Abstract
Syncarida are malacostracan crustaceans that live exclusively in fresh and brackish waters all over the world. With the exception of a few Anaspidacea that live in free freshwater (streams, ponds, superficial lakes and caves) the great majority inhabit the interstitial groundwater (they are stygobiont that live in groundwater in the wide sense). The Syncarida lack a carapace; have compound eyes (absent in subterranean taxa); the range in body size from 0.55 to 55 mm long and are more or less cylindrical in body shape; they have separate sexes with no free-swimming larval stage. Only the epigean Anaspidaea have coloration. Fossil Syncarida comprises two orders: Palaeocaridacea (five families, 15 genera and 20 species from Europe, USA and Brazil) and Anaspidacea (two monospecific genera from Australia). Anaspidacea also has present-day representatives: five families with 12 genera and 21 living species that live only in the Southern Hemisphere. Bathynellacea, the third order of Syncarida, with no fossil representatives, has two families, with 66 genera and 219 species that are widely distributed throughout all continents, except Antarctica. Since 1950, new species of Bathynellacea have been discovered with regularity, however many countries remain poorly sampled. The accumulation curves for Parabathynellidae, Bathynellidae and the whole of Bathynellacea demostrate that new species descriptions continue to accumulate at a rate that is well beyond the “plateau” level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Syncarida Packard, 1885 are malacostracan crustaceans that were highly diversified during the Palaeozoic. First known in fossil forms is Uronectes fimbriatus (Jordan, 1847), it took 35 years before a living representative was found: Bathynella natans Vejdovsky, 1882, in a water well in Prague, and a few years later, Anaspides tasmaniae Thompson, 1893 in a stream in Tasmania. Fossil forms were initially linked to isopods and amphipods until Packard described Syncarida in 1885. Later, Calman (1896) gave Syncarida the rank of order and included them in the Eumalacostraca. With the increasing number of species discovered (Chappuis, 1917–1929) the first classifications were proposed (Siewing, 1959; Brooks, 1962). Noodt (1964) provided the phylogenetic foundations for the present-day systematics of the group, erecting the order Stygocaridacea and introducing the first classification of the Bathynellacea Chappuis 1915. This was adopted by Brooks (1969) and completed by Schminke (1973, 1975, 1978a). Schram (1984) advanced several alternative cladograms for the fossil and present day families and Coineau (1996; 1998) synthesized all of these hypotheses and classifications. Currently, fossil and living Syncarida are included in three orders: Palaeocaridacea, that comprises only fossil forms, Anaspidacea with living and fossil representatives, and Bathynellacea with living forms only.
The Syncarida lack a carapace, and the cephalon typically show a mandibular seat. Compound eyes (absent in subterranean taxa) can be either pedunculate or sessile. The body ranges between 0.55 and 55 mm long and is more or less cylindrical, with the pereon comprising eight free somites in members of the orders Bathynellacea and Palaeocaridacea (fossil), and seven free somites in the order Anaspidacea, where the first thoracomere is incorporated into the cephalon to compose a cephalothorax. The pleon comprises six free somites plus a telson in the Anaspidacea, whereas the telson is incorporated into the 6th pleonite to form a pleotelson in the Bathynellacea (Fig. 1). The thoracopods of syncarids (7–8 pairs) are biramous, with the last pair transformed into a copulatory organ in males in some instances, in some cases, the 7th thoracopod is lacking. The pleopods (0–5 pairs) are biramous, uniramous, vestigial or absent; the first two pairs can make up the so-called petasma in males. The uropods are biramous and inserted on the 6th pleonite or on the pleotelson; if foliaceous, they form a caudal fan with the telson (the case of the telson is free); when the telson is incorporated into a pleotelson, the uropod branches are styliform, and there are two furcal branches on the terminal margin of the pleotelson.
Only the epigean Anaspidacea exhibit coloration, due to the presence of chromatophores.
The fossil syncarids lived in brackish water in estuaries and lagoons from the lower Carboniferous to the Permian (Schram, 1981). Present day representatives live mainly in freshwater; only a few Bathynellacea and Stygocaridacea live in estuaries in oligo to polyhaline water and are euryhaline and eurythermal. Except for a few Anaspididae that live in free freshwater (streams, ponds, superficial lakes and caves) the great majority of syncarids inhabit the interstitial groundwater (they are stygobionts that live in groundwater in the broad sense). The type of substrate used as ranges from coarse to fine, and the depth varies depending on the taxa, wiht the Bathynellacea inhabiting the subterranean aquifers, including two species living in Lake Baikal at depths between 20 and 1440 m.
The interstitial syncarids are detritivores, feeding on bacteria, animal and vegetal remains, and fungi adhered to sand grains; Bathynellidae filter and consume large pieces (Serban, 1980), some Parabathynellidae (Iberobathynella) eat harpaticoid copepods (Coineau, 1996), and the epigean Anaspidacea, living in caves, galleries of other crustaceans, or in gravel banks, can be herbivorous (algae), carnivorous or can feed on faecal pellets (Allanaspides).
The epigean Anaspidacea swim freely or walk among the vegetation and have an escape reaction known as a caridoid response. Interstitial forms need to be in contact with sand grains (positive thigmotaxis) and move by walking with their thoracopods.
Syncarids have separate sexes though mating has never been observed. Fecundation appears to take place in the oviduct (Smith, 1908) and eggs are deposited one by one. Metamorphosis takes place inside the egg and there are no free-swiming larvae. Forms leaving the egg are similar to the adult but smaller in size and successive moults are necessary to attain adult size. Descriptions of the development of Anaspides tasmaniae can be found in Hickman (1937), and of Bathynellacea in Jakobi (1954), Serban (1972), Schminke (1973) and Coineau (1998). The embryonic stage takes 9 months in Antrobathynella stammeri (Coineau, 1998) and life span can be up to 2.5 years in Iberobathynella (Coineau, 1998).
Species diversity
Fossil Syncarida comprises two orders: Palaeocaridacea (five families, 15 genera and 20 species from Europe, USA and Brazil), diversified in the Carboniferous, are the most primitive and do not have living representatives. The order Anaspidacea includes two monospecific genera, Anaspidites from the Australian Triassic and Koonaspides from the Australian lower Cretaceous, both belonging to the family Anaspididae. This order also has living representatives: five families (Table 1a and b; see Table in annex available on the website dedicated to FADA-Chapters) comprising 12 genera and 21 living species.
Bathynellacea, the third order of Syncarida, with no fossil representatives, comprises two families with 66 genera and 219 species: Parabathynellidae, 39 genera and 128 species, and Bathynellidae, 27 genera and 91 species. The latter necessitates a thorough revision.
Since 1950, new species of Bathynellacea have been discovered with regularity. Ten species of Parabathynellidae were known to occur in the Iberian Peninsula as of 1980. Following two decades of extensive work, the figure has risen to 30 species (Camacho 2003c). At present, there are 17 species of Bathynellidae known to occur in France, and five species in the Iberian Peninsula. However, this figure will soon exceed 20 species as we are currently describing 14 additional species. Nonetheless, many countries remain poorly sampled.
Thus, the understanding of the global syncarid taxonomic diversity is fraught with uncertainty derived from several sources:
-
First, some areas have been poorly sampled, especially Africa, South America and Asia.
-
Second, those that have been sampled on a more or less regular basis, probably have not been sampled enough, as demostrated by Camacho & Valdecasas (2003).
-
Third, some species are poorly described, and the genera known to date are in urgent need of revision.
(See Fig. 2 for a summary of number of species and genera by zoogeographical region.)
Figure 3a shows three accumulation curves for Parabathynellidae, Bathynellidae and the whole of Bathynellacea. There is no need to fit a function to the raw data to see that new species descriptions still accumulate at a rate that is well beyond the “plateau” level.
(a) Three accumulation curves for Parabathynellidae, Bathynellidae and the whole Bathynellacea; (b) rarefaction curve for all the species of Syncarida (PA = Palaearctic Region; NA = Nearctic Region; AT = Afrotropical Region; NT = Neotropical Region; OL = Oriental Region and AU = Australasian Region). (c) resampling curve (see text)
A generic diversity was calculated using rarefaction estimation (Simberloff, 1972). This procedure is frequently used in ecology, and takes the number of specimens per species in a collection to estimate the number of species in collections with smaller numbers of specimens. However, a useful taxonomic diversity index can be obtained by rarefacting species into genera, as is frequently done in paleontology (e.g. Favtosky et al., 2004). In our case we took the total Syncarid distribution of species per genera (see Table in annex available on the website dedicated to FADA-Chapters) to estimate generic distribution per biogeographical region, as defined by Wallace (1876). Only two regions show a clear discrepancy between observed and estimated number of genera (Table 2), the Paleartic and the Australasian regions. Rarefaction calculation is highly dependent on the “mother” list, so these figures could change drastically when some regions are better characterized. Figure 3b shows this curve for the whole range of species of Syncarida. It gives the number of genera predicted for a collection of species, if the real “universe” of Syncarida had a similar pattern to the sampling distribution. About 95% limits are also indicated in the figure. In general, the analysis points to the occurrence of undersampling, especially in the Palaearctic and Australasian region.
Finally, we have carried out a resampling scheme, with the whole Syncarid group, dividing the number of species by five year sampling periods, from 1880 to 2005 (55 sampling periods, s.p.), and resampling with replacement one thousand sets of 55 s. p. Results have been organized in 25 classes (Fig 3c). At the 5% significance level (for a two tail distribution test), the lowest values are 111 species and the highest is 341. Between this range lies the other 95% population figure. The highest number obtained within this 95% is 341, a number of species clearly above the present 240 species. However, this could be a poor estimation of the total Syncarid diversity, as around 100 species are still awaiting description.
Phylogeny and Historical proccesses
The Syncarida were already diversified during the Palaeozoic. They were one of the most important elements of the crustacean communities in brackish and littoral-marginal freshwaters of Laurentia during the Carboniferous. During the Permo-Trias they were present in several areas of the Gondwana, more specifically in Australia and Brazil, where Anaspidacea still live today (Schram, 1981, 1984). Present day distribution includes all continents, except Antarctica. Two alternative hypotheses have been proposed to explain Syncarid distribution on a continental scale.
In the first scenario, the littoral (surface) marine ancestor entered actively, through swimming larvae, into the continental waters and from there, through the hydrographic network of superficial waters into caves, karst and the alluvial porous systems (stygobionts of freshwater origin) (Schminke, 1981). The presence of syncarids in brackish interstitial waters, in this case, would be of secondary derivation. This hypothesis is supported by the presence of Anaspidacea in superficial freshwaters and of rare Bathynellacea in old mountain ranges (e.g. in Spain, see Camacho & Coineau, 1989).
The second scenario addresses the fact that present-day Anaspidacea and Bathynellacea live in areas that have suffered one or several trangression-regression events of the Tethys Sea after the Triassic. The biphase colonization model (Boutin & Coineau, 1990; Notenboom, 1991; Coineau & Boutin, 1992) states that in a first step the littoral marine ancestral syncarid actively colonized the littoral sediments and became interstitial; in a second step, considered passive, during the regression of the Tethys, a part of the littoral interstitial population remained in place and became phreatobitic. Meanwhile, the piezometric level fell and the rest of the population remained littoral as the shore line gradually retreated (thalassostygobiont origin). At the end of the regression the two populations were genetically isolated, and a speciation took place due to a vicariant event (this stage corresponds to the “Regression Model Evolution” of Stock, 1980). Many syncarids inhabit the same habitat as other stygobionts (of undeniable marine origin) that fit this model. The majority of present-day Bathynellacea from littoral waters and many fossil taxa known from littoral facies lend credence to this hypothesis (Boutin & Coineau, 1987; Camacho & Coineau, 1989; Coineau, 1996, 1998; Camacho, 2004; Camacho et al., 2006).
The Syncarida could have evolved by progenesis from an ancestral type of zoea larva. The Anaspidacea exhibit the typical caridoid facies (except for the caparace), and the Stygocarididae and Bathynellacea derive from repeated progenetic events. With the “Zoea theory” Schminke (1981) showed that the Bathynellacea have a larva-like morphology similar to their ancestral form. The five families of the Anaspidacea belong to an evolutive series connecting the surface living forms to the interstitial stygobionts; as they adapted to the interstitial subterranean life, the species of every derived clade looked similar to the juvenile stages of the more primitive sister groups in the adaptive phylogenetic series (Coineau, 1996, 2000).
The traditional phylogenetic tree assumes that the Syncarida are primitive Eumalacostraca (Coineau, 1996; Schram & Hof, 1998; Spears & Abele, 1999). Present day alternatives on syncarid phylogeny are that Syncarida includes the sister groups Bathynellacea and Anaspidacea (Richter & Scholtz, 2001) or that the Bathynellacea are the ancestor of the Malacostraca, including the Anaspidacea (Lange & Schram, 1999). In a recent phylogenetic analysis (Camacho et al., 2002) based on molecular data derived from the 16S rDNA of different Malacostraca, including a species of Bathynellacea and five species of Anaspidacea, Bathynellacea remained in a basal position and clearly split off from the Syncarida, but lay within the Eumalacostraca. Serban (1972) has previously advocated for a superorder Podophallocarida for the Bathynellacea outside of the Syncarida. More studies are clearly needed to resolve this question.
The phylogenetic relationships among the families of the Syncarida have been explored by several authors (Schminke, 1975, Schram, 1984; Coineau, 1996). Figure 4 shows a cladogram with the more commoly accepted relationships (Coineau, 1996).
Other phylogenetic studies within Bathynellacea and Anaspidacea include Grosso & Peralta (2002), Schminke (1973, 1975), Schram (1984), Coineau (1996), Camacho (1987, 2003c), Camacho et al. (1997, 2000), Guil and Camacho (2001) and Cho (1995, 2001), however, there is no global study that focuse on the relationships among all genera of both families.
Present distribution and main areas of endemicity
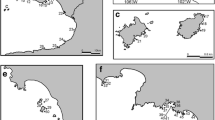
The distribution of both fossil and living Syncarida can be explained by a double vicariant biogeographic model, taking into account plate tectonics and the evolution of the Tethys Sea during the Mesozoic and the Cenozoic. The extinct taxa, endemic to the Laurentia in the Carboniferous, could have dispersed through the Pangea, since its split during the Permo-Trias. The fragmentation of Pangea during the Mesozoic could have produced a discontinuity in the distribution (Schram, 1977), and the Syncarida of each area could have evolved independently. The Anaspidacea, living only in the Southern Hemisphere (in South America and in the Australo-Tasmano-New Zealand area) (see Fig. 5) would reflect a Gondwanian distribution, similar to the distribution of the fossil Malacostraca of the Permo-Trias.
World distribution of Syncarida genera per biogeographical area (based on Wallace’s Region). White circle = Parabathynellidae: 1. Iberobathynella Schminke, 1973; 2. Californibathynella Camacho et Serban, 1998; 3. Califobathynella Cho, 1997; 4. Texanobathynella Delamare et al., 1975; 5. Guadalopebathynella Camacho et Serban, 1998; 6. Paraiberobathynella Camacho et Serban, 1998; 7. Hexaiberobathynella Camacho et Serban, 1998; 8. Hexabathynella Schminke, 1972; 9. Chilibathynella Noodt, 1963; 10. Atopobathynella Schminke, 1973; 11. Notobathynella Schminke, 1973; 12. Parabathynella Chappuis, 1926; 13. Eobathynella Birstein et Ljovuschkin, 1964; 14. Paraeobathynella Camacho, 2005; 15. Sketinella Camacho, 2005; 16. Kimberleybathynella Cho et al., 2005; 17. Issykkulibathynella Serban, 1984; 18. Batubathynella Schminke, 1973; 19. Allobathynella Morimoto et Miura, 1957; 20. Nipponbathynella Schminke, 1973; 21. Sabahbathynella Schminke, 1988; 22. Psalidobathynella Schminke, 1979; 23. Leptobathynella Noodt, 1965; 24. Parvulobathynella Schminke, 1973; 25. Thermobathynella Capart, 1951; 26. Cteniobathynella Schminke, 1973; 27. Nilobathynella Dumont, 1984; 28. Heterodontobathynella Schminke, 1973; 29. Brasilibathynella Jakobi, 1958; 30. Odontobathynella Delamare et Serban, 1979; 31. Noodtibathynella Schminke, 1973; 32. Haplophallonella Serban et Coineau, 1975; 33. Lamtobathynella Serban et Coineau, 1987; 34. Acantobathynella Coineau, 1967; 35. Ctenophallonella Coineau et Serban, 1978; 36. Racovitzaibathynella Serban et Coineau, 1994; 37. Afrobathynella Schminke, 1976; 38. Nunubathynella Schminke, 1976; 39. Habrobathynella Schminke, 1973. Grey square = Bathynellidae: 40. Gallobathynella Serban et al., 1971; 41. Clamousella Serban, 1989; 42. Vejdovskybathynella Serban et Leclerc, 1984; 43. Hispanobathynella Serban et al., 1971; 44. Meridiobathynella Serban et al., 1971; 45. Pseudobathynella Serban et al., 1971; 46. Vandelibathynella Serban et al., 1971; 47. Delamareibathynella Serban, 1989; 48. Parameridiobathynella Serban et Leclerc., 1984; 49. Bathynella Vejdovsky, 1882; 50. Baikalobathynella Birstein et Ljovuschkin, 1967; 51. Pacificabathynella Schminke et Noodt, 1988; 52. Pseudantrobathynella Schminke, 1988; 53. Sardobathynella Serban, 1973; 54. Antrobathynella Serban, 1966; 55. Tianschanobathynella Serban, 1993; 56. Austrobathynella Delamare Deboutteville, 1960; 57. Nannobathynella Noodt, 1969; 58. Agnatobathynella Schminke, 1980; 59. Transvaalthynella Serban et Coineau, 1975; 60. Transkeilthynella Serban et Coineau, 1975; 61. Uenobathynella Serban, 2000; 62. Parauenobathynella Serban, 2000; 63. Paradoxibathynella Serban, 2000; 64. Morimotobathynella Serban, 2000; 65. Nihobathynella Serban, 2000; 66. Serbanibathynella Reddy et Schminke, 2005. Black square = Anaspididae Thomson, 1884: 67. Anaspides Thomson, 1894; 68. Paranaspides Smith, 1908; 69. Allanaspides Swain et al., 1970. Koonungidae Sayce, 1880: 70. Koonunga Sayce, 1907; 71. Micraspides Nicholls,1931. Psammaspididae Schminke, 1974: 72. Psammaspides Schminke, 1974; 73. Eucrenonaspides Knott et Lake,1980; Stygocarididae Noodt, 1963: 74. Stygocaris Noodt, 1963; 75. Parastygocaris Noodt, 1963; 76. Oncostygocaris Schminke, 1980; 77. Stygocarella Schminke, 1980. Patagonaspididae Grosso et Peralte, 2002: 78. Patagonaspides Grosso et Peralta, 2002
The Palaearctic is the best known region for this fauna and here the distribution of Bathynellidae is broader than that of the Parabathynellidae (Fig. 5). Bathynellidae is more diversified and widely distributed in the northern hemisphere (21 genera and 82 species) than in the southern hemisphere (7 genera and 9 species). Parabathynellidae are widely distributed and diversified through the temperate and tropical regions. North America is the region with the lowest number of genera (6) and species (7) known.
Within Bathynellidae, the genus Bathynella Vejdovsky, 1882, has more than 50 species and subspecies, but only a few have been properly studied. The Asian species and a few others should be revised as some of them may belong to different genera (Serban, 2000). According to Serban (2000), Bathynella does not occur in Asia. In the European context, it spreads to southeast Europe, but is not present in the Iberian Peninsula. There is only one case of a genus of this family that is found in two very distinct areas, Nannobathynella being present with one species each in Brazil and the Ivory Coast.
Within the family Parabathynellidae there are a few genera with several species distributed through very different biogeographic regions; Chilibathynella and Atopobathynella being found in Chile and in Australia; Parvulobathynella, Cteniobathynella and Thermobathynella live in South America and Africa; and Habrobathynella, that was known only from Africa and has been found recently in India. The distribution of Iberobatinellids is amphi-Atlantic, and is related to the opening of the North Atlantic Ocean. The genera Iberobathynella, Guadalopebathynella and Hexaiberobathynella are restricted to the Iberian Peninsula and the Balearic islands, while some related genera such as Texanobathynella and Californibathynella live in North America and Paraiberobathynella in the Iberian Peninsula and North Africa (Coineau, 1998; Camacho et al. 2000).
The genus Hexabathynella is the only one displaying a worldwide distribution. However, the majority of the species has been found in just one locality or in localities of a limited area (Camacho, 2003a). The majority of genera live in very limited areas, although this can be, due to the temporaly and spatialy sporadic nature of the collecting. For this reason, it does not seem appropiate to designate them endemics. When sampling is done intensively and extensively in different seasons over different years and with different sampling techniques in different habitats (caves, springs, wells and the interstitial environment associated with streams), previously restricted species have been shown to have a wider distribution, e.g. Iberobathynella imuniensis, Paraiberobathynella fagei or Hexaiberobathynella mateusi (Camacho, 2003c).
Figure 5 shows the general distribution of the genera of known Syncarida represented in the classic biogeographical areas (based on the Wallace’s Region).
References
Main References (See complete list of references -auxiliary references – in annex available on the website dedicated to FADA-Chapters)
Boutin, C., & N. Coineau, 1987. Iberobathynella (Crustacea: Syncarida: Bathynellacea) sur le continent africain. Implications paléobiogéographiques. Comptes Rendus de l’ Académie des Sciences, Paris 304: 355–358.
Boutin, C. & N. Coineau, 1990. “Regression model”, “Modèle Biphase” d’évolution et origine des micro-organismes stygobies interstitiels continentaux. Revue de Micropaléontologie 33 (3–4): 303–322.
Brooks, H. K., 1962. On the fossil Anaspidacea, with a revision of the classification of the Syncarida. Crustaceana 4: 229–242.
Brooks, H. K., 1969. Syncarida. In Moore, R. C. (ed.), Treatise on Invertebrate Paleontology, Part R, Arthropoda 4, Vol. 1. Geological Society of America and University of Kansas, Lawrence: 345–359.
Calman, W. T., 1896. On the genus Anaspides and its affinities with certain fossil Crustacea. Transaction of the Royal Society of Edimburgh 38: 787–802.
Camacho, A. I., 1987. La Familia Parabathynellidae en la Península Ibérica: Taxonomía, Filogenia y Biogeografía. Tesis Doctoral (unpublished), Universidad Autonoma de Madrid, 890 pp.
Camacho, A. I., 2003a. Historical biogeography of Hexabathynella, a cosmopolitan genus of groundwater Syncarida (Crustacea, Bathynellacea, Parabathynellidae). Biological Journal of the Linnean Society 78: 457–466.
Camacho, A. I., 2003c. An overview of the distribution of the Parabathynellidae (Crustacea Syncarida Bathynellacea) on the Iberian Peninsula and Balearic Islands. Graellsia, 59(1): 63–78.
Camacho, A. I., 2004. An overview of Hexabathynella (Crustacea, Syncarida, Parabathynellidae) with the description of a new species. Journal of Natural History 28: 1249–1261.
Camacho, A. I. & N. Coineau, 1989. Les Bathynellacea d’Espagne: Répartition et Biogéographie. Mémoires de Biospéléologie XVI(43): 111–124.
Camacho, A. I., E. Bello, & G. F. Estabrook, 1997. A statistical approach to the evaluation of characters to estimate evolutionary relationships among the species of the aquatic subterranean genus Iberobathynella (Crustacea, Syncarida). Biological journal of the Linnean Society 60: 221–241.
Camacho, A. I. & A. G. Valdecasas, 2003. Evaluating extinction in rare habitats: an essay. Graellsia 59(2–3): 409–414.
Camacho, A. I., E. Serban & N. Guil, 2000. Phylogenetical review and biogeographic remarks on the interstitial and subterranean freshwater iberobathynells (Crustacea, Syncarida, Parabathynellidae). Journal of Natural History 34: 563–585.
Camacho A. I., I. Rey, B. A. Dorda, A. Machordom & A. G. Valdecasas, 2002. A note on the systematic position of the Bathynellacea (Crustacea, Malacostraca) using molecular evidence. Contribution to Zoology 71(4): 123–129.
Camacho, A. I., T. Torres, E. Ortiz, C. Puch & A. G. Valdecasas, 2006. Small-scale biogeographical pattern in groundwater Crustacea (Syncarida, Parabathynellidae). Biodiversity and Conservation, 15: 3527–3541.
Coineau, N., 1996. Sous-Classe des Eumalacostracés (Eumalacostraca Grobben, 1892). Super-Ordre des Syncarides (Syncarida Packard, 1885). In Forest, J. (ed.), Traité de Zoologie, Crustacés VII(2): 897–954.
Coineau, N., 1998. Syncarida. In Juberthie, C. & V. Decu (eds), Encyclopaedia Biospeologica, Tome II, Moulis, Bucarest, Société Biospéologie: 863–876.
Coineau, C., 2000. In Wilkens, H., D.C. Culver & W.F. Humphreys (eds), Subterranean Ecosystems, Ecosystems of the world 30, Elsevier : 189–210.
Coineau, N. & C. Boutin, 1992. Biological processes in space and time: colonization. evolution and speciation in interstitial stygobionts. In Camacho A. I. (ed.), The Natural History of Biospeleology. Monografías del M.N.C.N. 7(CSIC), Madrid: 423–451.
Chappuis, P. A., 1929. Anaspidacea. In Kükenthal-Krumbach, Handb Zoology 3(6): 594–606.
Cho, J. L., 1995. Systematik und Biogeographie von Hexabathynella Schminke, 1973 sowie ein Beitrag zur Taxonomie der “Leptobathynellinae” Noodt, 1964 (Bathynellacea, Syncarida, Malacostraca). Kiel, Doctoral Thesis (unpublished).
Cho, J. L., 2001. Phylogeny and zoogeography of three new species of the genus Hexabathynella (Crustacea, Malacostraca, Bathynellacea) from North America, Zoologica Scripta 30(2), 145–157.
Fastovsky, D. E., Y. Huang, J. Hsu, J. Martin-McNaughton, P. M. Sheehan & D. B. Weishampel, 2004, Shape of Mesozoic dinosaur richness: Geology 32: 877–884.
Grosso, L. E., & M. Peralta, 2002. Patagonaspides gen.n.; P. sandroruffoi sp.n. (Crustacea, Syncarida). First phreatobite species of a new anaspidacean family discovered in Patagonia with cladistic analisis of Stygocaridinea (Anaspidacea). Bulletino del Museo Cívico di Storia Naturale di Verona 26: 105–118.
Guil, N., & A. I. Camacho, 2001. Historical Biogeography of Iberobathynella (Crustacea, Syncarida, Bathynellacea), an aquatic subterranean genus of Parabathynellids, endemic to the Iberian Peninsula. Global Ecology and Biogeography 10: 487–501.
Hickman, V. V., 1937.The embriology of the Syncarid Crustacean Anaspides tasmaniae. Papers and Proceedings of the Royal Society of Tasmania: 1–36.
Jakobi, H., 1954. Biologie, Entwicklungsgeschichte und Systematik von Bathynella natans Vejd. Zoologisches Jahrbuch, Systematic 83(1/2): 1–62.
Jordan, H., 1847. Entdeckung fossiler Crustaceen im Saarbrücken’schen Steinkohlengebirge. Verhandlungen des naturhistorischen Vereines preussischen Rheinlande 4: 89–92.
Lange, S. & F. Schram, 1999. Evolución y filogenia de los crustáceos. Boletín de la Sociedad Entomológica Aragonesa 26: 235–254.
Noodt, W., 1964. Natürliches System und Biogeographie der Syncarida. Gewässer und Abwässer 37/38: 77–186.
Notenboom, J., 1991. Marine regressions and the evolution of groundwater dwelling amphipods (Crustacea). Journal of Biogeography 18: 437–454.
Richter, S., & G. Scholtz, 2001. Phylogenetic analysis of the Malacostraca (Crustacea). Journal of Zoological Systematics and Evolutionary Research 39: 113–136.
Schram, F. R., 1977. Paleozoogeography of the late Paleozoic and Triassic Malacostraca. Systematic Zoology 26: 367–379.
Schram, F. R., 1981. On the classification of Eumalacostraca. Journal of Crustacean Biology 1(1): 1–10.
Schram, F. R., 1984. Fossil Syncarida. Transaction of the San Diego Society of Natural History 20: 189–246.
Schram, F. R., & C. H. J. Hof, 1998. Fossils and the interrelationships of Major Crustacean groups. In: Edgecombe, G. D. (ed.) Arthropod Fossils and Phylogeny. Columbia University Press, New York: 233–302.
Schminke, H. K., 1973. Evolution, System und Verbreitungsgeschichte der Familie Parabathynellidae (Bathynellacea, Malacostraca). Mikrofauna Meeresboden 24: 1–192.
Schminke, H. K., 1975. Phylogenie und Verbreitungsgeschichte der Syncarida (Crustacea, Malacostraca). Verhandlungen Zoologische Gesellschaft, Bochum: 384–388.
Schminke, H. K., 1978a. Die phylogenetische Stellung der Stygocarididae (Crustacea: Syncarida). Unter besonderer Berücksichtingung morphologischer Ähnlichkeiten mit Larvenformen der Eucarida. Zeitschrift für zoologische Systematik und Evolutionforschung 16: 225–239.
Schminke, H. K., 1981. Adaptation of Bathynellacea (Crustacea, Syncarida) to the life in the interstitial (“Zoea Theory”). International Revue Gesamter Hydrobiologie 66(4): 576–637.
Serban, E., 1972. Bathynella (Podophallocarida, Bathynellacea). Travaux de l’Institut de Spéologie “Émile Racovitza” 11: 1–398.
Serban, E., 1980. La mandibule et l’individualisation des ensembles évolutifs majeurs dans l’ordre des Bathynellacea (Malacostraca: Podophallocarida). Bijdragen tot de Dierkunde 50(1): 155–189.
Serban, E., 2000. Uenobathynella n.g., Parauenobathynella n.g., Morimotobathynella n.g., Nihobathynella n.g. et Paradoxibathynella n.g., Bathynellinae du Japón (Bathynellidae, Bathynellacea, Podophallocarida). Travaux de l’Institut de Spéologie Émile Racovitza 36: 3–61.
Siewing, R., 1959. Syncarida. In Bronn, H. G. (ed.), Klassen und Ordnungen des Tierreichs. Akademische Verlagsgesellsch. Geest & Portig, Leipzig, Thomson, 5(1): 121; 4(2): 1–121.
Simberloff, D., 1972. Models in biogeography. In: Schopf, J. M. (ed.), Models in Paleobiology. Freeman, Cooper & Company, San Francisco: 160–191.
Smith, G., 1908. On the Anaspidacea. Living and Fossil. Quaternaly Journal of Microscopy Sciences 53: 489–578.
Spears, T. & L. G. Abele, 1999. Crustacean phylogeny inferred from 18s rDNA. In: Fortey R. A. & R. H. Thomas (eds), Arthropod relationship. Chapman and May, London: 160–187.
Stock, J. H., 1980. Regression model evolution as exemplified by genus Pseudoniphargus (Amphipoda). Bijdragen tot Dierkunde 50(1): 105–144.
Wallace, A. R. 1876. The geographical distribution of animals. Harper, New York, xxiii +503, xi + 553 pp (reprinted 1962, Hafner, New York).
Acknowledgements
We gratefully acknowledge the assistance of C. Puch, J.M. Becerra and M.L. Peláez. This study was supported by projects EVK2-CT-2001-00121 (PASCALIS) and Convenio Junta de Castilla y León- CSIC (2002–2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: E. V. Balian, C. Lévêque, H. Segers & K. Martens
Freshwater Animal Diversity Assessment
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Camacho, A.I., Valdecasas, A.G. Global diversity of syncarids (Syncarida; Crustacea) in freshwater. Hydrobiologia 595, 257–266 (2008). https://doi.org/10.1007/s10750-007-9021-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9021-5