate the implementation of management actions that maximize N removal in headwater streams. To achieve our objective, sediments from three study streams located within the same agricultural watershed were collected, returned to the laboratory, and underwent various treatments. We hypothesized that denitrification rates would respond positively to increased temperature, \( {\text{NO}}^{ - }_{{\text{3}}} \), glucose, and humic acid. However, we expected denitrification rates to be higher in presence of a more labile C source, glucose, than in presence of a more recalcitrant source of C, humic acid. Finally, we hypothesized that denitrification rates would be maximized at neutral pH.
Methods
Study sites
Three 50-m reaches were selected on primary headwater streams in the Upper Sugar Creek Watershed in Northeast Ohio, USA (40°51′42″ N, 81°50′29″ W; Fig. 1). Over 85% of the land-use in the Upper Sugar Creek Watershed is agriculture, and most is heavily tile drained (Ohio EPA, 2002). Even if the watershed of each site is dominated by agriculture (Table 1), land use immediately adjacent to each reach varies. Adjacent to Orr Run Creek is a road on one side and row crop agriculture on the other. The riparian zone of Smith Creek is residential lawn, fed upstream by agricultural fields. Potato Creek is located within a small woodlot, which is surrounded by row crop agriculture and potato farms. All three sites are channelized, have no sinuosity, and little slope (<2%), and their soil parent material is dominated by glacial till. Sediment type is highly variable among streams (Table 1). The sites were selected as they represent the variability of headwater streams in the watershed. The reaches were periodically monitored for discharge, water chemistry, and sediment characteristics (Table 1). Stream velocity was measured using a SonTek Acoustic Doppler Velocimeter and discharge was measured using the USGS standard six-tenths method (Buchanan & Somers, 1969). At all sites, baseflow discharge was low (<21 l s−1), and \( {\text{NO}}^{ - }_{{\text{3}}} \) was the dominant inorganic N species (Table 1). Concentrations of \( {\text{NO}}^{ - }_{{\text{3}}} \) increased at Orr Run Creek and at Smith Creek as discharge increased, while concentrations remained elevated at Potato Creek regardless of changes in stream flow.
Denitrification assays
Sediments from the stream reaches were collected in late January—early February 2006. At each reach, the top 3–5 cm of sediment was collected at eight random locations along the 50-m reach and homogenized, providing one sample per stream. The sediments were stored at 4°C until processed in the laboratory. Prior to denitrification assays, sediments were covered with deionized water and incubated in the dark at room temperature (22–25°C) for 48 h. This time period allowed the microbial populations to acclimate to warm temperatures and become active. After 48 h, sediment slurries were created using deionized water. Approximately 25 mL of slurry was added to each 150 ml borosilicate glass media bottle.
Denitrification was measured using the acetylene inhibition method (Knowles, 1990). Prior to sediment addition, chloramphenicol was added to a final concentration of 5 mM. The use of chloramphenicol reduces bottle effects by eliminating enzyme growth and allows denitrification to be measured over short incubation periods (Bernot et al., 2003). Deionized water was added to each bottle with a corresponding amendment to reach a final volume of 75 ml. Using deionized water as compared to stream water allowed us to calculate the exact amount of substrate each media bottle received and easily manipulate pH.
Oxygen was purged from each bottle by continuously pumping helium at 35 ml min−1 for 5 min and shaking the bottles every 30 s. About 15 ml of acetylene was added to reach a final concentration of 10%, and bottles were placed in a Torrey Pines Scientific temperature controlled benchtop incubator at a set temperature in the dark. After 20 min of equilibration time, 5 ml of gas sample was taken from each bottle every hour for 3 h. Prior to sampling, bottles were shaken and given 3 min to equilibrate. Immediately after sampling, 5 ml of replacement gas (10 parts acetylene/90 parts helium) was added.
Treatment experiments
The following parameters were manipulated to determine their effect on denitrification: \( {\text{NO}}^{ - }_{{\text{3}}} \), temperature, pH, and C quantity and quality. The response of denitrification was tested at four different concentrations of \( {\text{NO}}^{ - }_{{\text{3}}} \) (0, 2, 10, and 20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1); all treatments received non-limiting concentrations of C (20 mg glucose-C l−1) and were incubated at 25°C. The effect of temperature on denitrification was tested at high (25°C) and low (8°C) temperatures on slurries receiving non-limiting concentrations of \( {\text{NO}}^{ - }_{{\text{3}}} \) (20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1) and C (20 mg glucose-C l−1). The effect of pH on denitrification was tested at low (5.5), neutral (7.5), and high (9.5) pH values, on slurries receiving non-limiting concentrations of \( {\text{NO}}^{ - }_{{\text{3}}} \) (20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1) and C (20 mg glucose-C l−1). Either H2SO4 or NaOH was used to adjust pH, following the enrichments with \( {\text{NO}}^{ - }_{{\text{3}}} \) and C. The effect of two C substrates (glucose and humic acid) on denitrification was tested at four different concentrations (0, 2, 10, and 20 mg C l−1); all treatments received non-limiting concentrations of \( {\text{NO}}^{ - }_{{\text{3}}} \) (20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1) and were incubated at 25°C. From the homogenized sediments, four pseudo-replicates were used to determine a mean denitrification rate at each site. This pseudo-replication was conducted, as recommended by Tiedje (1982), to account for the variability of the denitrification process. However, the level of replication is the number of study sites (n = 3).
Sample analysis
Gas samples were placed in pre-evacuated vials and stored at 4°C for no longer than seven days until analysis. Samples were analyzed for N2O on a Shimadzu Gas Chromatograph (Alltech Poropak-Q column 80/100 mesh 12′ × 1/8″ × 0.085; Alltech Poropak-Q column 80/100 mesh 12′ × 1/8″ × 0.085″ SS; 40°C; carrier gas: 95% argon/5% methane; flow rate: 35 ml min−1) equipped with an ECD detector and a 1 ml sample loop.
Water samples were analyzed for N species on a Lachat QuikChem 8500 Autoanalzyer and dissolved organic C (DOC) on a Dorhmann-Rosemont Carbon Analyzer. Samples analyzed for dissolved constituents were filtered through 0.45 μm membrane filters and preserved with H2SO4. To determine total N, unfiltered samples were digested using an autoclave following the persulfate method (AHPA, 1998).
After gas analysis, the sediment from each assay bottle was dried at 60°C for 24 h, weighed, combusted at 550°C for 6 h, and reweighed to determine ash free dry mass (AFDM). Total C and N were measured in sediments dried at 60°C for 24 h with a CE Instruments CHN Analyzer. After sediments were dispersed in sodium hexametaphosphate, size distribution was determined by mechanical wet sieving.
Statistics
ANOVA was used to determine significance in the different treatment experiments using Systat 11.0. The data met the assumption of the analysis (normality and homoscedasticity). The effect of the different factors on denitrification rates was tested as follows: effect of \( {\text{NO}}^{ - }_{{\text{3}}} \) concentration (one-way ANOVA; 0, 2, 10, and 20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1; df = 3), effect of temperature (one-way ANOVA; 25°C and 8°C; df = 1), effect of pH (one-way ANOVA; pH of 5.5, 7.5, and 9.5; df = 2), and effect of C concentration and C quality (two-way ANOVA; glucose and humic acid; 0, 2, 10, and 20 mg C l−1; effect of substrate quality df = 1; effect of concentration df = 3; effect of substrate quality*concentration df = 3). When appropriate, post hoc analysis was done using pairwise Tukey tests. Denitrification rates in the \( {\text{NO}}^{ - }_{{\text{3}}} \) enrichment experiment were fit to Michaelis-Menten type kinetics using a nonlinear regression function on Sigma Plot 8.0 with the following equation:
The two parameters in the Michaelis-Menten equation describe how denitrification responds logarithmically to increasing \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations until reaching saturation. The first parameter, Vmax, is the maximum denitrification rate achieved and the second, km, is the \( {\text{NO}}^{ - }_{{\text{3}}} \) concentration at which the denitrification rate is one-half Vmax. Lower km values are indicative of denitrifiers that become saturated at lower \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations. Since denitrification rates are expressed in units that are not easily converted between each other (e.g., expressed in a dry mass basis, an AFDM basis, or an aerial basis), Vmax is difficult to compare among studies. Conversely, km is almost always expressed in units of aqueous concentration making it relatively easy to compare.
Results
Nitrate amendments
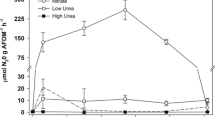
Significant differences were detected in the \( {\text{NO}}^{ - }_{{\text{3}}} \) enrichment experiment (F3,11 = 6.190; P = 0.018), with the control (0.1 ± 0.1 μg N g AFDM−1 h−1; all data henceforth will be mean ± standard deviation unless otherwise indicated) having a lower denitrification rate than the treatments receiving 10 and 20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1 but similar to the treatment receiving 2 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1 (Tukey’s P < 0.05). No differences were detected in the treatments receiving enrichments of 2, 10, and 20 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1, for which denitrification rates were 7.4 ± 1.3, 10.7 ± 5.4, and 10.5 ± 4.2 μg N g AFDM−1 h−1, respectively. The response of denitrification rates to increasing \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations followed Michaelis-Menten kinetics (Fig. 2). Fitting Michaelis-Menten type kinetics to the data produced a significant, positive correlation (P < 0.001, r2 = 0.70). On the basis of regression line, Vmax was estimated to be 11.4 μg N g AFDM−1 h−1 and km was estimated to be 1.0 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1.
Denitrification rates from the sites under varying nitrate concentrations. Significant differences were detected in the denitrification rates (F3,11 = 6.190; P = 0.018). Treatments with the same letter are not significantly different (Tukey’s P < 0.05). Regression analysis was conducted using Michaelis-Menten type kinetics. Each treatment received a glucose amendment (20 mg glucose-C l−1), and all incubations were done at 25°C
Temperature and pH
Denitrification rates were twice as high (F1,5 = 11.208; P < 0.029) at 25°C (22.1 ± 4.7 μg N g AFDM−1 h−1) compared to those at 8°C (11.2 ± 3.1 μg N g AFDM−1 h−1). Denitrification rates were similar at all three pH levels tested. At pH values of 5.5, 7.5, and 9.5, the denitrification rates were 21.7 ± 4.6, 22.1 ± 4.7, and 23.1 ± 5.5 μg N g AFDM−1 h−1, respectively.
Carbon amendments
Carbon quality and C concentration had no effect on denitrification rates. Denitrification rates in the control averaged 11.6 ± 5.8 μg N g AFDM−1 h−1. The glucose treatments had denitrification rates of 11.0 ± 4.2, 13.3 ± 5.7, and 10.8 ± 4.3 μg N g AFDM−1 h−1 for 2, 10, and 20 mg glucose-C l−1, respectively. In the humic acid treatments, the denitrification rates were 10.6 ± 4.6, 10.4 ± 5.4, and 10.9 ± 4.8 μg N g AFDM−1 h−1 for 2, 10, and 20 mg humic acid-C l−1, respectively.
Discussion
Comparison of denitrification rates in low order streams
Several methods are available to measure rates of sediment denitrification, each of which has strengths and weaknesses depending on specific research questions and site conditions (Groffman et al., 2006). We chose a denitrification method frequently used in stream sediments to facilitate comparison to other studies in the literature. Denitrification rates in the study sites displayed substantial spatial and temporal variability that is inherent in most microbial communities (García-Ruiz et al., 1998; Kemp & Dodds, 2002; Royer et al., 2004). Sediments used in this study were collected during winter. Although some studies have identified seasonal effects on denitrification (Martin et al., 2001), we found that the range of denitrification rates obtained during the experiment was similar to that obtained from these same streams during spring, summer, and fall (<0.1–22.4 μg N g AFDM−1 h−1; Herrman, 2007). Either denitrification in the study streams was not seasonally affected or the effects of seasonality were minimized because we incubated the sediments at room temperature prior to experimentation.
Denitrification rates observed in the study sites were within the same order of magnitude (but generally higher) than rates reported for low order streams (see review in Table 2). The denitrification rate (12.0 μg N g AFDM−1 h−1) closest to ours was measured in a stream that had maximum \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations of 5 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)–N l-1 (Inwood et al., 2005). The sites we examined, however, routinely had in-stream concentrations >10 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)–N l−1. Consistent with other research in agricultural streams, we attributed our high denitrification rates to heavy anthropogenic N loading (Schaller et al., 2004). Overall, the denitrification rates measured in the study reaches were comparable to the rates in the literature, but were probably enhanced as a result of the surrounding agricultural activity in the watershed.
Effect of nitrate on denitrification
Denitrification in the study sites, followed Michaelis-Menten kinetics as \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations increased. In the literature, we only identified one study that did not document a \( {\text{NO}}^{ - }_{{\text{3}}} \) limitation (Table 3). Sediments from the South Platte River were reported to be unaffected by \( {\text{NO}}^{ - }_{{\text{3}}} \) (Pfenning & McMahon, 1996), but this conclusion may be limited by the experimental design. Sediments were enriched with increasing \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations, but were not amended with C, even though the sediments were C limited (Pfenning & McMahon, 1996). After denitrification did not respond to the increasing \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations, the authors concluded that the sediments were not \( {\text{NO}}^{ - }_{{\text{3}}} \) limited, but the non-response may have been a result of C limitation. In our study, we supplied a non-limiting concentration of organic C to determine the response of denitrification to increasing \( {\text{NO}}^{ - }_{{\text{3}}} \). Such an experimental design minimizes confusion regarding co-limitation and allows us to definitively conclude \( {\text{NO}}^{ - }_{{\text{3}}} \) affected denitrification in our headwater streams.
Few studies have examined the response of denitrification to \( {\text{NO}}^{ - }_{{\text{3}}} \) using Michaelis-Menten kinetics. Studies that have used Michaelis-Menten kinetics have utilized a variety of denitrification methods from a range of terrestrial, riparian, wetland, intertidal, and stream ecosystems (Laverman et al., 2006). As previously stated, Vmax is measured with differing units making this parameter difficult to compare between studies. Values of km, however, are relatively easy to compare and yield two pieces of information. First, the magnitude of km indicates how quickly denitrifiers are saturated with \( {\text{NO}}^{ - }_{{\text{3}}} \). The values we measured appear to be in agreement with most of the literature for hydric soils and sediments, and suggest that denitrification in the sediments of the study streams becomes saturated at moderate \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations (Table 4). The highest value of km we identified in the literature, was measured using intact sediment cores (García-Ruiz et al., 1998). Intact cores yield higher km values than sediment slurries because slurries provide greater exposure to the microbial population to \( {\text{NO}}^{ - }_{{\text{3}}} \) (Laverman et al., 2006). Second, values of km give an indication of how close denitrification is to saturation. If in-stream \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations are below km, then denitrification is not near saturation. Conversely, if in-stream \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations are above km, then denitrification is saturated. The median \( {\text{NO}}^{ - }_{{\text{3}}} \) concentration for our sites is well above km. Therefore, denitrification in these agricultural headwater streams is most likely saturated with \( {\text{NO}}^{ - }_{{\text{3}}} \). Other studies using various methods have also determined that agricultural headwater streams are \( {\text{NO}}^{ - }_{{\text{3}}} \) saturated or at least saturated during certain periods of the year (Royer et al., 2004; Bernot et al., 2006).
Effect of temperature on denitrification
We chose 8°C and 25°C as incubation temperatures because they represented the range of temperature observed in the study streams from spring to fall. Consistent with other published research, we observed that denitrification was higher under increased temperature (Schipper et al., 1993; Holmes et al., 1996; Maag et al., 1997; Seitzinger, 1988; Martin et al., 2001). We also determined that denitrifiers, when supplied with adequate organic C and \( {\text{NO}}^{ - }_{{\text{3}}} \), can maintain significant activities at low temperatures. Overall, temperature appears to be a critical factor regulating denitrification, and our study demonstrates that it should be incorporated into N removal models as it is sometimes overlooked (e.g., RivR-N see Seitzinger et al., 2002).
Effect of pH on denitrification
Denitrification could be affected by pH because protons are consumed during the reaction. For aquatic sediments, there is some agreement that denitrification is not inhibited at neutral and high pH (Knowles, 1982; Almeida et al., 1995; Wang et al., 1995; Glass & Silverstein, 1998), but is inhibited at low pH (Napier & Bustamante, 1988). Most of these studies were conducted on bacteria isolated in the laboratory or on wastewater treatment sludge, making their applicability to natural systems challenging.
Over three pH values (5.5, 7.5, and 9.5), we did not document a pH effect on denitrification. This finding is supported by a study conducted in forested headwater streams with pH values as low as 5.76 (Martin et al., 2001). However, a study of a stream receiving acid mine drainage did identify pH effects on denitrification at values below four (Baeseman et al., 2006). Neither our study nor that of Martin et al. (2001) was able to observe an effect, probably because the pH values included may not have been low enough to inhibit denitrification. We selected pH values for the experiment that were above and below the maximum and minimum pH values recorded in the study streams (range = 7.7–8.7). This allows us to conclude that within the study streams, current pH levels do not affect denitrification.
Effect of carbon on denitrification
Our study and several others determined that C concentration has no effect on denitrification; however, some studies have documented a C limitation (Table 3). We concluded that our inability to identify a C limitation in the study streams was probably a result of the abundant amount of DOC (3.4–3.7 mg C l−1) and sedimentary C (23.4–34.7 mg C g soil−1). Even though we removed DOC from the overlying water column for the control treatment, C was still available to the denitrifiers via the sediment. Sediment with C higher than 10 mg C g soil−1 has been suggested to be the boundary between oligotrophic and eutrophic systems (Piña-Ochoa & Álvarez-Cobelas, 2006). Therefore, the sedimentary C in the study sites most likely provided sufficient substrate to support denitrification. We also examined C quality by using a labile (i.e., glucose) and recalcitrant (i.e., humic acid) C source for denitrification. This experiment did not yield significant information because C was not a limiting factor in the sediments we examined.
Comparing our results to the literature is difficult: few denitrification studies have published residual C data and when they did, it was typically DOC and not sedimentary C. Two studies that have documented a C limitation on denitrification were conducted in sites with low C inputs. The first was a study of riverine sediments with low residual sedimentary C (∼0.67 mg C g soil−1, Pfenning & McMahon, 1996). The second study examined a stream receiving acid mine drainage, and the authors generally concluded that the stream had low C supplies (Baeseman et al., 2006). Studies that have not found a C limitation were conducted in streams in forested or agricultural watersheds and received moderate to high C inputs. Specifically, one study looked at streams with organic rich sediments (24.1 mg organic matter g soil−1, Martin et al., 2001), and two others examined streams with high DOC concentrations (2.5–5.5 mg C l−1, Royer et al., 2004; 1.73–3.54 mg C l−1, Holmes et al., 1996). Ultimately, denitrification in the agricultural headwater streams we investigated was not affected by C, probably because they received adequate supply of C from sediment sources.
Conclusion
Denitrification in the agricultural headwater streams we identified is not affected by pH and C quantity and quality but is controlled by temperature and \( {\text{NO}}^{ - }_{{\text{3}}} \) concentration. Specifically, the effect of \( {\text{NO}}^{ - }_{{\text{3}}} \) on denitrification followed Michaelis-Menten kinetics. Despite high denitrification rates, these streams were most likely saturated with \( {\text{NO}}^{ - }_{{\text{3}}} \) because in-stream \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations were well above km. Agricultural headwater streams could potentially remove substantial N during warm summer months when in-stream \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations are low (<1 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1), but would remove insignificant amounts of N during cooler periods of winter, spring, and fall when \( {\text{NO}}^{ - }_{{\text{3}}} \) concentrations are high (>1 mg \( {\text{NO}}^{ - }_{{\text{3}}} \)-N l−1). In the Upper Sugar Creek Watershed, N loads are highest during spring and fall (Herrman, 2007) and combined with our findings, this suggests that the headwater streams we examined are not effective at removing in-stream N.
References
Alexander, R. B., R. A. Smith & G. E. Schwarz, 2000. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403: 758–761.
Almeida, J., A. Reiss & M. Carrondo, 1995. Competition between nitrate and nitrite reduction in denitrification by Pseudomonas flourescens. Biotechnology and Bioengineering 46: 476–484.
Ambus, P., 1993. Control of denitrification enzyme activity in a streamside soil. FEMS Microbiology Ecology 102: 225–234.
American Public Health Administration, 1998. Standard Methods for the Examination of Water and Wastewater. 18th ed. APHA, Washington D.C.
Baeseman, J. L., R. L. Smith & J. Silverstein, 2006. Denitrification potential in stream sediments impacted by acid mine drainage: effects of pH, various electron donors, and iron. Microbial Ecology 51: 232–241.
Bernot, M. J., W. K. Dodds, W. S. Gardner, M. J. McCarthy, D. Sobolev & J. L. Tank, 2003. Comparing denitrification estimates for a Texas estuary by using acetylene inhibition and membrane inlet mass spectrometry. Applied and Environmental Microbiology 69: 5950–5956.
Bernot, M. J., J. L. Tank, T. V. Royer & M. B. David, 2006. Nutrient uptake in streams draining agricultural catchments of the Midwestern United States. Freshwater Biology 51: 499–509.
Boyer, E. W., R. B. Alexander, W. J. Parton, C. Li, K. Butterbach-Bahl, S. D. Donner, R. W. Skaggs & S. J. Del Grossoh, 2006. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecological Applications 16: 2123–2142.
Buchanan, T. J. & W. P. Somers, 1969. Discharge Measurements at Gauging Stations: U.S. Geological Survey Techniques of Water-Resources Investigations, Book 3, Chap. A8, pp. 65.
Burkhart, M. R. & D. E. James, 1999. Agricultural-nitrogen contributions to hypoxia in the Gulf of Mexico. Journal of Environmental Quality 28: 850–859.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559–568.
Duff, J. H., F. J. Triska & R. S. Oremland, 1984. Denitrification associated with stream periphyton: chamber estimates from undirsrupted communities. Journal of Environmental Quality 13: 514–518.
Eriksson, P. G. & S. E. B. Weisner, 1999. An experimental study on effects of submersed macrophytes on nitrification and denitrification in ammonium-rich aquatic systems. Limnology and Oceanography 44: 1993–1999.
Galloway, J. N. & E. B. Cowling, 2002. Reactive nitrogen and the world: 200 years of change. Ambio 31: 72–78.
García-Ruiz, R., S. N. Pattinson & B. A. Whitton, 1998. Kinetic parameters of denitrification in a river continuum. Applied and Environmental Microbiology 64: 2533–2538.
Glass, C. & J. Silverstein, 1998. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Research 32: 831–839.
Goolsby, D. A., W. A. Battaglin, G. B. Lawrence, R. S. Artz, B. T. Aulenbach, R. P. Hooper, D. R. Keeney & G. J. Stensland, 1999. Flux and sources of nutrients in the Mississippi-Atchafalaya River Basin. Topic 3 report for the Integrated Assessment on Hypoxia in the Gulf of Mexico, Decision Analysis Ser. 17. NOAA Coastal Ocean Office, Silver Spring, MD.
Groffman, P. M., M. A. Altabet, J. K. Böhlke, K. Butterbach-Bahl, M. B. David, M. K. Firestone, A. E. Giblin, T. M. Kana, L. P. Nielsen & M. A. Voytek, 2006. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecological Applications 16: 2091–2122.
Herrman, K. S., 2007. Mechanisms controlling nitrogen removal in agricultural headwater streams. Ph.D. Dissertation. The Ohio State University.
Holmes, R. M., J. B. Jones, S. G. Fisher & N. B. Grimm, 1996. Denitrification in a nitrogen-limited stream ecosystem. Biogeochemistry 33: 125–146.
Hordijk, C. A., M. Schnieder, J. M. van Engelen & T. E. Cappenberg, 1987. Estimation of bacterial nitrate reduction rates at in situ concentrations in freshwater sediments. Applied and Environmental Microbiology 53: 217–223.
Inwood, S. E., J. L. Tank & M. J. Bernot, 2005. Patterns of denitrification associated with land use in 9 midwestern headwater streams. Journal of the North American Benthological Society 24: 227–245.
Kemp, M. J. & W. K. Dodds, 2002. Comparisons of nitrification and denitrification in prairie and agriculturally influenced streams. Ecological Applications 12: 998–1009.
Knowles, R., 1982. Denitrification. Microbiological Reviews 46: 43–70.
Knowles, R., 1990. Acetylene Inhibition Techniques: Development, Advantages, and Potential Problems. Denitrification in Soil and Sediment. Plenum Press, New York, NY.
Laverman, A. M., P. Van Cappellen, D. van Rotterdam-Los, C. Pallud & J. Abell, 2006. Potential rates and pathways of microbial nitrate reduction in coastal sediments. FEMS Microbiology Ecology 58: 179–192.
Maag, M., M. Mainovsky & S. M. Nielsen, 1997. Kinetics and temperature dependence of potential denitrification in riparian soils. Journal of Environmental Quality 26: 215–223.
Martin, L. A., P. J. Mulholland, J. R. Webster & H. M. Valett, 2001. Denitrification potential in sediments of headwater streams in the southern Appalachian Mountains, USA. Journal of the North American Benthological Society 20: 505–519.
Mitsch, W. J., J. W. Day Jr., J. W. Gilliam, P. M. Groffman, D. L. Hey, G. W. Randall & N. Wang, 2001. Reducing nitrogen loading to the Gulf of Mexico from the Mississippi River Basin: strategies to counter a persistent ecological problem. BioScience 51: 373–388.
Napier, J. & R. Bustamante, 1988. In-situ biodenitrification of the S-3 ponds. Environmental Progress 7: 13–16.
Ohio EPA, 2002. Total Maximum Daily Loads for the Sugar Creek Basin: Division of Surface Water, Columbus, OH.
Oremland, R. S., C. Umberger, C. W. Culbertsen & R. L. Smith, 1984. Denitrification in San Francisco Bay intertidal sediments. Applied and Environmental Microbiology 47: 1106–1112.
Peterson, B. J., W. M. Wollheim, P. J. Mulholland, J. R. Webster, J. L. Meyer, J. L. Tank, E. Martí, W. B. Bowden, H. M. Valett, A. E. Hershey, W. H. McDowell, W. K. Dodds, S. K. Hamilton, S. Gregory & D. D. Morall, 2001. Control of nitrogen export from watersheds by headwater streams. Science 292: 86–90.
Pfenning, K. S. & P. B. McMahon, 1996. Effect of nitrate, organic carbon, and temperature on potential denitrification rates in nitrate-rich riverbed sediments. Journal of Hydrology 187: 283–295.
Piña-Ochoa, E. & M. Álvarez-Cobelas, 2006. Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81: 111–130.
Rabalais, N. N., R. E. Turner & W. J. Wiseman Jr., 2002. Gulf of Mexico hypoxia, a.k.a. “The dead zone.” Annual Review of Ecological Systems 33: 235–263.
Risgaard-Petersen, N., & K. Jensen, 1997. Nitrification and denitrification in the rhizosphere of the aquatic macrophyte Lobelia dortmanna L. Limnology and Oceanography 42: 529–537.
Risgaard-Petersen, N., 2003. Coupled nitrification–denitrification in autotrophic and heterotrophic estuarine sediments: on the influence of benthic microalgae. Limnology and Oceanography 48: 93–105.
Royer, T. V., J. L. Tank & M. B. David, 2004. Transport and fate of nitrate in headwater agricultural streams in Illinois. Journal of Environmental Quality 33: 1296–1304.
Schaller, J. L., T. V. Royer, M. B. David & T. L. Tank, 2004. Denitrification associated with plants and sediments in an agricultural stream. Journal of the North American Benthological Society 23: 667–676.
Schipper, L. A., A. B. Cooper, C. G. Harfoot & W. J. Dyck, 1993. Regulators of denitrification in an organic riparian soil. Soil Biology and Biochemistry 25: 925–933.
Seitzinger, S. P., 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S. P., R. V. Styles, E. W. Boyer, R. B. Alexander, G. Billen, R. W. Howarth, B. Mayer & N. van Breemen, 2002. Nitrogen retention in rivers: model development and application to watersheds in the northeastern U.S.A. Biogeochemistry, 57–58, 199–237.
Tiedje, J. S., 1982. Denitrification. Methods of Soil Analysis, Part II. American Society of Agronomy: Soil Science Society of America, Madison, Wisconsin.
Wang, J., B. Baltzis & G. Lewandowski, 1995. Fundamental denitrification kinetic studies with Pseudomonas denitrificans. Biotechnology and Bioengineering 47: 27–41.
Ward, M. H., S. D. Mark, K. P. Cantor, D. D. Weisenburger, A. Correa-Villaseñor & S. H. Zahm, 1996. Drinking water nitrate and the risk of non-Hodgkin’s lymphoma. Epidemiology 7: 465–471.
Acknowledgments
We thank two anonymous reviewers and the Associate Editor for their valuable comments on the manuscript. Special thanks to Lance Williams, Anne Carey, and Tom Koontz whose suggestions greatly improved the manuscript. We would also like to thank Gwen Dubelko, Becky Fauver, and Nickla Louisy for assistance in the laboratory and the field, and Scott Long for obtaining site permissions. This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (award # 2005-35102-16325) and the Water Resources Research Institute of the USGS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: D. Ryder
Rights and permissions
About this article
Cite this article
Herrman, K.S., Bouchard, V. & Moore, R.H. Factors affecting denitrification in agricultural headwater streams in Northeast Ohio, USA. Hydrobiologia 598, 305–314 (2008). https://doi.org/10.1007/s10750-007-9164-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9164-4