Abstract
Eelgrass beds represent important habitats for marine organisms, but are in decline in many coastal areas around the world. On Cortes Island, British Columbia, Canada, oysters coexist regionally with native eelgrass (Zostera marina L.), but eelgrass is typically absent directly seaward of oyster beds (the “below-oyster cobble zone”). We compared assemblage structure of nekton (fish and swimming macroinvertebrates) and epibenthos (macroinvertebrates and macroalgae) between eelgrass bed and below-oyster habitats. We sampled the intertidal zone on Cortes Island at low tide using two methods: quadrats to enumerate epibenthic macroinvertebrates and macroalgae, and beach seines to enumerate fish and swimming macroinvertebrates. Using multivariate analysis of similarity (ANOSIM), we found that the structure of nektonic and epibenthic assemblages associated with below-oyster cobble zones were significantly different from those in eelgrass-beds. Univariate measures showed that nektonic species richness and abundance were significantly higher in eelgrass beds than in below-oyster cobble habitat, whereas epibenthic species richness and abundance were significantly higher in below-oyster habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass beds stabilize sediments, slow ocean currents, provide marine animals refuge from predation, and trap planktonic larvae and suspended particulate matter (Jones et al., 1994; Coleman & Williams, 2002). The relatively high diversity and abundance of fish and invertebrates associated with eelgrass (Zosteraceae: Zostera spp.) beds are frequently attributed to these characteristics (Marsh, 1973; Heck & Orth, 1980; Lubbers et al., 1990; Griffin, 1997). In coastal British Columbia and the Pacific Northwest of the United States, eelgrass beds serve as habitat for many economically important species such as juvenile salmon (Oncorhynchus spp.) and as spawning grounds for Pacific herring (Clupea harengus L.) (Reise et al., 1989; Griffin 1997; Murphy et al., 2000; Sheridan & Minello, 2003; Wyllie-Echeverria et al., 2003). The suspended particulate matter and planktonic organisms trapped by eelgrass blades nourish many suspension-feeding invertebrates, and promote the development of distinct eelgrass-associated epibenthic and epifaunal assemblages (Marsh, 1973; Lubbers et al., 1990; Griffin, 1997; Ruesink et al., 2005). In turn, these complex invertebrate assemblages provide prey for crabs and fish (Marsh, 1973; Trianni, 1996). A diverse infaunal assemblage is protected against large epibenthic predators by eelgrass root and rhizome structure (Summerson & Peterson, 1984; Reise, 1985).
In coastal British Columbia and the American Pacific Northwest, beds of eelgrass (Z. marina L.) co-occur at a larger scale with the Pacific oyster Crassostrea gigas (Thunberg). C. gigas was introduced to North America from Japan in the early 20th century for aquaculture, and has since become a prominent feature of the intertidal zone in many areas (Quayle, 1964). C. gigas beds on the west coast of North America occur as either beach-cultivated sites, in which oysters are grown directly on the substrate in the intertidal zone (BCSGA, 2003), or as feral oyster beds, which have resulted from the sporadic spawning of cultured oysters (Quayle, 1964; Dumbauld et al., 2001). Oyster culture also occurs off-bottom on rafts or stakes (BCSGA, 2003).
Oysters and eelgrass are both found in sheltered coastal areas of relatively low wave energy. Oysters are typically found in the mid-to-high intertidal zone, while eelgrass is constrained to the low intertidal to shallow subtidal zones. While eelgrass can be found in close proximity to oyster beds on Cortes Island, it is typically absent directly seaward from these beds (pers. obs.). Long-term Cortes Island residents report that existing small eelgrass beds formed a continuous band along much of the Cortes coastline as recently as 15 years ago.
Significant eelgrass losses have been noted in coastal British Columbia and the American Pacific Northwest (Griffin, 1997; reviewed in Wyllie-Echeverria et al., 2003). Dredging and filling for harbour construction, shading by docks, sedimentation due to logging, introduction of toxic chemicals in runoff, increased nutrient input from septic systems, bioturbation by burrowing invertebrates and grazing pressure from Canada geese (Branta canadensis L.) have been cited as factors contributing to eelgrass loss in these areas (Wyllie-Echeverria et al., 2003). While some of these factors exist on Cortes Island, none of them adequately explains the pattern of eelgrass absence seaward of oyster beds when eelgrass is present in adjacent areas not seaward of oyster beds. Everett et al. (1995) found a negative effect of cultured oysters on eelgrass cover and shoot density in Oregon, but the mechanisms behind this effect were unknown. Oysters may contribute to the apparent exclusion of eelgrass through amplification of sulphide in the sediment, as the addition of organic matter to the sediment through production of faeces and pseudofaeces creates conditions that favour growth of sulphide-producing bacteria (Ingold & Havill, 1984; Castel et al., 1989; deZwaan & Babarro, 2001). High sulphide levels are associated with reduced photosynthesis and growth in eelgrass (Goodman et al., 1995; Holmer & Bondgaard, 2001).
If oyster beds are linked to a reduction in eelgrass cover, expansion of feral and farmed oyster beds could result in a reduction both in eelgrass beds and in their associated ecological functions. The first step in understanding potential impacts of such a shift is to quantify differences in community structure between eelgrass beds and below-oyster areas. The objective of this study was to assess the extent to which assemblages of fish, swimming macroinvertebrates, epibenthic macroinvertebrates and macroalgae differ between eelgrass beds and below-oyster cobble habitats. If a causal link between oyster presence and eelgrass loss exists, results of this study can be used to assess potential impacts on intertidal community structure of replacing eelgrass with below-oyster cobble zones.
Methods
Study sites
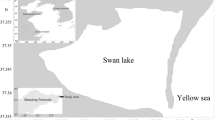
All study sites were located on the southwest coast of Cortes Island, British Columbia, Canada (50°4′ N, 124°56′ W) (Fig. 1). The intertidal zone directly seaward from oyster beds (the “below-oyster cobble zone”) at the study sites was characterized by large cobble, and often dominated by macroalgae such as Ulva sp. and Sargassum muticum (Yendo). Below-oyster sites were located directly seaward of feral or cultured oyster beds and were at the same tidal height as adjacent eelgrass beds (Fig. 2). The substratum of eelgrass-bed sites was characterized by a layer of mud or sand covering a layer of medium to large cobble. The substratum found in the below-oyster zone was characterized by medium to large cobble similar to the cobble found under the mud/sand layer at eelgrass-bed sites; a layer of finer sandy sediment was found under this cobble layer.
Map of Cortes Island with detail of study sites. Unfilled circles indicate eelgrass bed (EEL) sites; filled circles indicate below-oyster (BO) sites. Sites BO-1 and 3 were below feral oyster beds, while sites BO-2 and 4 were located below cultured oyster beds. Sampling sites EEL-1, 2, 3 and 4, and BO-1 were located in Manson’s Landing Provincial Park, and BO-2 was immediately south of the park; sites EEL-5 and BO-3 and 4 were located directly north of Smelt Bay Provincial Park
Epibenthic macroinvertebrates and macroalgae
Surveys were conducted at low tide at five eelgrass beds and four adjacent below-oyster sites to enumerate epibenthic macroinvertebrates and macroalgae (Fig. 1). At each site, we laid two 15 m transects perpendicular to the shoreline beginning at the low-tide line and extending through the intertidal zone on to shore. Five 0.5 m × 0.5 m quadrats were placed at random along each transect. Epibenthic macrofauna within the quadrats were identified to species except for limpets (to family), and shore crabs and hermit crabs (to genus). Barnacles, limpets and littorine snails were very abundant, particularly in below oyster-transects. We categorized these taxa as rare (0–20 individuals; exact number reported), common (21–100 individuals), abundant (101–300 individuals) or very abundant (300–1,000 individuals). The exact number of individuals was recorded for all other taxa. Macroalgae were identified to species, and abundance was recorded as estimated percent cover for each species. Abundance of eelgrass was also recorded as percent cover. Eelgrass blades were visually inspected for macroscopic fauna, and each eelgrass blade within a quadrat was lifted to count any epibenthic fauna under blades.
Fish and swimming macroinvertebrates
We sampled fish and swimming macroinvertebrates using a 12 m × 2 m beach seine with 1-cm mesh at eelgrass beds (N = 3 sites) and in below-oyster cobble zones (N = 3 sites). Fish and invertebrates were identified to species and released immediately. Two seine hauls were made at each site, and all hauls were made mid-day on an incoming tide for consistency across sampling events.
Statistical methods
Repeated measures ANOVA was used to compare benthic macroinvertebrate abundance and macroalgal percent cover between eelgrass and below-oyster cobble habitats. Benthic macroinvertebrate abundance data were log-transformed to correct for unequal variances. Two-sample t-tests were used to compare fish species richness and abundance, and swimming macroinvertebrate species richness and abundance between the two habitats. ANOVA and t-tests were performed using the software package SPSS 11.5 (SPSS, 2002). The Bonferroni-Holm sequential correction for multiple comparisons was applied to t-test results (Romano & Wolf, 2005).
We analyzed assemblage structure of epibenthic organisms associated with the below-oyster cobble zone and eelgrass beds, and fishes and swimming macroinvertebrates associated with each habitat in order to identify community-level variation in species composition and abundance. Multivariate analyses were performed using the PATN 3.2 multivariate software package (Belbin, 1993). Eelgrass and oysters were excluded as factors from ordination analyses. Category midpoints were used for epibenthic organisms for which abundance was estimated (barnacles, limpets and littorines): common = 60, abundant = 200, and very abundant = 500. Bray–Curtis distances between the quadrats were calculated based on raw, log-transformed, and presence–absence data, and semi-strong hybrid multidimensional scaling (SSH-MDS) with 1,000 random starts was used to create ordinations. Unlike basic MDS, which applies linear regression and ordinal regression to all dissimilarity values below a threshold value, SSH-MDS uses linear regression for values below a threshold and ordinal regression for values greater than the threshold value (Belbin, 1991, 1993). It was chosen as the appropriate algorithm due to its robustness to the high number of zeroes in the data matrix (Belbin, 1993). We used the PATN default threshold value of 0.9 for our analyses. We used multivariate analysis of similarity (ANOSIM) with 1,000 permutations to determine whether assemblage structure in below-oyster habitat was significantly different from that in eelgrass beds.
The number of replicate samples for fishes and swimming macroinvertebrates was inadequate for ordination, so Bray–Curtis distances among sites were used to create a dendrogram showing multivariate relationships among sites.
Results
Epibenthic macroinvertebrates and macroalgae
Plant and macroalgal cover in eelgrass bed transects was significantly higher than in below-oyster transects (P = 0.037; Table 1). In eelgrass bed transects, cover was dominated by Z. marina followed by the green alga Ulva and Japanese eelgrass Z. japonica. In total, 11 plant and macroalgal species were found in eelgrass bed transects (Table 2). Below-oyster transects contained eight macroalgal species and were dominated by Ulva and the invasive brown alga Sargassum muticum (Table 2).
Eleven animal taxa were found in eelgrass bed transects, while a total of 20 were found in below-oyster transects (Electronic supplementary material—Appendix 1). Eelgrass bed transects were dominated by the bamboo worm Spiochaetopterus costarum, the dove snail Alia carinata, hermit crabs Pagurus spp. and the bubble snail Haminoea vesicula. Average animal abundance per below-oyster transect was significantly higher than that per eelgrass transect (P < 0.0001), largely due to numerical dominance of barnacles (Balanus glandula) in below-oyster transects (Table 3). In addition to barnacles, shore crabs Hemigrapsus nudus and H. oregonensis, limpets (Lottidae) and hermit crabs (Pagurus spp.) were common in below-oyster transects.
Analysis of similarity found that the assemblage of epibenthic plants and animals associated with the below-oyster areas was significantly different from that associated with eelgrass beds regardless of whether raw, log-transformed or presence–absence data were used (P < 0.001 for all analyses) (see Fig. 3 for ordination based on raw data). In total, 29 taxa were found in below-oyster cobble zones compared to 22 taxa in eelgrass beds (Electronic supplementary material—Appendix 1).
Two-dimensional ordination created using SSH-MDS (semi-strong hybrid multidimensional scaling) on log-transformed data with 1,000 random starts in PATN software. Z. marina was excluded as an intrinsic factor for ordination and mapped onto two-dimensional ordination post-analysis. Filled circles represent eelgrass bed transects (n = 30); open circles represent below-oyster transects (n = 24). ANOSIM P < 0.001. Vectors represent the taxa that were significantly correlated with the ordination (P < 0.05)
Fish and swimming macroinvertebrates
Eelgrass and below-oyster seines had few taxa in common (Electronic supplementary material—Appendix 1), as illustrated by the dendrogram (Fig. 4), which confirms that assemblages of fish and swimming macroinvertebrates differ markedly between eelgrass and adjacent below-oyster cobble zones. Eelgrass-bed sites were significantly higher than below-oyster sites in overall species richness (P = 0.006), number of fish species (P = 0.002) and number of macroinvertebrate species (P = 0.02) (Tables 4 and 5). Abundance of fish was also significantly higher in eelgrass-bed seines than in below-oyster seines (P = 0.01). Although the overall abundance of swimming macroinvertebrates in eelgrass-bed seines was an order of magnitude higher than in below-oyster sites, our low-sample size and high variance resulted in the difference being only marginally statistically significant (P = 0.06) (Table 6).
Representatives of 11 fish families were found in eelgrass beds. The most abundant species were the plainfin midshipman (Porichthys notatus, Batrachoididae), shiner perch (Cymatogaster aggregata, Embiotocidae) and staghorn sculpin (Leptocottus armatus, Cottidae). Four fish families were collected in below-oyster seines; each family was represented by a single species with the staghorn sculpin being the most abundant.
The macroinvertebrate assemblage captured by seine in eelgrass beds consisted mainly of bubble snails (Haminoea vesicula), a hydrozoan jelly (Aequoria victoria) and kelp crabs (Pugettia producta) (Fig. 5). Common species in below-oyster seines included A. victoria and the ctenophore Pleurobrachia bachei (Fig. 5).
Discussion
Eelgrass beds and the below-oyster cobble zone on Cortes Island are distinct in their community structure, both in terms of epibenthic assemblages of macroinvertebrates and macroalgae (Fig. 2), and of fish and swimming macroinvertebrates (Fig. 3). The epibenthic assemblages in below-oyster cobble zones displayed higher species richness and animal abundance than those of eelgrass beds, and the two habitats had few species in common. In contrast, fish and swimming macroinvertebrates were more abundant and their assemblages more species-rich in eelgrass than in below-oyster habitat. ANOSIM confirmed that epibenthic assemblages in eelgrass and the below-oyster cobble zone are significantly different; these differences persist even when data are transformed to reduce or eliminate the impact of high abundance of taxa such as barnacles, shore crabs and limpets. This is consistent with other studies that have found assemblages associated with seagrass beds to be distinct from those associated with adjacent unvegetated sediments (Orth & vanMontfrans, 1982; Summerson & Peterson, 1984; Villarreal, 1995; Pihl et al., 2006). Differences in community structure could not be attributed to differences in depth, as both eelgrass and below-oyster cobble zones were at the same intertidal depth; similarly, major discrepancies in wave action between the two types of sites were unlikely because of their proximity and similarity in depth and slope. Minor differences in wave energy between eelgrass sites and below-oyster sites were likely due to the wave-dampening effect of eelgrass blades.
Many eelgrass-associated macroinvertebrates require either the soft sediment of an eelgrass bed or eelgrass blades as substrates. For example, partially buried suspension feeders (bamboo worms, Spiochaetopterus costarum), and gastropods that are cryptic on eelgrass blades (Haminoea vesicula) or exposed roots (Alia carinata) were among the most common eelgrass-associated fauna. The most common below-oyster associated macroinvertebrates either required a hard surface for attachment (e.g. barnacles and limpets) or preyed on small attached fauna (e.g. shore crabs).
Although, to our knowledge, no other studies have focussed on the below-oyster cobble zone, others have compared fauna within oyster beds to that on adjacent mudflats, sandy areas or eelgrass beds (Simenstad & Fresh, 1995; Villarreal, 1995; Trianni, 1996; Dumbauld et al., 2001). Villarreal (1995) and Trianni (1996) found higher invertebrate biomass and species richness in eelgrass beds than in oyster beds and unvegetated areas; however, both of these studies included infauna as well as epibenthic species. Simenstad & Fresh (1995) attribute the higher diversity of benthic invertebrates and macroalgae on oyster beds than on sandy areas or mudflats to increased habitat complexity created by oyster shells. Dumbauld et al. (2001) suggest that the increased hard surface area provided by oyster shells for attachment of macroalgae and associated invertebrates facilitates higher abundance of these organisms as compared to bare mud areas. Although the below-oyster cobble zone is generally devoid of oyster shells, bare rocks provide a similar hard surface area for attachment of barnacles, limpets and encrusting macroalgae. In contrast, eelgrass blades and the soft, muddy substratum found in eelgrass beds are unsuitable for attachment of these organisms. Cobble underlying the muddy layer of eelgrass-bed substratum was similar in size and appearance to cobble at below-oyster sites, suggesting that the top layer of sand and mud could have eroded from below-oyster sites following eelgrass loss.
Our observation that eelgrass beds display higher fish abundance and species richness than below-oyster cobble sites is supported by other studies comparing eelgrass bed fauna to that of nearby unvegetated sediments (Orth et al., 1984; Summerson & Peterson, 1984; Lubbers et al., 1990; Mattila et al., 1999; Manderson et al., 2000; Murphy et al., 2000; Joseph et al., 2006; Pihl et al., 2006). Several experimental studies have found significantly lower predation on fish in seagrass beds than in unvegetated areas (Rooker et al., 1998; Manderson et al., 2000; Linehan et al., 2001). The complex physical structure of an eelgrass bed provides protection from predation for fish as well as for swimming and epifaunal macroinvertebrates (Heck & Orth, 1980; Orth et al., 1984; Summerson & Peterson, 1984; Lubbers et al., 1990; Sogard & Able, 1991; Connolly, 1994; Rooker et al., 1998; Murphy et al., 2000; Sheridan & Minello, 2003).
Differences in availability of food for fish and swimming macroinvertebrates in eelgrass beds as compared to unvegetated areas may also be a contributing factor in the higher abundance of swimming organisms in seines through eelgrass (Marsh, 1973; Orth & vanMontfrans, 1982; Lubbers et al., 1990; Connolly, 1994). Slower water currents above eelgrass beds trap plankton and particulate matter in the water column, making them available to eelgrass-bed inhabitants including juvenile fish and suspension-feeding epifauna (Summerson & Peterson, 1984; Lubbers et al., 1990; Jones et al., 1994). Eelgrass leaves provide substantial surface area for attachment of epiphytic algae and small grazers, which are important sources of food for kelp crabs and juvenile fishes (Hines, 1982; Lubbers et al., 1990).
The results of this study reinforce the importance of eelgrass conservation in coastal British Columbia. Our observations and those of longtime residents of Cortes Island suggest that the establishment of feral oysters has resulted in interruption of the previously contiguous band of Zostera beds by patches of cobble. We hypothesize that waste products from oysters result in loss of Zostera seaward of oyster beds, and that the underlying cobble is revealed due to subsequent erosion of fine sediments that were originally trapped by seagrass rhizomes. Further research is required to determine whether a causal link exists between oyster bed establishment and replacement of eelgrass beds by below-oyster habitat. However, the precautionary principle states that management decisions should be made to address potential environmental threats even when full scientific evidence is lacking (Environment Canada, 2001), and as such, further expansion of ground-culture oyster farms should be limited to areas well away from eelgrass beds until more information is available.
References
BCSGA, 2003. Information resource centre. BC Shellfish Growers Association http://www.bcsga.ca. Accessed 21 Feb. 2005.
Belbin, L., 1991. Semi-strong hybrid scaling, a new ordination algorithm. Journal of Vegetation Science 2: 491–496.
Belbin, L., 1993. PATN (Pattern Analysis Package) Technical Reference. CSIRO, Australia.
Castel, J., J.-P. Labourg, V. Escaravage, I. Auby & M. E. Garcia, 1989. Influence of seagreass beds and oyster parks on the abundance and biomass patterns of meio- and macrobenthos in tidal flats. Estuarine, Coastal and Shelf Science 28: 71–85.
Coleman, F. C. & S. L. Williams, 2002. Overexploiting marine ecosystem engineers: potential consequences for biodiversity. Trends in Ecology and Evolution 17: 40–44.
Connolly, R. M., 1994. The role of seagrass as preferred habitat for juvenile Sillaginodes punctata (Cuv. & Val.) (Sillaginidae, Pisces): habitat selection or feeding? Journal of Experimental Marine Biology and Ecology 180: 39–47.
deZwaan, A. & J. M. F. Babarro, 2001. Studies on the causes of mortality of the estuarine bivalve Macoma balthica under conditions of (near) anoxia. Marine Biology 138: 1021–1028.
Dumbauld, B., K. M. Brooks & M. H. Posey, 2001. Response of an estuarine benthic community to application of the pesticide carbaryl and cultivation of Pacific oysters (Crassostrea gigas) in Willapa Bay, Washington. Marine Pollution Bulletin 42: 826–844.
Environment Canada, 2001. A Canadian Perspective on the Precautionary Approach/Principle. http://www.ec.gc.ca/econom/pamphlet_e.htm. Accessed 21 February 2007.
Everett, R., G. M. Ruiz & J. T. Carlton, 1995. Effect of oyster mariculture on submerged aquatic vegetation – an experimental test in a Pacific Northwest estuary. Marine Ecology Progress Series 125: 205–217.
Goodman, J. L., K. A. Moore & W. C. Dennison, 1995. Photosynthetic responses of eelgrass (Zostera marina L.) to light and sediment sulfide in a shallow barrier island lagoon. Aquatic Botany 50: 37–47.
Griffin, K., 1997. Eelgrass ecology and commercial oyster cultivation in Tillamook Bay, Oregon. Tillamook Bay National Estuary Project Report #11-97.
Heck, K. L. & R. J. Orth, 1980. Seagrass habitats: the roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In Kennedy, V. S. (ed.), Estuarine Perspectives. Academic Press, New York, 449–464.
Hines, A. H., 1982. Coexistence in a kelp forest: size, population dynamics, and resource partitioning in a guild of spider crabs (Brachyura, Majidae). Ecological Monographs 52: 179–198.
Holmer, M. & E. J. Bondgaard, 2001. Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquatic Botany 70: 29–38.
Ingold, A. & D. C. Havill, 1984. The influence of sulphide on the distribution of higher plants in salt marshes. Journal of Ecology 72: 1043–1054.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Joseph, V., A. Locke & J.-G. J. Godin, 2006. Spatial distribution of fishes and decapods in eelgrass (Zostera marina L.) and sandy habitats of a New Brunswick estuary, eastern Canada. Aquatic Ecology 40: 111–123.
Linehan, J. E., R. S. Gregory & D. C. Schneider, 2001. Predation risk of age-0 cod (Gadus) relative to depth and substrate in coastal waters. Journal of Experimental Marine Biology and Ecology 263: 25–44.
Lubbers, L., W. R. Boynton & W. M. Kemp, 1990. Variations in structure of estuarine fish communities in relation to abundance of submersed vascular plants. Marine Ecology Progress Series 65: 1–14.
Manderson, J. P., B. A. Phelan & A. W. Stoner, 2000. Predator–prey relations between age-1+ summer flounder (Paralichthys dentatus L.) and age-0 winter flounder (Pseudopleuronectes americanus Walbaum): predator diets, prey selection and effects of sediments and macrophytes. Journal of Experimental Marine Biology and Ecology 251: 17–39.
Marsh, G. A., 1973. The Zostera epifaunal community in the York River, Virginia. Chesapeake Science 14: 87–97.
Mattila, J., G. Chaplin, M. R. Eilers, K. L. Heck, J. P. O’Neal & J. F. Valentine, 1999. Spatial and diurnal distribution of invertebrate and fish fauna of a Zostera marina bed and nearby unvegetated sediments in Damariscotta River, Maine (USA). Journal of Sea Research 41: 321–332.
Murphy, M. L., S. W. Johnson & D. J. Csepp, 2000. A comparison of fish assemblages in eelgrass and adjacent subtidal habitats near Craig, Alaska. Alaska Fishery Research Bulletin 7: 11–21.
Orth, R. & J. vanMontfrans, 1982. Structural analysis of benthic communities associated with vegetated and unvegetated habitats. In Orth, R. J. & J. vanMontfrans (eds), Interactions of Resident Consumers in a Temperate Estuarine Seagrass Community. Virginia Institute of Marine Sciences, Vaucluse Shores, Virginia, USA, 232 pp.
Orth, R., K. L. Heck & J. Montfrans, 1984. Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator–prey relationships. Estuaries 7: 339–350.
Pihl, L., S. Baden, N. Kautsky, P. Rönnbäck, T. Söderqvist, M. Troell & H. Wennhage, 2006. Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science 67: 123–132.
Quayle, D., 1964. Distribution of introduced marine mollusca in British Columbia waters. Journal of the Fisheries Research Board of Canada 21: 1155–1164.
Reise, K., 1985. Tidal Flat Ecology. Springer-Verlag, Berlin, 191 pp.
Reise, K., E. Herre & M. Sturm, 1989. Historical changes in the benthos of the Wadden Sea around the island of Sylt in the North Sea. Helgoländer Meeresuntersuchungen 43: 413–433.
Romano, J. P. & M. Wolf, 2005. Exact and approximate stepdown methods for multiple hypothesis testing. Journal of the American Statistical Association 100: 94–108.
Rooker, J. R., G. J. Holt & S. A. Holt, 1998. Vulnerability of newly settled red drum (Sciaenops ocellatus) to predatory fish: is early-life survival enhanced by seagrass meadows? Marine Biology 131: 145–151.
Ruesink, J. L., H. S. Lenihan, A. C. Trimble, K. W. Heiman, F. Micheli, J. E. Byers & M. C. Kay, 2005. Introduction of non-native oysters: ecosystem effects and restoration implications. Annual Review of Ecology, Evolution and Systematics 36: 643–689.
Sheridan, P. & T. J. Minello, 2003. Nekton use of different habitat types in seagrass beds of Lower Laguna Madre, Texas. Bulletin of Marine Science 72: 37–61.
Simenstad, C. & K. I. Fresh, 1995. Influence of intertidal aquaculture on benthic communities in Pacific Northwest estuaries: scales of disturbance. Estuaries 18: 43–70.
Sogard, S. M. & K. W. Able, 1991. A comparision of eelgrass, sea lettuce macroalgae, and marsh creeks as habitats for epibenthic fishes and decapods. Estuarine, Coastal and Shelf Science 33: 501–519.
Summerson, H. C. & C. H. Peterson, 1984. Role of predation in organizing benthic communities of a temperate-zone seagrass bed. Marine Ecology Progress Series 15: 63–77.
Trianni, M. S., 1996. The Influence of Commercial Oyster Culture Activities on the Benthic Infauna of Arcata Bay. Humboldt State University, Humboldt, CA.
Villarreal, G., 1995. Alterations in the structure of the macrobenthic community at Bahia Falsa, Mexico, related to the culture of Crassostrea gigas. Ciencias Marinas 21: 373–386.
Wyllie-Echeverria, S., T. Mumford, J. Gaydos & S. Buffum, 2003. Z. marina declines in San Juan County, WA. Westcott Bay Taskforce Mini-Workshop.
Acknowledgements
We wish to acknowledge logistical and field support of Mathew Brechtel, Michael, Sierra, Marg and Sully Sullivan, Heidi, Ruth and Fred Zwickel, and Chris Williamson. Research was supported by an NSERC Post Graduate Scholarship to JRK and an NSERC Discovery Grant and NSERC/SSHRC MCRI to JPV. We also thank two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. Trexler
Electronic supplementary material
Below is the link to the electronic supplementary material
10750_2007_9057_MOESM1_ESM.doc
Classification and taxonomic authorities of taxa found in field surveys. Presence (+) or absence (-) is indicated for each habitat type found (eelgrass bed and below-oyster habitat). (DOC 85 KB)
Rights and permissions
About this article
Cite this article
Kelly, J.R., Proctor, H. & Volpe, J.P. Intertidal community structure differs significantly between substrates dominated by native eelgrass (Zostera marina L.) and adjacent to the introduced oyster Crassostrea gigas (Thunberg) in British Columbia, Canada. Hydrobiologia 596, 57–66 (2008). https://doi.org/10.1007/s10750-007-9057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9057-6