Abstract
Diatoms are among the most widely used indicators of human and climate induced wetland salinity history in the world. This is particularly as a result of the development of diatom-based models for inferring past salinity. These models have primarily been developed from relationships between diatoms and salinity measured at the time of sampling or during the preceding year. Although within site variation in salinity has the potential to reduce the efficacy of such models, its influence has been rarely considered. Hence, diatom–conductivity relationships in eight seasonally monitored wetlands have been investigated. In developing a diatom–conductivity transfer function from these sites, we sought to assess the influence of conductivity variation on diatom inference model performance. Our sites were characterised by variability in conductivity that was not correlated to its range and thus were well suited to an investigation of this type. We found, contrary to expectations, that short-term (seasonal) changes in conductivity which were often dramatic did not result in unduly reduced transfer function performance. By contrast, sites that were more variable in the medium term (5–6 years) tended to have larger model errors. In addition, we identified a secondary ecological gradient in the diatom data which could not be related to any measured variable (including pH, turbidity or nutrient concentrations).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinisation of the aquatic and terrestrial environment is regarded as the most significant threat to the health of Australian ecosystems. Landscape salinisation has a variety of different causes, but essentially the majority of human induced salinisation derives from rising water tables that bring dissolved salts near or to the surface, resulting in reductions in biodiversity. Soil and lake salinisation is a natural process (Bowler, 1981; Crowley, 1994) but is being accelerated by human agency in many localities. Palaeolimnology is an important means of determining the nature and extent of aquatic ecosystem degradation arising from secondary (human-induced) salinisation.
Diatoms are one of the key indicators in documenting the full extent of changes associated with secondary salinisation in Australia from pre-impact conditions, through early settlement to the present day (Gell et al., 2005a, b). Importantly, in relation to the objectives of the International Geosphere-Biosphere Programme’s LIMPACS (“Human Impacts on Lake Ecosystems”) project to integrate understanding of past, present and future lake ecosystem change, diatoms are used routinely in both palaeosalinity studies (Gasse et al., 1997) and modern biomonitoring of salt lakes (Gell et al., 2002). As a result, diatom studies are potentially able to bridge the temporal divide between historical and process studies. This is particularly through the application of diatom–salinity transfer functions that permit quantitative estimates of past lake salinity (Fritz et al., 1999). However, despite the demonstrated strengths of diatom–salinity transfer functions, a number of factors serve to mediate relationships between diatoms and salinity. These include factors which are shared with other diatom–environment relationships such as confounding by water quality variables not of primary interest (notably pH, ionic composition (Gasse et al., 1995) and nutrients (Saros & Fritz, 2000a, b)), the seasonality of diatom production and preservation (Wilson et al., 1996; Gasse et al., 1997) and mismatches between the length of time represented by diatom surface samples and water quality data (with the upper centimetre in dated River Murray wetlands representing between <1 and >10 years (Reid et al., 2002; Gell et al., 2006)). Other issues, such as problems associated with diatom dissolution (Reed, 1998; Ryves et al., 2001), are of greater importance in salt lakes. However, this study is particularly focussed on examining the effect of within-site salinity variability on the strength of diatom–environment models.
Aquatic environments in Australia are among the most hydrologically variable in the world, particularly on timescales greater than 1 year (Puckridge et al., 1998). This observation may be applied more widely than is appropriate, given that it is particularly true of the arid and semi-arid zone where traditionally less aquatic research has been undertaken. Nevertheless, it is clear that aquatic environments associated with even large river systems are subject to substantial hydrological variability, with, for example, the Murray River which has the largest drainage basin in Australia regularly drying in its downstream reaches under pre-regulation flow conditions (Powell, 1989).
This variability, in combination with some observed lack of clearly explicable shifts in lower Murray diatom records, led Fluin (2002) to suggest that diatom species with broad ecological niches are advantaged in lower River Murray wetlands. This, in turn, was seen to potentially inhibit the efficacy of diatom–salinity relationships in these wetlands. Although two diatom–salinity models have been developed in the Murray-Darling Basin (Tibby & Reid, 2004; Philibert et al. 2006), these have focussed on in-stream samples and are therefore unlikely to have widespread applicability in wetlands.
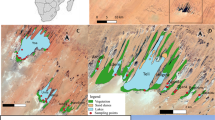
In light of the above, this study develops a diatom–salinity model from a small selection of wetlands in north-west Victoria (Fig. 1a and b) and particularly focuses on investigating factors which may lead to reduced performance in such models. A subset of these wetlands have already been the subject of analysis by Gell et al. (2002) who have demonstrated the primacy of salinity in influencing the diatom community. Given that these wetlands are subject to substantial hydrological variability (with changes in electrical conductivity in some sites exceeding 10,000 μS cm−1 over a 3-month period), we sought to evaluate three hypotheses:
-
1.
That the conductivity gradient (represented by site mean and/or median conductivity) is not related to conductivity variability in these wetlands,
-
2.
That the greatest salinity variability would be associated with the greatest errors in diatom–salinity models and,
-
3.
That, through a combination of factors unrelated to salinity, individual sites have distinctive communities that are unresponsive to salinity change.
Materials and methods
Data from the eight most perennial wetlands monitored under a Victorian State Government salinity management programme were selected for analysis. Three wetlands (Callendar’s Swamp, Karadoc Swamp; Little Mullaroo Creek) are closely linked to the main channel of the Murray River while two basins (Psyche Bend Lagoon, Lake Ranfurly) are used to retain saline groundwater seepage. Lake Cullulleraine is remote from the River but maintained by an irrigation channel. Cardross Basin is maintained by the diversion of irrigation drainage water runoff and the occasional environmental flow. Lake Hattah is periodically connected to the Murray River by the sinuous anabranch Chalka Creek. All but two sites are set in a landscape of intensive horticultural irrigation. Lake Hattah is located within Hattah-Kulkyne National Park, whilst Little Mullaroo Creek is located within Murray-Sunset National Park. Field sampling and laboratory methods are detailed in Gell et al. (2002) and are briefly summarised here.
Diatom and water samples were taken from the wetland edge in approximately 50 cm water depth quarterly except where sites were dry or occasionally where flooding prevented access (see Table 1). Surface sediment diatoms were sampled, with approximately the top 5 mm of the surface mud collected in an attempt to focus on collection of living or recently deceased diatoms. Electrical conductivity (as a proxy for salinity), pH, temperature and dissolved oxygen were measured in the field with a Horiba U-10 water quality meter. Frozen water samples were sent to National Association of Testing Agencies (Australia) accredited laboratories for analysis of turbidity, total phosphorus, total kjeldahl nitrogen, nitrate, nitrite and total nitrogen. Diatoms were processed using a modification of the method outlined in Battarbee et al. (2001) and were counted at between 1,000 and 1,500×, predominantly on a Zeiss Axiolab or Olympus BH-2 microscope with, respectively, phase contrast optics or Normaski differential interference contrast.
Statistical analyses were undertaken in SPSS for Windows version 13.0 (SPSS, 2004), Canoco for Windows 4.02 (ter Braak & Smilauer, 1999), PRIMER version 5.0 (Clarke & Gorley, 2001) and C2 (Juggins, 2004). SPSS (SPSS, 2004) was used to determine summary statistics for the data and for Pearson correlation calculations. The relative standard deviation (standard deviation/mean × 100) was used as a measure of conductivity variability in the data. Canonical Correspondence Analysis was implemented in Canoco for Windows 4.02 to assess the extent to which measured variables influence diatom assemblage composition. In particular, it was used to assess whether conductivity explained the greatest variance in the diatom data and which other measured variables were important. Only taxa with relative abundances exceeding 1% in any sample were included, with rare species down weighted. Unrestricted Monte Carlo permutations (n = 1999) of species–environment relationships were used to assess the significance of diatom–environment relationships. PRIMER (Clarke & Gorley, 2001) was used to ordinate the diatom relative abundance data via multidimensional scaling (MDS) where Bray–Curtis similarity (using all taxa >1% without transformation or standardisation) was used to derive the similarity matrix from which the MDS (with 20 restarts) was generated. Given that our 2-dimensional MDS output was characterised with a high level of stress (see results), we utilised Procrustes rotation to compare this solution with that of a correspondence analysis (implemented in Canoco for Windows 4.02 with Hill’s scaling focussed on samples, with rare taxa downweighted). We used PROTEST (Jackson, 1995) to implement the Procrustes analysis and undertake Monte Carlo permutation tests (n = 1999) to assess whether any similarity might arise due to chance.
C2 (Juggins, 2004) was used to derive weighted-averaging based diatom–conductivity models. We selected the best model as that with the lowest root-mean-squared error of prediction (RMSEP). Since the major purpose of our study was to investigate the environmental factors that affected the development of transfer functions we opted not to attempt to improve the model via outlier removal.
Results
The wetlands in this data set vary from fresh (Little Mullaroo Creek, Lake Cullulleraine and Callendar’s Swamp) to hypersaline (Lake Ranfurly West) (see Table 2 and Fig. 2). All are alkaline and eutrophic (based on mean TP concentrations, OECD, 1982), with some experiencing very high turbidity (Lakes Cullulleraine and Hattah and Callendar’s Swamp). Diatom assemblages in these sites are diverse (Fig. 3), with over 130 taxa that have a maximum relative abundance of greater than 5%.
Distribution of salinity in the study sites. Boxes represent the interquartile range, horizontal lines represent the upper, middle and lower horizontal lines represent the 90th percentile, median and 10th percentile, respectively. Filled circles represent maximum, minimum and 5th and 95th percentiles
Relative abundances of the dominant diatom taxa and conductivity in the north-west Victorian wetlands. Only the most abundant (>60% in any sample) taxa are shown, with the exception of Staurosirella pinnata, included for discussion purposes. Sites are ordered in terms of increasing mean conductivity. Taxa are ordered according to their optima (in log10 g salt l−1 from Gell, 1997). Gell’s (1997) optima and tolerances (in log10 g salt l−1) are shown in parentheses. Where optima for subspecies are not published, the information for the nominate variety is provided
Summary conductivity data are presented in Table 3 and Fig. 2. These reveal that there is no simple relationship between mean or median conductivity and variability. For example, although Lake Ranfurly has the highest mean and median conductivity of the sites, it is the fourth least variable (relative standard deviation: 24). Indeed for the data set, there is no significant correlation between mean conductivity and relative standard deviation (r 2 = 0.06, P = 0.99).
Canonical correspondence analysis (CCA) indicates that conductivity explains the largest (3.86%), significant (P = 0.001) amount of variance in the data set (Table 4). Other variables important in explaining diatom variance independent of that associated with conductivity include pH (variance explained 2.21%, P = 0.001), nitrate, total nitrogen, turbidity and dissolved oxygen. Given that conductivity explains the greatest amount of variance in our data set, it is appropriate to develop a diatom model for inferring this variable (Birks, 1998).
A simple weighted averaging model with inverse deshrinking produced the best performing diatom–conductivity model (determined by leave-one-out replacement) with a RMSEP of 0.318 log10 μS cm−1 and a jacknifed r 2 between measured and predicted conductivity of 0.89 (see Table 5). Since our study focussed on factors leading to model errors, no attempts were made to further improve model performance (e.g. through the deletion of outliers). Despite this, it is clear that some samples have very high measured minus diatom–inferred conductivity residuals. For example, a single sample from Hattah Lake has a predicted conductivity that overestimates the measured value almost 1.5 log10 μS cm−1 (Fig. 4). Conversely, diatoms in two samples from Psyche Bend Lagoon under-predict conductivity by greater than 0.7 log10 μS cm−1.
Discussion
As can be seen by the distribution of diatoms in relation to salinity in Fig. 3, the salinity preferences of diatoms in this study appear broadly similar to those observed in Gell (1997). Sites with low salinity (e.g. Little Mullaroo Creek, Lake Cullulleraine and Hattah Lake are dominated by taxa (e.g. Aulacoseira granulata and Pseudostaurosira brevistriata) with low salinity optima in Gell (1997). Similarly, taxa with high optima (e.g. Nitzschia etoshensis and Navicula salinicola) are largely restricted to the more saline sites. This outcome is despite the observed diversity in diatom assemblages and the contrasting hydrological regime of the riverine wetlands in this study and the predominantly closed drainage wetlands described in Gell (1997). Furthermore, it suggested that the diatom–salinity relationships outlined in Gell (1997) can therefore be applied to a broad range of wetland types in south-eastern Australia.
Assessment of hypothesis 1
The range of wetland salinity (measured by mean or median) bears little relationship to salinity variability in our data set (Table 3, Fig. 2). This outcome supports hypothesis 1. Importantly, in terms of investigating the effect of variability on the performance of diatom transfer functions (hypothesis 2), this is a useful phenomenon, since conductivity variability is independent from the range of salinity in these wetlands (as measured by mean or median). This observation is in contrast to those made about diatom–nutrient relationships where it is has been observed that increases in concentration are associated with substantial increases in range (e.g. Gibson et al., 1996 cited in Anderson, 1997).
Assessment of hypothesis 2
Hypothesis 2 states that sites exhibiting the greatest salinity variability would be associated with the greatest errors in diatom–salinity inference models. This hypothesis stems from Fluin (2002) who suggested that hydrological and water quality variability in Murray River wetlands advantages taxa with broad ecological tolerance, permitting them to dominate. This observation includes, but is not restricted to, taxa in the Fragilariacae identified in the literature as less suited to diatom-based reconstructions (Bennion et al., 2001; Sayer, 2001). To investigate this notion, we used the magnitude of the difference (residual) between measured and diatom–inferred conductivity in the diatom–conductivity inference model (Fig. 4) as measure of “error”. The relationship between model errors and salinity variability was assessed in two ways. First, the degree of short-term, often rapid, conductivity changes preceding sampling was compared with the absolute residual for a given sample. Second, the effect of longer-term variability on model performance was assessed by comparing the mean of the diatom–conductivity residuals over the sampling period to the mean residual error for each site over that period.
It is clear from Fig. 5 that there is only a very weak positive relationship between conductivity change and the size of the measured minus diatom–inferred conductivity residuals. It appears, therefore, that salinity change on a seasonal timeframe is not an important explanatory factor in understanding sources of model diatom–conductivity errors. Notably, this is despite the fact that some sites experienced major changes in salinity (often >10,000 μS cm−1 in 3 months, Fig. 3), with the potential that a flora may have been preserved reflecting a very different water chemistry. There is poor preservation of diatoms in sediment cores from some of these sites (Tibby, Fluin and Gell unpublished data), suggesting that silica may be rapidly recycled from diatom valves deposited on the sediment surface to the water column.
Figure 6 illustrates the relationship between conductivity variability and the mean of the diatom–conductivity residuals over the sampling period. As noted above, this assessment is independent of the effect of gradient length since there is no significant relationship between mean conductivity and variability (r 2 = 0.06, P = 0.99). There is, however, a positive relationship between site variability and model residuals (r 2 = 0.19, P = 0.01), indicating that sites with variable conductivity over timeframes of several years are likely to have lower predictive power in diatom–salinity inference models. In total, this analysis provides support for hypothesis 2, that greatest salinity variability would be associated with the greatest errors in diatom–salinity models, but only over longer timescales.
Assessment of hypothesis 3
Given the significant relationship between model errors and longer-term salinity variability, we sought, finally, to assess whether individual sites were characterised by distinctive assemblages that were unrelated to salinity. Figure 7 illustrates the MDS biplot. Although this solution has a high stress level, Procrustes analysis indicates that it was highly significantly correlated with an output from correspondence analysis (m 12 = 0.24, P = 0.0005) and suggests that it adequately summarises the main patterns in our diatom data. As can be seen from Fig. 7, the MDS indicates that, although there is some partitioning of individual localities, samples with similar salinity for the most part have similar species assemblages, providing further support for the notion that salinity exhibits a strong influence on the diatom flora from these wetlands. Indeed, there was a strong relationship between the first axis of the MDS ordination and conductivity (r 2 = 0.53, P < 0.005), considerably stronger than that exhibited by the next most important variable, total nitrogen (TN) (r 2 = 0.23, P < 0.005). At the high salinity end of the gradient, samples from Lake Ranfurly, Psyche Bend Lagoon and Karadoc Swamp overlap. Similarly the low salinity sites of Little Mullaroo River, Callendar’s Swamp, and Lake Hattah exhibit considerable overlap of sample scores that are, for the most part, positive on axis 1 and negative on axis 2. Given its low mean salinity (second lowest in the data set), Lake Cullulleraine is somewhat isolated from the other low salinity sites (on axis 2) and has a closer association with the flora from the high salinity sites, Psyche Bend Lagoon and Karadoc Swamp. Lake Cullulleraine samples are dominated by Pseudostaurosira brevistriata, a taxon which can thrive in a wide variety of environments (Bennion et al., 2001; Weckstrom & Juggins, 2006). As was noted earlier, this site has high turbidity. However, despite the fact that turbidity was identified as a significant explanatory variable in CCA, there is little relationship between turbidity and axis 2 scores (r 2 = 0.01, P = 0.34) which might point to this being a factor in this site’s separation on this axis. Indeed, the strongest relationship exhibited between any variables and axis 2, again related to TN, was weak, although significant (r 2 = 0.06, P = 0.01). It appears therefore that other (unmeasured) variables have an important influence on the diatom flora in these wetlands, particularly in Lake Cullulleraine.
Sample scores for a multidimensional scaling of the north-west Victorian wetland diatom data. (a) Scores from Psyche Bend Lagoon, Lake Ranfurly, Little Mullaroo Creek and Lake Cullulleraine, (b) scores from Callendar’s Swamp, Cardross Basin, Hattah Lake and Karadoc Swamp. Bubble sizes are proportional to the conductivity at time of sampling
Conclusions
Salinity exerts a dominant influence on diatom communities in north-west Victorian wetlands. An apparently reliable diatom–conductivity transfer function can be derived from diatom samples regularly taken from a relatively small number of wetlands. Our analysis indicates that salinity variability exerts a moderate influence on the performance of diatom–salinity models, particularly over medium length temporal scales. Certainly, this outcome supports Fluin’s (2002) original call for caution when interpreting records dominated by taxa with broad ecological tolerances, a conclusion drawn about some of the same taxa in diverse localities around the globe (e.g. Bennion et al., 2001; Reid, 2005). Nevertheless, diatoms are highly important in demonstrating the extent of post-settlement salinity impact in Murray-Darling Basin wetlands (Gell et al., 2005a, b; Fluin et al., 2007; Gell et al., 2007).
Few diatom transfer functions have been developed from a small number of intensely monitored sites (though see Mackay et al., 2003 for an examination of this possibility). Despite this, it appears from this study that transfer functions with strong performance can be developed from small numbers of sites where substantial within-site variation creates long environmental gradients. Such an outcome is desirable particularly where few sites analogous to fossil conditions exist (such as in the Yarra River catchment east of Melbourne, Victoria, Leahy et al., 2005).
References
Anderson, N. J., 1997. Historical changes in epilimnetic phosphorus concentrations in six rural lakes in Northern Ireland. Freshwater Biology 38: 427–440.
Battarbee, R. W., V. J. Jones, R. J. Flower, N. G. Cameron, H. Bennion, L. Carvalho & S. Juggins, 2001. Diatoms. In Stoermer E. F., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments. Volume 3: Terrestrial, Algal and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht, The Netherlands, 155–202.
Bennion, H., P. G. Appleby & G. L. Phillips, 2001. Reconstructing nutrient histories in the Norfolk Broads, UK: implications for the role of diatom-total phosphorus transfer functions in shallow lake management. Journal of Paleolimnology 26: 181–204.
Birks, H. J. B., 1998. Numerical tools in palaeolimnology – progress, potentialities, and problems. Journal of Paleolimnology 20: 307–332.
Bowler, J. M., 1981. Australian salt lakes. A palaeohydrological approach. Hydrobiologia 82: 431–444.
Clarke, K. R. & R.N. Gorley, 2001. Primer v5: User Manual/Tutorial. Plymouth, PRIMER-E Ltd.
Crowley, G. M., 1994. Quaternary soil salinity events and Australian vegetation history. Quaternary Science Reviews 13: 15–22.
Fluin, J., 2002 A Diatom-based Palaeolimnological Investigation of the Lower River Murray, South Eastern Australia. Ph.D. Thesis, School of Geography & Environmental Science, Monash University, Victoria, Australia.
Fluin, J., P. Gell, D. Haynes, J. Tibby & G. Hancock, 2007. Palaeolimnological evidence for the independent evolution of neighbouring terminal lakes, the Murray Darling Basin, Australia. Hydrobiologia 591: 117–134.
Fritz, S. C., B. F. Cumming, F. Gasse & K. R. Laird, 1999. Diatoms as indicators of hydrologic and climatic change in saline lakes. In: Stoermer E. F., J. P. Smol (eds) The Diatoms: Applications for Environmental and Earth Sciences. Cambridge University Press, London, 41–72.
Gasse, F., S. Juggins & L. BenKhelifa, 1995. Diatom-based transfer functions for inferring past hydrochemical characteristics of African lakes. Palaeogeography, Palaeoclimatology, Palaeoecology 117: 31–54.
Gasse, F., P. A. Barker, P. A. Gell, S. C. Fritz & F. Chalie, 1997. Diatom-inferred salinity of palaeolakes, an indirect tracer of climate change. Quaternary Science Reviews 16: 547–563.
Gell, P. A., 1997. The development of diatom database for inferring lake salinity, Western Victoria, Australia: Towards a quantitative approach for reconstructing past climates. Australian Journal of Botany 45: 389–423.
Gell, P. A., I. R. Sluiter & J. Fluin, 2002. Seasonal and inter-annual variations in diatom assemblages in Murray River-connected wetlands in northwest Victoria, Australia. Marine and Freshwater Research 53: 981–992.
Gell, P., S. Bulpin, P. Wallbrink, S. Bickford & G. Hancock, 2005a. Tareena Billabong – a palaeolimnological history of an everchanging wetland, Chowilla Floodplain, lower Murray-Darling Basin. Marine and Freshwater Research 56: 441–456.
Gell, P., J. Tibby, J. Fluin, P. Leahy, M. Reid, K. Adamson, S. Bulpin, A. MacGregor, P. Wallbrink & B. Walsh, 2005b. Accessing limnological change and variability using fossil diatom assemblages, south-east Australia. River Research and Applications 21: 275–269.
Gell, P., J. Tibby, F. Little, D. Baldwin & G. Hancock, 2007. The impact of regulation and salinisation on floodplain lakes: the lower River Murray, Australia. Hydrobiologia 591: 135–146.
Gell, P., J. Fluin, J. Tibby, D. Haynes, S. Khanum, B. Walsh, G. Hancock, J. Harrison, A. Zawecki & F. Little, 2006. Changing fluxes of sediments and salts as recorded in lower River Murray wetlands, Australia. International Association of Hydrological Sciences 306: 416–424.
Gibson, C. E., R. H. Foy & A. E. Bailey Watts, 1996. An analysis of the total phosphorus cycle in some temperate lakes – the response to enrichment. Freshwater Biology 35: 525–532.
Jackson, D. A., 1995. PROTEST: a PROcrustean randomization TEST of community environment concordance. Ecoscience 2: 297–303.
Juggins, S., 2004. C2 data analysis. Newcastle, University of Newcastle.
Leahy, P. J., J. Tibby, A. P. Kershaw, H. Heijnis & J. S. Kershaw, 2005. The impact of European settlement on Bolin Billabong, a Yarra River floodplain lake, Melbourne, Australia. River Research and Applications 21: 131–149.
Mackay, A. W., R. W. Battarbee, R. J. Flower, N. G. Granin, D. H. Jewson, D. B. Ryves & M. Sturm, 2003. Assessing the potential for developing internal diatom-based transfer functions for Lake Baikal. Limnology & Oceanography 48: 1183–1192.
Organisation for Economic Co-operation and Development (OECD), 1982. Eutrophication of waters: monitoring, assessment and control OECD, Paris.
Philibert, A., P. Gell, P. Newall, B. Chessman & N. Bate, 2006. Development of diatom-based tools for assessing stream water quality in south-eastern Australia: assessment of environmental transfer functions. Hydrobiologia 572: 103–114.
Powell, J. M., 1989. Watering the Garden State. Water, land and community in Victoria, 1834–1988. Allen and Unwin, Sydney.
Puckridge, J. T., F. Sheldon, K. F. Walker & A. J. Boulton, 1998. Flow variability and ecology of large rivers. Marine & Freshwater Research 49: 55–72.
Reed, J. M., 1998. Diatom preservation in the recent sediment record of Spanish Saline Lakes – implications for palaeoclimate study. Journal of Paleolimnology 19: 129–137.
Reid, M., 2005. Diatom-based models for reconstructing past water quality and productivity in New Zealand lakes. Journal of Paleolimnology 33: 13–38.
Reid, M., J. Fluin, R. Ogden, J. Tibby & P. Kershaw, 2002. Long-term perspectives on human impacts on floodplain-river ecosystems, Murray-Darling Basin, Australia. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 28: 710–716.
Ryves, D. B., S. Juggins, S. C. Fritz & R. W. Battarbee, 2001. Experimental diatom dissolution and the quantification of microfossil preservation in sediments. Palaeogeography, Palaeoclimatology, Palaeoecology 172.
Saros, J. E. & S. C. Fritz, 2000a. Changes in the growth rates of saline-lake diatoms in response to variation in salinity, brine type and nitrogen form. Journal of Plankton Research 22: 1071–1083.
Saros, J. E. & S. C. Fritz, 2000b. Nutrients as a link betwen ionic concentration/composition and diatom distributions in saline lakes. Journal of Paleolimnology 23: 449–453.
Sayer, C. D., 2001. Problems with the application of diatom-total phosphorus transfer functions: examples from a shallow English lake. Freshwater Biology 46: 743–757.
SPSS, 2004. SPSS for Windows 13.0. Chicago, SPSS.
ter Braak, C. J. F. & P. Smilauer, 1999. Canoco for Windows Version 4.02. Wageningen, The Netherlands, Centre for Biometry, Wageningen.
Tibby, J. & M. Reid, 2004. A model for inferring past conductivity in low salinity waters derived from Murray River diatom plankton. Marine and Freshwater Research 55: 587–607.
Weckström, K. & S. Juggins, 2006. Coastal diatom–environment relationships from the Gulf of Finland, Baltic Sea. Journal of Phycology 42: 21–35.
Wilson, S. E., B. Cumming & J. P. Smol, 1996. Assessing the reliability of salinity inference models from diatom assemblages: an examination of a 219-lake data set from western North America. Canadian Journal of Fisheries & Aquatic Sciences 53: 1580–1594.
Acknowledgements
Diatom samples and water quality were collected under the auspices of monitoring programmes supported by The Victorian Department of Sustainability and Environment under regional Salinity Management Action Plans. Chris Crothers drafted Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tibby, J., Gell, P.A., Fluin, J. et al. Diatom–salinity relationships in wetlands: assessing the influence of salinity variability on the development of inference models. Hydrobiologia 591, 207–218 (2007). https://doi.org/10.1007/s10750-007-0803-6
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0803-6